|
||
|
Applications of synthetic biology to stem-cell-derived modeling of early embryonic development
Synthetic Biology Journal
2025, 6 (3):
669-684.
DOI: 10.12211/2096-8280.2025-013
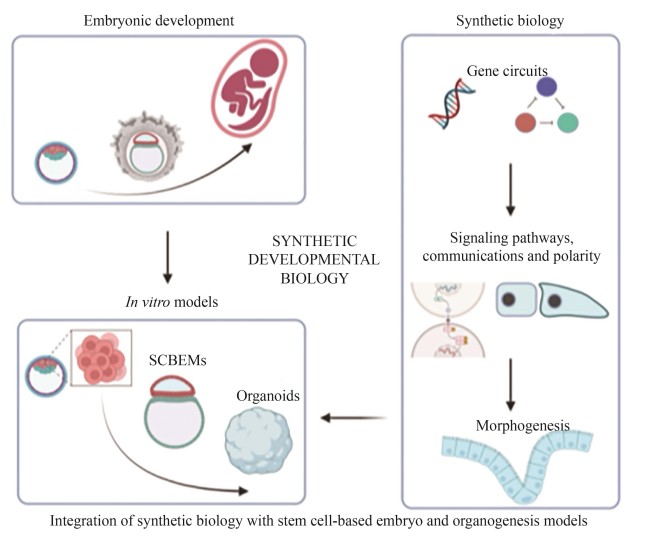
Understanding how a fertilized egg develops from a single cell into complex tissues and organs remains a central question in developmental biology. However, in mammals, especially in humans, technical and ethical constraints limit in utero investigation of the post-implantation development and ex utero culture beyond organogenesis as well. As a result, the molecular and cellular mechanisms underpinning spatiotemporal regulation during these stages remain poorly understand. This knowledge gap underscores the urgent need for high-fidelity in vitro models that not only recapitulate in vivo developmental processes but also allow for precise experimental perturbations. Recent advances in stem cell-based embryo models and organoids leverage the developmental potential and intrinsic self-organizing capabilities of pluripotent stem cells to mimic aspects of early embryonic and organ development, offering new platforms for studying those complex processes. Concurrently, synthetic biology provides powerful tools, such as programmable gene circuits, optogenetics, and engineered signaling pathways, to control gene expression, cell differentiation, intercellular communications, and tissue patterning with unprecedented precision. This review highlights recent progress in integrating synthetic biology with in vitro models to dissect and reconstitute fundamental mechanisms of embryonic development. By harnessing synthetic biology tools, researchers can now modulate specific pathways with temporal and spatial precision, enabling a deeper understanding of processes such as signal transduction dynamics, cellular adhesion networks, symmetry breaking, and the establishment of polarity. This bottom-up “build-to-learn” approach shifts the paradigm from observational to predictive developmental biology. Such innovations have collectively given rise to the emerging field of synthetic developmental biology. This field not only provides mechanistic insights into developmental events that were previously inaccessible but also opens new avenues for building artificial tissues and structures with tailored functions. We also discuss current limitations in mimicking the morphology and function of natural embryonic structures, emphasizing the need for robust evaluation systems and refined strategies to precisely control cell behavior. Finally, we explore how synthetic developmental biology can elucidate key principles of embryogenesis and accelerate future applications in regenerative medicine.
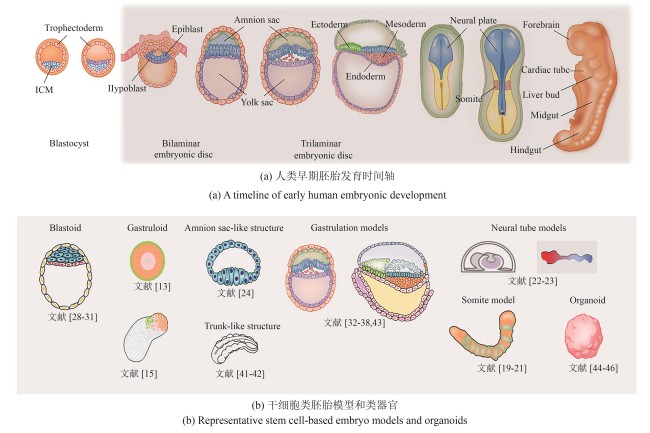
Fig.1
Early embryonic development models and organoids
(Human embryonic development begins with the blastocyst implanting into the uterine wall, followed by the formation of the bilaminar disc composed of the epiblast and hypoblast. Gastrulation then transforms the disc into a trilaminar structure: ectoderm, mesoderm, and endoderm, laying the foundation for early organogenesis. Here are representative stem cell-based embryo models selected by the authors, which recapitulate key developmental stages ranging from pre-implantation and peri-implantation to gastrulation and early organogenesis. Due to space constraints, we regret that not all relevant studies could be included.)
