|
||
|
Synthetic biology and applications of high-adhesion protein materials
Synthetic Biology Journal
2025, 6 (4):
806-828.
DOI: 10.12211/2096-8280.2025-043
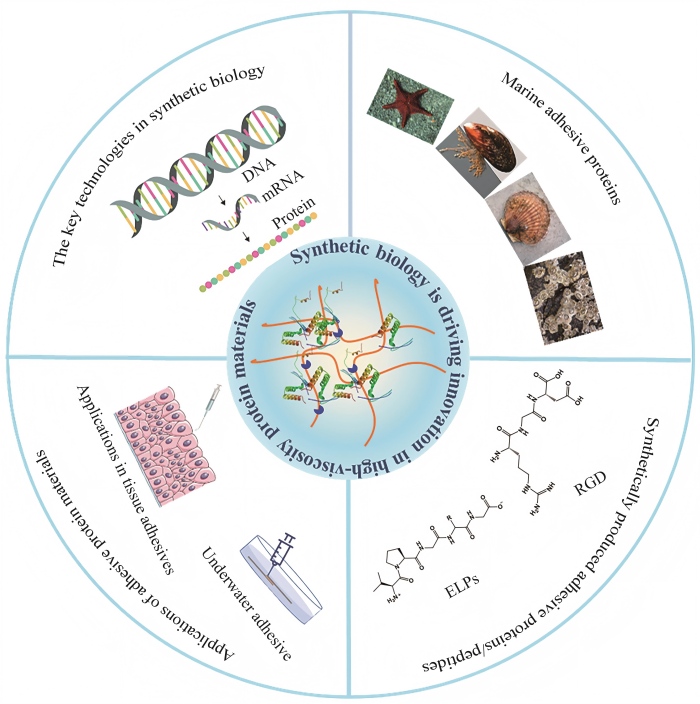
Due to their exceptional bioadhesive properties and potential biocompatibility, high-viscosity protein materials exhibit significant application prospects in the fields of biomedical materials and adhesives. However, traditionally sourced high-viscosity protein materials encounter numerous challenges, including low yields, structural complexity, and difficulties in scaling up production. Synthetic biology, as an emerging interdisciplinary field, offers innovative strategies to address these bottlenecks. This review systematically summarizes recent advances in the biosynthesis, modification, and applications of high-viscosity protein materials, focusing on the advantages of synthetic biology in addressing issues related to the yield, controllability, and functional diversity of these materials. The precise design and efficient expression of adhesive proteins, such as mussel adhesive proteins, barnacle cement proteins, and scallop foot proteins, achieved through genetic engineering, are comprehensively reviewed, demonstrating the overcoming of limitations in the production and controllability of high-viscosity protein materials. Furthermore, the unique advantages of these protein materials in bioadhesives and functional medical coatings, such as the wet adhesion of mussel proteins, the strong adhesion of barnacle cement proteins, and the tunable properties of elastin-like proteins, are summarized. By employing synthetic biology approaches, limitations in the yield, performance, and functionality of high-viscosity protein materials can be overcome, thereby accelerating their