|
||
|
Applications of the recombinant human collagen type Ⅲ-based trimerization motif in the design of vaccines to fight against SARS-CoV-2 and influenza virus
Synthetic Biology Journal
2024, 5 (2):
385-395.
DOI: 10.12211/2096-8280.2023-058
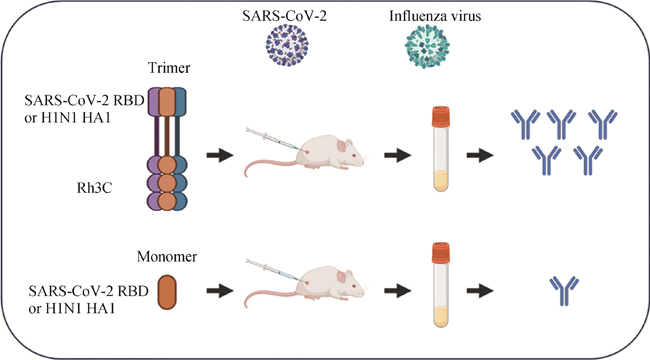
Glycoproteins with enveloped viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza virus, and human immunodeficiency virus (HIV), display a trimeric conformation. Different from the monomeric form, the trimeric proteins exhibit superior immunogenicity. Several trimerization motifs, such as Foldon derived from phage T4 fibritin, have been used to promote the formation of trimeric proteins with natural conformations. Although the Foldon-induced trimeric proteins are stable, their high immunogenicity limits applications in the development of vaccine antigens. In a previous study, we developed a recombinant human collagen type Ⅲ protein and determined its crystal structure, revealing a triple-helix conformation. However, the potential of this recombinant protein as a trimerization motif remained unknown. In this study, we demonstrated that the recombinant humanized type Ⅲ collagen (Rh3C) was able to act as a trimerization motif, facilitating the spontaneous trimer formation of the Rh3C-conjugated receptor-binding domain (RBD) within the spike (S) protein of SARS-CoV-2. This trimeric protein could induce a stronger SARS-CoV-2 RBD-specific IgG, IgG1, and IgG2a immune response, when compared with the monomeric RBD protein in the immunized mice. Notably, the Rh3C-RBD protein, when adjuvanted with the novel STING agonist CF501, also elicited significantly higher neutralizing antibody responses against both the pseudotyped SARS-CoV-2 (D614G) and its variant Omicron (BA.2.2) in the immunized mice. To showcase the broad applications of the Rh3C trimerization motif, we further demonstrated that the Rh3C-conjugated HA1 of the influenza virus could also elicit a stronger antibody