|
||
|
Applications of synthetic biology to stem-cell-derived modeling of early embryonic development
Synthetic Biology Journal
2025, 6 (3):
669-684.
DOI: 10.12211/2096-8280.2025-013
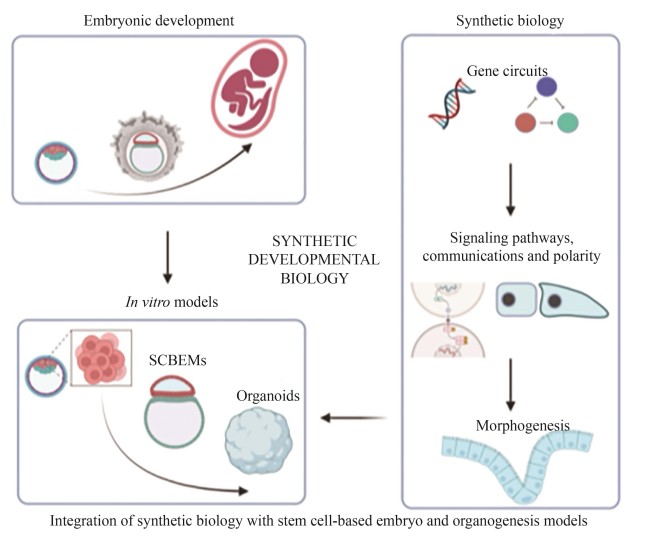
Understanding how a fertilized egg develops from a single cell into complex tissues and organs remains a central question in developmental biology. However, in mammals, especially in humans, technical and ethical constraints limit in utero investigation of the post-implantation development and ex utero culture beyond organogenesis as well. As a result, the molecular and cellular mechanisms underpinning spatiotemporal regulation during these stages remain poorly understand. This knowledge gap underscores the urgent need for high-fidelity in vitro models that not only recapitulate in vivo developmental processes but also allow for precise experimental perturbations. Recent advances in stem cell-based embryo models and organoids leverage the developmental potential and intrinsic self-organizing capabilities of pluripotent stem cells to mimic aspects of early embryonic and organ development, offering new platforms for studying those complex processes. Concurrently, synthetic biology provides powerful tools, such as programmable gene circuits, optogenetics, and engineered signaling pathways, to control gene expression, cell differentiation, intercellular communications, and tissue patterning with unprecedented precision. This review highlights recent progress in integrating synthetic biology with in vitro models to dissect and reconstitute fundamental mechanisms of embryonic development. By harnessing synthetic biology tools, researchers can now modulate specific pathways with temporal and spatial precision, enabling a deeper understanding of processes such as signal transduction dynamics, cellular adhesion networks, symmetry breaking, and the establishment of polarity. This bottom-up “build-to-learn” approach shifts the paradigm from observational to predictive developmental biology. Such innovations have collectively given rise to the emerging field of synthetic developmental biology. This field not only provides mechanistic insights into developmental events that were previously inaccessible but also opens new avenues for building artificial tissues and structures with tailored functions. We also discuss current limitations in mimicking the morphology and function of natural embryonic structures, emphasizing the need for robust evaluation systems and refined strategies to precisely control cell behavior. Finally, we explore how synthetic developmental biology can elucidate key principles of embryogenesis and accelerate future applications in regenerative medicine.
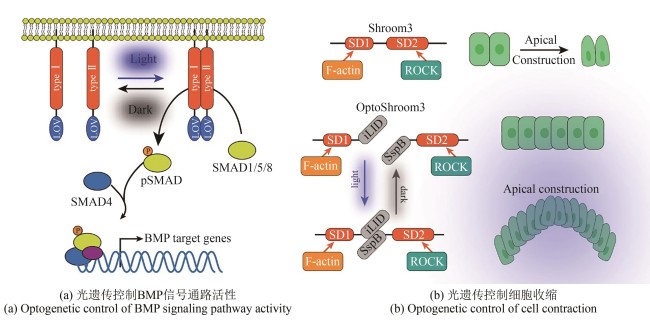
Fig. 3
Applications of optogenetic tools in developmental biology
(Dimerization mediated by the light-oxygen-voltage (LOV) sensing domain regulates intracellular tyrosine kinase activity, leading to SMAD1/5 phosphorylation and their translocation into the nucleus to initiate downstream BMP target gene expression. Optogenetically activated Shroom3 drives apical constriction through ROCK recruitment at apical junctions.)
Extracts from the Article
光遗传学(optogenetics)是一种结合光学与遗传学的前沿技术,近年来广泛用于发育生物学研究。其核心原理是利用光敏蛋白精准调控细胞内信号,可结合成像技术实时研究细胞行为和基因调控,能够以高时空分辨率进行控制,使其成为探索发育信号通路的强大工具[80-81]。BMP是发育过程中的关键信号分子。然而,传统外源添加BMP蛋白或小分子的方法无法精准模拟其动态激活模式。鉴于BMP信号依赖Ⅰ型和Ⅱ型受体的四聚化驱动下游SMAD蛋白磷酸化[82-83],研究人员设计了OptoBMP受体系统[图3(a)]。该系统锚定BMP受体胞内激酶结构域于细胞膜,并在其C端融合藻类光敏蛋白LOV。蓝光刺激诱导LOV二聚化,使Ⅰ型和Ⅱ型受体接近,从而精准启动BMP信号通路[84]。
为探究胚胎形态发生机制,研究人员开发了体外重建形态发生行为的方法。在后生动物发育中,顶端收缩(apical constriction)是形成弯曲结构的核心机制[85-86]。顶端收缩的发生由肌动蛋白收缩驱动,通常由顶侧的Rho-ROCK信号通路引起,发生于胚胎发育的特定阶段和区域。体外重构弯曲形态的组织要求对细胞的收缩有很强的时空控制性。光遗传技术恰是一种能在分子乃至多细胞水平上实现精确时空控制的强大方法[87-90]。诱导具有复杂组织结构的形态发生过程具有较大技术挑战,为克服技术难点,研究人员开发了新的光遗传学工具OptoShroom3。其设计基于光敏蛋白iLID与其结合蛋白SspB,Shroom3包含两个结构域:F-Actin结合域SD1(Shroom domain 1)和ROCK结合域SD2(Shroom domain 2)。N端结构域SD1与iLID融合,C端结构域SD2与SspB融合,这种类似剪切体的形式构成OptoShroom3。在蓝光刺激下iLID发生构象改变从而与SspB结合,激活OptoShroom3引起顶端收缩[91]。该研究表明,诱导顶面收缩的时空控制可以根据初始组织背景触发多种类型的3D组织形变,为研究细胞形态和组织发育提供了新的工具[图3(b)]。
Other Images/Table from this Article
|