|
||
|
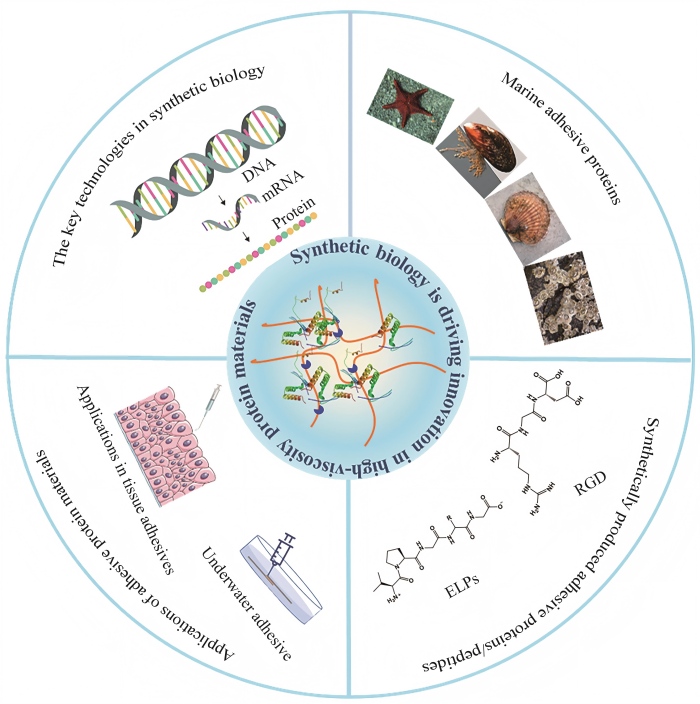
Synthetic biology and applications of high-adhesion protein materials
Synthetic Biology Journal
2025, 6 (4):
806-828.
DOI: 10.12211/2096-8280.2025-043
Due to their exceptional bioadhesive properties and potential biocompatibility, high-viscosity protein materials exhibit significant application prospects in the fields of biomedical materials and adhesives. However, traditionally sourced high-viscosity protein materials encounter numerous challenges, including low yields, structural complexity, and difficulties in scaling up production. Synthetic biology, as an emerging interdisciplinary field, offers innovative strategies to address these bottlenecks. This review systematically summarizes recent advances in the biosynthesis, modification, and applications of high-viscosity protein materials, focusing on the advantages of synthetic biology in addressing issues related to the yield, controllability, and functional diversity of these materials. The precise design and efficient expression of adhesive proteins, such as mussel adhesive proteins, barnacle cement proteins, and scallop foot proteins, achieved through genetic engineering, are comprehensively reviewed, demonstrating the overcoming of limitations in the production and controllability of high-viscosity protein materials. Furthermore, the unique advantages of these protein materials in bioadhesives and functional medical coatings, such as the wet adhesion of mussel proteins, the strong adhesion of barnacle cement proteins, and the tunable properties of elastin-like proteins, are summarized. By employing synthetic