response than free HA1. Considering the wide distribution of the Rh3C protein in human bodies, its use as a trimerization motif would not induce an immune response due to immune tolerance, thereby allowing the immune response to concentrate on targeted viral proteins. Therefore, this Rh3C-based trimerization motif holds great potential for the design and optimization of vaccines consisting of trimeric protein antigens. {L-End}
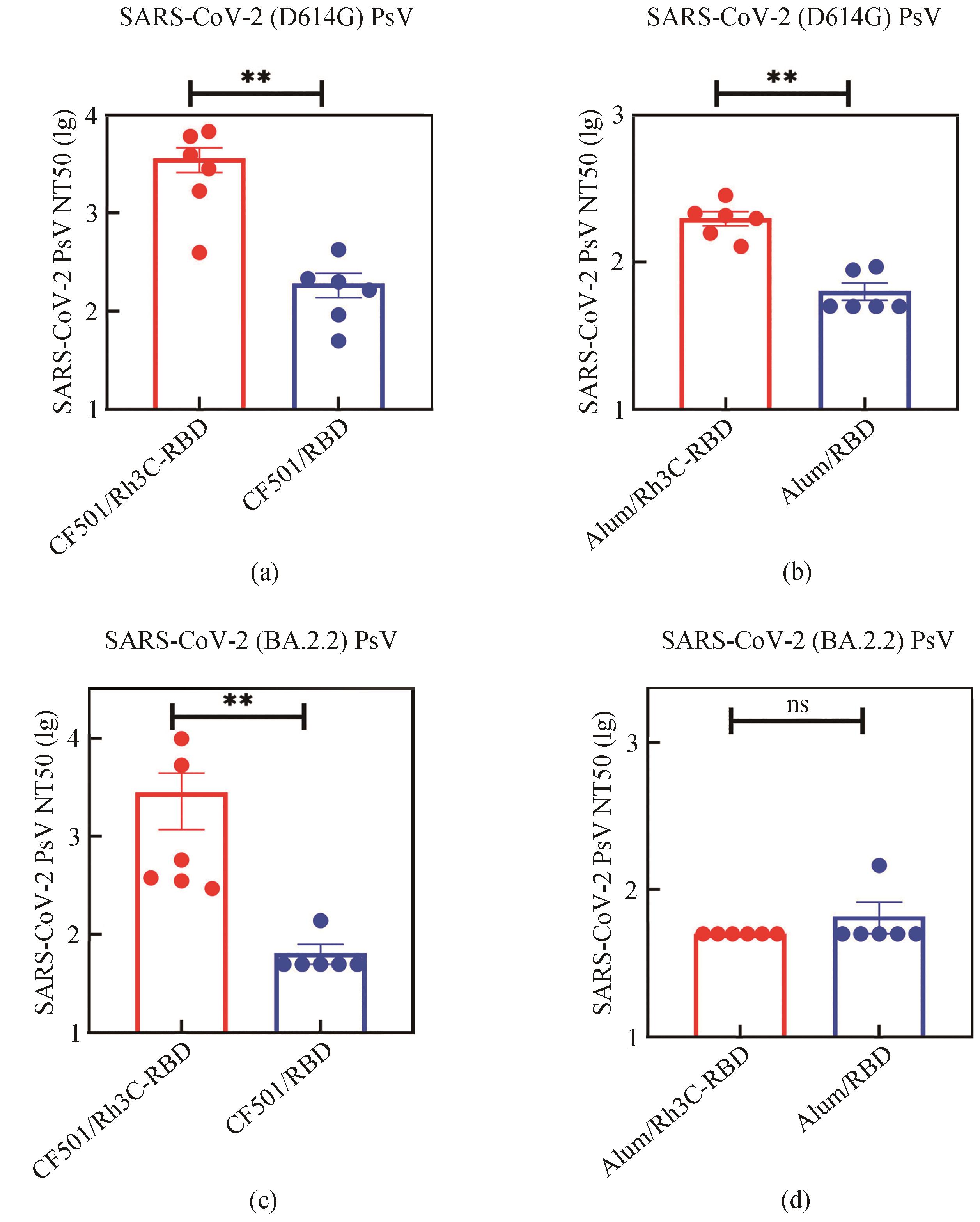
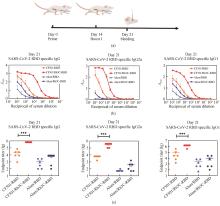
Fig. 3
Evaluation of the neutralizing antibodies against the pseudovirus SARS-CoV-2 (D614G) in the sera of immunized mice
[Assessment of the neutralizing antibody titers against SARS-CoV-2 (D614G) in the sera of mice immunized with CF501/RBD or CF501/Rh3C-RBD (a), Alum/RBD or Alum/Rh3C-RBD (b), CF501/RBD or CF501/Rh3C-RBD (c), and Alum/RBD or Alum/Rh3C-RBD (d). Statistical analysis was performed using the Student t test. ** indicating the statistical significance at P<0.001.]
Extracts from the Article
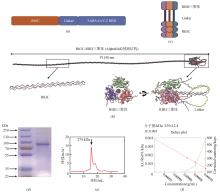
[(a)Ⅲ型胶原蛋白Rh3C同SARS-CoV-2 RBD融合蛋白的设计。(b)利用AlphaFold2预测的Rh3C-RBD三聚体结构。整个结构由多次的分段预测结果拼接而成。放大的图分别展示了串联重复的胶原三螺旋基序结构和RBD三聚体结构。(c)Ⅲ型人源化胶原蛋白Rh3C同RBD融合表达后的三聚体结构模式图。(d)Ⅲ型人源化胶原蛋白Rh3C同RBD融合表达后的SDS-PAGE图片。(e)凝胶过滤色谱分析Rh3C-RBD分子量。(f)粒径分析Rh3C-RBD融合蛋白获得蛋白分子量]
为了进一步鉴定Rh3C-RBD三聚体是否会诱导更高的中和抗体水平,将SARS-CoV-2(D614G)的刺突蛋白表达在HIV骨架蛋白表面,构建了SARS-CoV-2的假病毒。并利用SARS-CoV-2(D614G)和Omicron(BA.2.2)假病毒系统检测小鼠血清中的中和抗体效价。结果如图3所示,CF501/Rh3C-RBD组小鼠血清中对D614G和BA.2.2假病毒的平均中和抗体效价为3620和2807,而CF501/RBD组小鼠的平均效价仅为191和64。同样地,Alum/Rh3C-RBD组小鼠血清对D614G假病毒的平均中和抗体效价为198,而Alum/RBD组小鼠血清的平均中和抗体效价仅为63。Alum/Rh3C-RBD和Alum/RBD组小鼠血清对BA.2.2假病毒都未产生强效的中和作用。以上数据提示了,Rh3C-RBD不仅可显著提高抗原特异性结合抗体,还可以有效增强中和抗体的免疫应答。
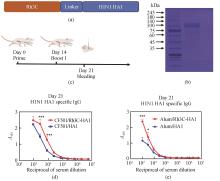
[(a)基于Rh3C连接的H1N1 HA1重组融合蛋白(Rh3C-HA1)的设计。(b)SDS-PAGE图片验证Rh3C-HA1蛋白的表达。(c)小鼠的免疫程序。(d)以CF501为佐剂免疫小鼠后血清中针对HA1特异性的IgG抗体水平。(e)以Alum为佐剂免疫小鼠后血清中针对HA1特异性的IgG抗体水平。利用Two-way ANOWA进行统计学分析。***P<0.0001,*P<0.05]
Other Images/Table from this Article
|