application in areas such as tissue engineering and surface modification. Finally, the latest advancements and innovations in the field of synthetic biology for high-viscosity protein materials are summarized, and future development directions are envisioned, offering new ideas and strategies for the development of high-performance, multifunctional high-viscosity protein materials.
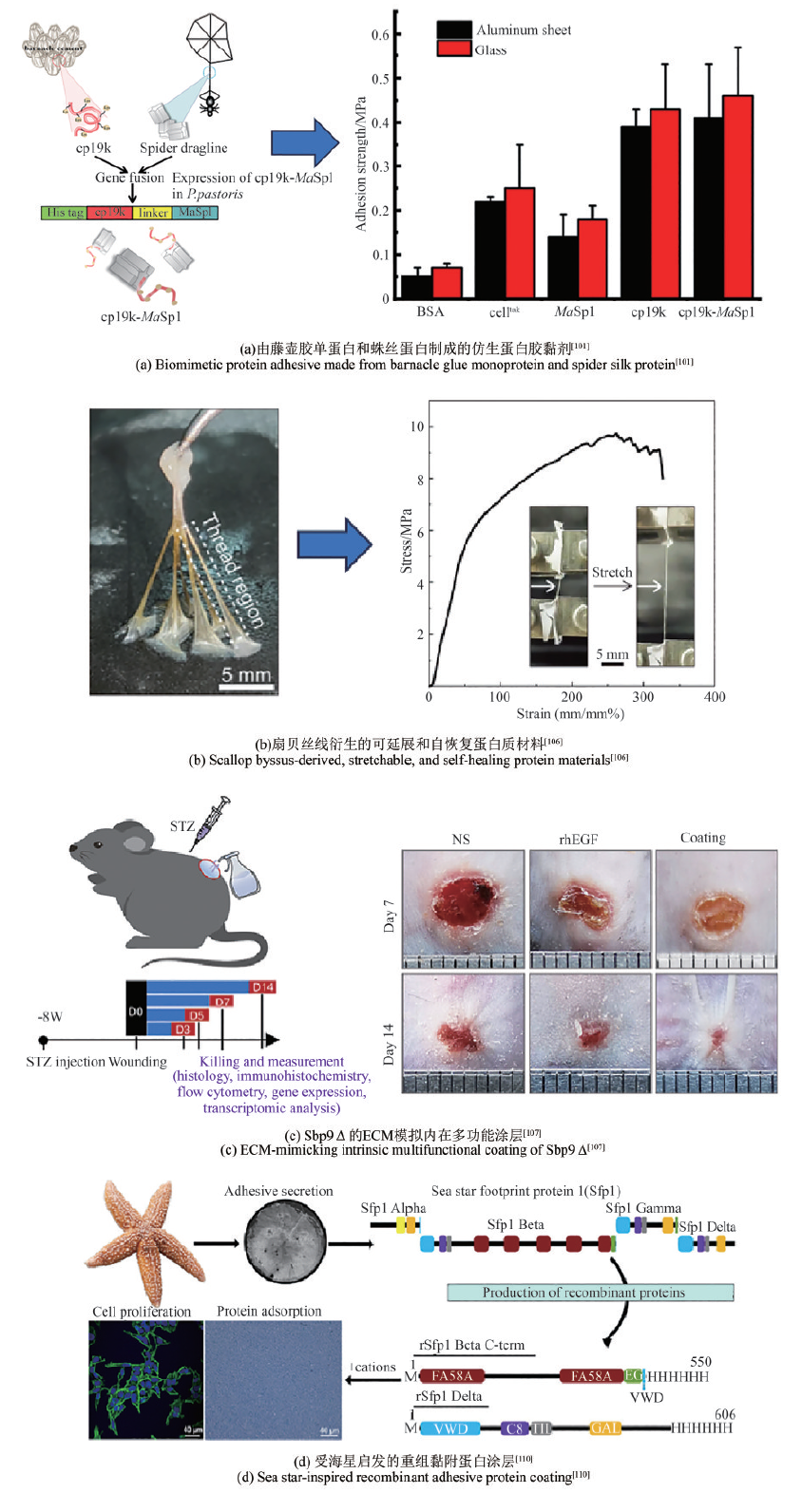
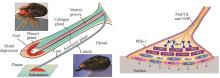
Fig. 4
Applications of other marine adhesive proteins, such as those from barnacles and scallops
Extracts from the Article
对藤壶胶黏附机制和黏胶蛋白的研究成果,为水下黏附剂的研发提供了坚实的理论基础。Ye等[101]通过基因设计并过表达了一种由cp19k和蛛丝蛋白(MaSp1)组成的融合蛋白(cp19k-MaSp1),并且通过静电纺丝制备的cp19k-MaSp1蛋白纤维支架表现出非常高的极端拉伸强度[(112.7±11.6)MPa]和优异的延展性(438.4%± 43.9%)[图4(a)]。Yan团队[102]为了提高Mrcp19k的水下黏弹性能与非水性环境下的黏附性能,利用基因工程学的方法,将不同的蛋白功能模块与Mrcp19k融合,构建了多种融合型黏合蛋白。结果显示,功能模块SpyCatcher/SpyTag以及ELP与黏合蛋白Mrcp19k融合,均能进一步提高黏合蛋白Mrcp19k的水下黏弹性能与非水性环境下的黏附性能。
Sbp的水下黏附能力,使其成为理想的生物医用黏合剂候选材料。例如,钟超团队[106]的研究揭示了扇贝足丝蛋白Sbp5-2中TRM结构赋予材料高延展性和自恢复特性。他们利用基因工程构建了含7个TRM串联的重组蛋白rTRM7,并在大肠杆菌中高效表达后,通过拉丝工艺制备的仿生纤维再现了天然材料的力学性能。研究发现,氢键、金属配位和二硫键构成的分子交联网络是性能调控的关键。通过嵌入石墨烯,他们开发出e-rTRM7导电纤维,并成功应用于人体运动监测传感器。该工作为生物基高性能材料设计提供了新思路,其可扩展生产和环境适应性在智能穿戴、医疗监测领域展现出潜在的应用前景[图4(b)]。Wang等[107]则聚焦于扇贝足丝蛋白Sbp9Δ(含10~13个表皮生长因子样EGFL模块),发现其二价钙离子可在30 min内触发原位形成多孔网络涂层。该涂层具有优异的湿吸附性、生物相容性及抗氧化能力,并能促进细胞增殖和迁移。通过融合抗菌肽LL37,涂层展现出抗菌和免疫调节功能。动物实验证实,该涂层通过调控巨噬细胞M2极化、清除活性氧和抑制创面细菌,显著改善糖尿病伤口微环境,加速愈合。EGFL模块作为细胞外基质(ECM)的共性组分,其自组装特性为开发新型多功能生物材料提供了新思路,尤其在创面修复领域展现出重要的应用潜力[图4(c)]。
Sfp1是目前为止了解最为深入的一种海星黏附蛋白,其在海星分泌型黏附蛋白中含量最为丰富。Sfp1可以通过其亚基(如Beta和Delta)在水下基质上形成高效黏附,Hennebert团队[110]利用大肠杆菌重组产生Sfp1的Beta C端和Delta亚基,发现这些蛋白质在NaCl存在下能自组装成寡聚体和聚集体,并在Na+或Ca2+作用下吸附于玻璃和聚苯乙烯表面,形成不同结构的涂层。这些涂层对HeLa细胞无细胞毒性,甚至促进细胞增殖,显示出在细胞培养和生物医学应用中的潜力[图4(d)]。
Other Images/Table from this Article
|