Extracts from the Article
胚胎发育始于受精卵与卵裂,随后分化为滋养外胚层(trophectoderm,TE)和内细胞团(inner cell mass,ICM)。其中,内细胞团进一步分化为上胚层(epiblast)和原始内胚层(primitive endoderm,又称下胚层hypoblast)。上胚层将发育成所有成体细胞类型,而滋养外胚层和下胚层分别贡献于胎盘和卵黄囊的形成。在胚胎植入后,上胚层后部形成原条(primitive streak),上胚层细胞通过原条迁移,形成中胚层(mesoderm)和定型内胚层(definitive endoderm),而留在上胚层的细胞最终发育为外胚层(ectoderm)[图1(a)]。这一过程被称为原肠运动(gastrulation),是细胞命运决定和形态发生的关键步骤。然而,由于技术和伦理限制,对这一发育关键时期的研究长期受阻,导致相关认知仍较为有限。为突破这一瓶颈,研究人员尝试在体外构建可控、可观察的简化模型,以模拟早期胚胎发育过程。经过近几十年的发展,科学家建立了具有不同发育潜能的PSC,包括原始态(na?ve)、中间态/形成态(intermediate/formative)和始发态(primed),这些干细胞在可控培养环境下能够模拟胚胎发育,为研究早期细胞命运决定机制提供了重要工具[8-10]。早期研究发现,由数百个ESC形成的聚集体(即胚状体,embryoid bodies),虽然能分化出多种胚胎组织,但整体结构与自然胚胎仍有较大差异[11-12]。Warmflash等[13]在直径约700 μm的碟形微图案区域上培养人类胚胎干细胞(hESC),并通过BMP4信号诱导生成类似人类原肠胚胚盘(embryonic disc)的细胞命运图式(cell fate patterning),揭示了多能性干细胞在二维(2D)培养环境下的自组装能力,这一模型被称为二维类原肠胚(2D-gastruloid)。与此同时,Martinez Arias课题组的一系列研究表明,在三维(3D)培养条件下,多能性干细胞团经Wnt信号激活后可打破对称性(symmetry breaking),形成轴向极性(axial polarity)[14-18]。基于这些研究,科学家们进一步构建了模拟体节(somite)发育的部分胚胎模型(partial embryo model)[19-21]。这些模型分别用于轴向延长(axial elongation)、体节发生(somitogenesis)和分节时钟(segmentation clock)的研究。此外,基于芯片和微流控技术的神经管(neural tube)模型也成功再现了早期神经发育的关键过程,使研究人员能够在受控环境中探索神经模式化(neural patterning)、神经管形态发生及谱系特化(lineage specification)[22-23]。上述类原肠胚及部分胚胎模型中因不包含滋养外胚层和下胚层等胚胎外细胞类型,因而无法模拟上胚层与胚外组织的相互作用。来自Fu实验室的系列工作表明,在微流控系统中,人胚胎干细胞在BMP4信号作用下可产生类似人的羊膜腔结构,该结构可用于研究羊膜细胞在上胚层分化过程中的作用[24-26]。此外,Rivron等[27]通过将小鼠的滋养层细胞干细胞(trophoblast stem cell)与胚胎干细胞进行共培养得到类似小鼠囊胚的结构,称之为类囊胚(blastoid)。随后的工作证实,通过人类原始态干细胞自组装可以得到人的类囊胚结构[28-31]。近两年来,人的类胚胎模型成功重现了围植入期(peri-implantation)的形态发生过程,整合了胚胎和胚胎外细胞类型(extra-embryonic cell type),包括上胚层和下胚层[32-35]。在某些情况下,这些模型还包含滋养层和胚外中胚层(extra-embryonic mesoderm,ExM)[36-38]。通过引入滋养层、下胚层和胚外中胚层,类胚胎进一步揭示了细胞分化、细胞间相互作用,组织形成和形态发生的关键分子机制。尤其在胚盘形成、对称性破缺、发育轴建立和原始造血(primitive hematopoiesis)方面。这些创新模型为未来解析人类胚胎发育的基本机制提供了全新的工具,相关内容可参考已有的中文综述文章[39-40]。此外,由干细胞或祖细胞在三维培养系统中自组织形成的类器官(organoid),现已模拟出多种类似自然器官的结构[图1(b)]。因篇幅有限,本文重点介绍类胚胎模型,关于类器官的详细综述请参看文献[44,47-53]。
Other Images/Table from this Article
|