biology approaches, limitations in the yield, performance, and functionality of high-viscosity protein materials can be overcome, thereby accelerating their application in areas such as tissue engineering and surface modification. Finally, the latest advancements and innovations in the field of synthetic biology for high-viscosity protein materials are summarized, and future development directions are envisioned, offering new ideas and strategies for the development of high-performance, multifunctional high-viscosity protein materials.
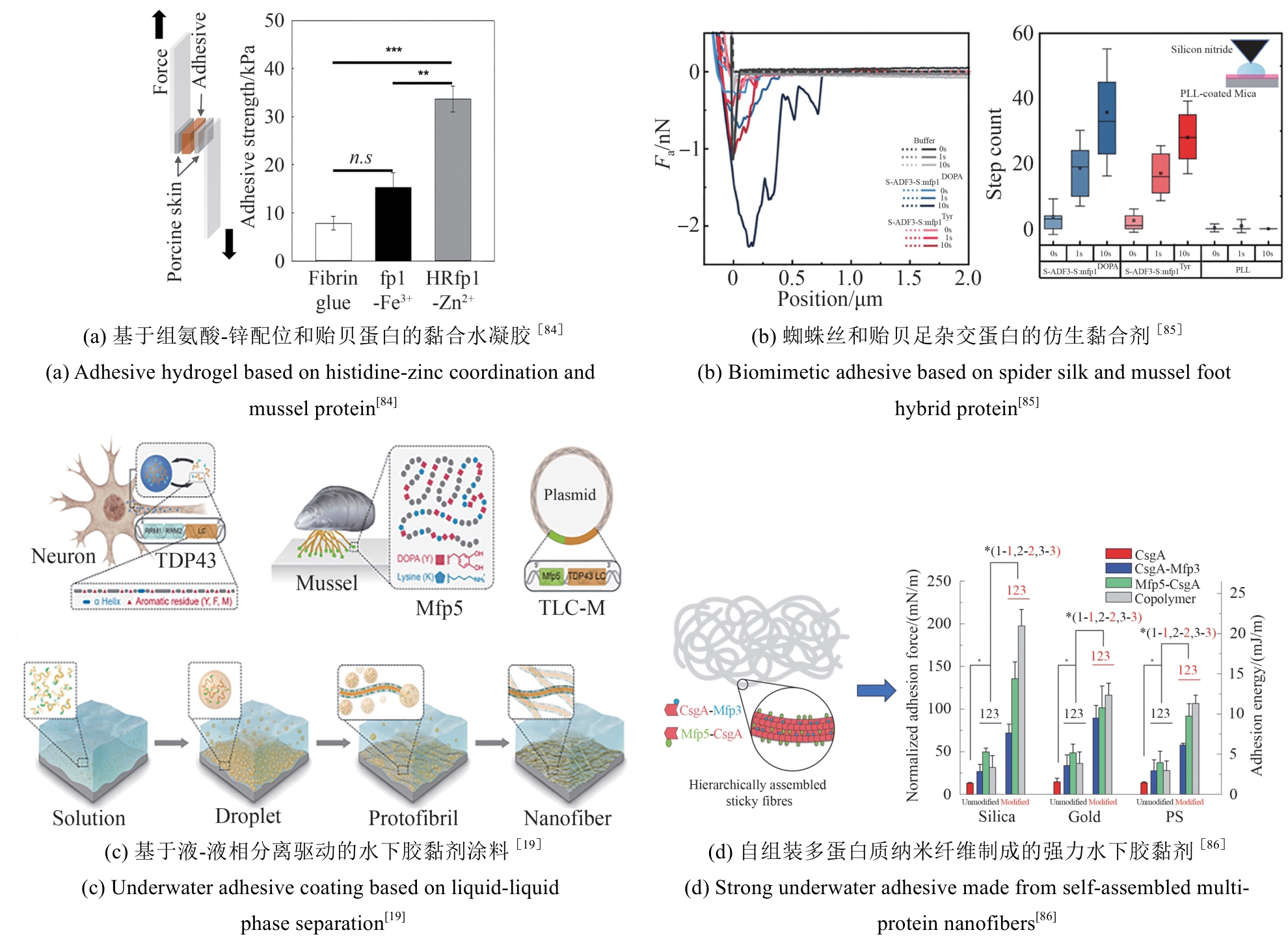
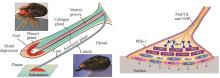
Fig. 2
Applications of Mfps in underwater adhesives
Extracts from the Article
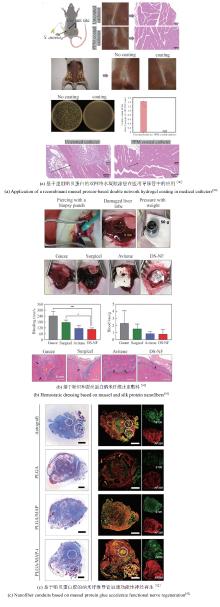
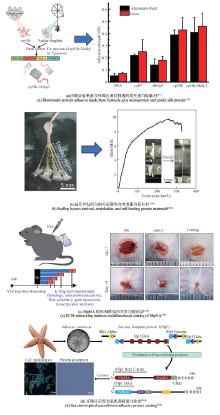
近年来,一些以DOPA为基础设计的多肽、水凝胶、聚合物等材料不断涌现,在界面黏附领域引起了广泛关注[83]。例如,Cha团队[84]通过基因融合富含组氨酸的连接蛋白Mfp-4结构域与富含DOPA的足斑蛋白Mfp-1,开发出一种新型混合黏附蛋白HRfp1。该蛋白利用组氨酸-Zn金属配位形成水凝胶,由于DOPA未参与交联而保留了黏附活性,从而克服了传统Mfp研究中黏附性与交联相互制约的难题,同时维持了优异的水下高黏附性能。这种组氨酸协同DOPA的多维设计策略为开发兼具自愈能力和强黏附性的仿生材料提供了新思路[图2(a)]。类似地,Yin等[85]利用合成生物技术将蛛丝蛋白(ADF3)与Mfp-1相结合,开发了一种高性能仿生黏合剂。他们利用SpyCatcher-SpyTag系统将两种蛋白共价偶联,并通过氧化贻贝足蛋白中的酪氨酸生成DOPA以增强黏附性[图2(b)]。
同时,一种利用理性设计结合模块基因策略的方法被用于水下黏合材料。通过这种方法构建的多结构域蛋白能够自组装形成性能媲美甚至超越天然材料的分子材料。钟超教授团队[19]将哺乳动物细胞DNA结合蛋白TDP43的低复杂结构域LC与Mfp-5融合,制备了重组蛋白TLC-M。TLC-M融合蛋白在低温下通过LC结构域的液-液相分离性质形成蛋白浓度很高的凝结体。该液态凝结体易逐层吸附在基底表面,最终能够进一步脱水组装成致密的淀粉样蛋白纤维涂层,表现出很强的水下黏附性能。这为构建基于液固相转变和自组装驱动的可控功能蛋白材料提供了新方向[图2(c)]。该团队还将Mfp与大肠杆菌淀粉样蛋白CsgA融合,构建了多功能水下黏合剂。该杂化材料能够自组装成Mfp黏附域暴露于CsgA淀粉样蛋白核心之外的高阶结构,水下黏附能可达20.9 mJ/m2,是目前生物衍生蛋白质黏合剂的1.5倍。其性能优于单独的Mfp或CsgA纤维,且在pH≥7.0时具有更强的抗氧化性[86][图2(d)]。
Other Images/Table from this Article
|