|
||
|
Applications of the recombinant human collagen type Ⅲ-based trimerization motif in the design of vaccines to fight against SARS-CoV-2 and influenza virus
Synthetic Biology Journal
2024, 5 (2):
385-395.
DOI: 10.12211/2096-8280.2023-058
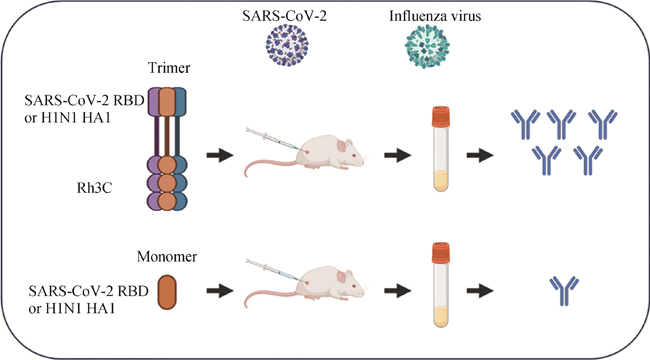
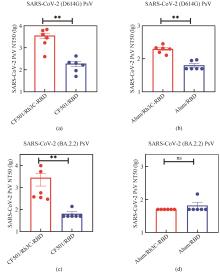
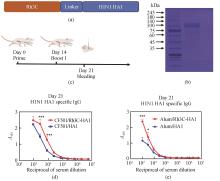
Glycoproteins with enveloped viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza virus, and human immunodeficiency virus (HIV), display a trimeric conformation. Different from the monomeric form, the trimeric proteins exhibit superior immunogenicity. Several trimerization motifs, such as Foldon derived from phage T4 fibritin, have been used to promote the formation of trimeric proteins with natural conformations. Although the Foldon-induced trimeric proteins are stable, their high immunogenicity limits applications in the development of vaccine antigens. In a previous study, we developed a recombinant human collagen type Ⅲ protein and determined its crystal structure, revealing a triple-helix conformation. However, the potential of this recombinant protein as a trimerization motif remained unknown. In this study, we demonstrated that the recombinant humanized type Ⅲ collagen (Rh3C) was able to act as a trimerization motif, facilitating the spontaneous trimer formation of the Rh3C-conjugated receptor-binding domain (RBD) within the spike (S) protein of SARS-CoV-2. This trimeric protein could induce a stronger SARS-CoV-2 RBD-specific IgG, IgG1, and IgG2a immune response, when compared with the monomeric RBD protein in the immunized mice. Notably, the Rh3C-RBD protein, when adjuvanted with the novel STING agonist CF501, also elicited significantly higher neutralizing antibody responses against both the pseudotyped SARS-CoV-2 (D614G) and its variant Omicron (BA.2.2) in the immunized mice. To showcase the broad applications of the Rh3C trimerization motif, we further demonstrated that the Rh3C-conjugated HA1 of the influenza virus could also elicit a stronger antibody response than free HA1. Considering the wide distribution of the Rh3C protein in human bodies, its use as a trimerization motif would not induce an immune response due to immune tolerance, thereby allowing the immune response to concentrate on targeted viral proteins. Therefore, this Rh3C-based trimerization motif holds great potential for the design and optimization of vaccines consisting of trimeric protein antigens. {L-End}

Fig. 1
Design, construction and identification of the Rh3C-conjugated RBD in the S protein of SARS-CoV-2 as a trimeric vaccine antigen
[(a) Schematic representation of the design of Rh3C-conjugated SARS-CoV-2 RBD (Rh3C-RBD) as a trimeric vaccine antigen.(b) Predicted trimeric structure of Rh3C-RBD using AlphaFold2. The entire structure is assembled from multiple segment predictions. The magnified images show the triple-helix motifs of the collagen tandem repeat and the trimeric conformation of RBD. (c) Schematic illustration of the Rh3C-RBD structure. (d) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis for the visualization of the Rh3C-RBD protein. (e) Representative elution chromatograph of the Rh3C-RBD protein using a calibrated Superose 6 Increase 10/300 column. (f) Determination of the molecular weight of Rh3C-RBD through the particle size analysis.]
Extracts from the Article
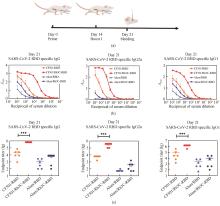
前期研究中,筛选获得了人源化Ⅲ型胶原蛋白Gly483-Pro512功能区,并通过将该区域进行16重复的串联表达获得性质更好的Rh3C[21]。本研究将Rh3C通过Linker与SARS-CoV-2的RBD蛋白以融合蛋白的形式表达(Rh3C-RBD),期望在胶原蛋白三聚化的作用下促使RBD以三聚体的形式表达,提高RBD的免疫原性[图1(a)]。利用AlphaFold2程序[24],预测了Rh3C-RBD融合蛋白的三聚体结构[图1(b)]。结果显示,整个Rh3C-RBD分子可以形成规则的三聚体结构,长度大约140 nm。分子的前端为相互错位1个氨基酸的胶原三聚体结构,后端为3重中心轴对称的RBD三聚体结构,两个结构由柔性Linker连接。该分子整体结构与设计预期基本吻合[图1(c)]。接下来的实验结果显示,Rh3C-RBD蛋白可被高效重组表达[图1(d)]。SDS-PAGE呈现的Rh3C-RBD蛋白单体分子量与预期蛋白分子量一致,约为85 kDa左右。通过对该蛋白进行纯化,获得了高纯度的Rh3C-RBD蛋白。通过凝胶过滤色谱检测了所表达的Rh3C-RBD的分子量,约为274 kDa,为所预测分子量的3倍左右,提示了Rh3C-RBD以三聚体形式成功被表达[图1(e)]。进一步通过测量该蛋白的粒径,估算出了该蛋白在天然情况下的分子量大小,约为255 kDa[图1(f)],与凝胶过滤色谱结果一致,符合预期的三聚体分子量。结果提示,Rh3C-RBD以三聚体的形式被表达及制备。
Other Images/Table from this Article
|