|
||
|
Applications of the recombinant human collagen type Ⅲ-based trimerization motif in the design of vaccines to fight against SARS-CoV-2 and influenza virus
Synthetic Biology Journal
2024, 5 (2):
385-395.
DOI: 10.12211/2096-8280.2023-058
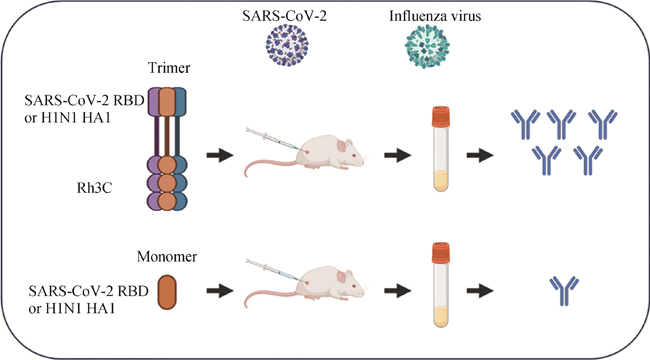
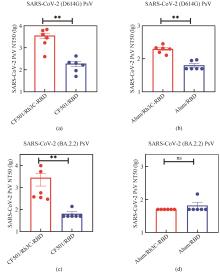
Glycoproteins with enveloped viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza virus, and human immunodeficiency virus (HIV), display a trimeric conformation. Different from the monomeric form, the trimeric proteins exhibit superior immunogenicity. Several trimerization motifs, such as Foldon derived from phage T4 fibritin, have been used to promote the formation of trimeric proteins with natural conformations. Although the Foldon-induced trimeric proteins are stable, their high immunogenicity limits applications in the development of vaccine antigens. In a previous study, we developed a recombinant human collagen type Ⅲ protein and determined its crystal structure, revealing a triple-helix conformation. However, the potential of this recombinant protein as a trimerization motif remained unknown. In this study, we demonstrated that the recombinant humanized type Ⅲ collagen (Rh3C) was able to act as a trimerization motif, facilitating the spontaneous trimer formation of the Rh3C-conjugated receptor-binding domain (RBD) within the spike (S) protein of SARS-CoV-2. This trimeric protein could induce a stronger SARS-CoV-2 RBD-specific IgG, IgG1, and IgG2a immune response, when compared with the monomeric RBD protein in the immunized mice. Notably, the Rh3C-RBD protein, when adjuvanted with the novel STING agonist CF501, also elicited significantly higher neutralizing antibody responses against both the pseudotyped SARS-CoV-2 (D614G) and its variant Omicron (BA.2.2) in the immunized mice. To showcase the broad applications of the Rh3C trimerization motif, we further demonstrated that the Rh3C-conjugated HA1 of the influenza virus could also elicit a stronger antibody response than free HA1. Considering the wide distribution of the Rh3C protein in human bodies, its use as a trimerization motif would not induce an immune response due to immune tolerance, thereby allowing the immune response to concentrate on targeted viral proteins. Therefore, this Rh3C-based trimerization motif holds great potential for the design and optimization of vaccines consisting of trimeric protein antigens. {L-End}
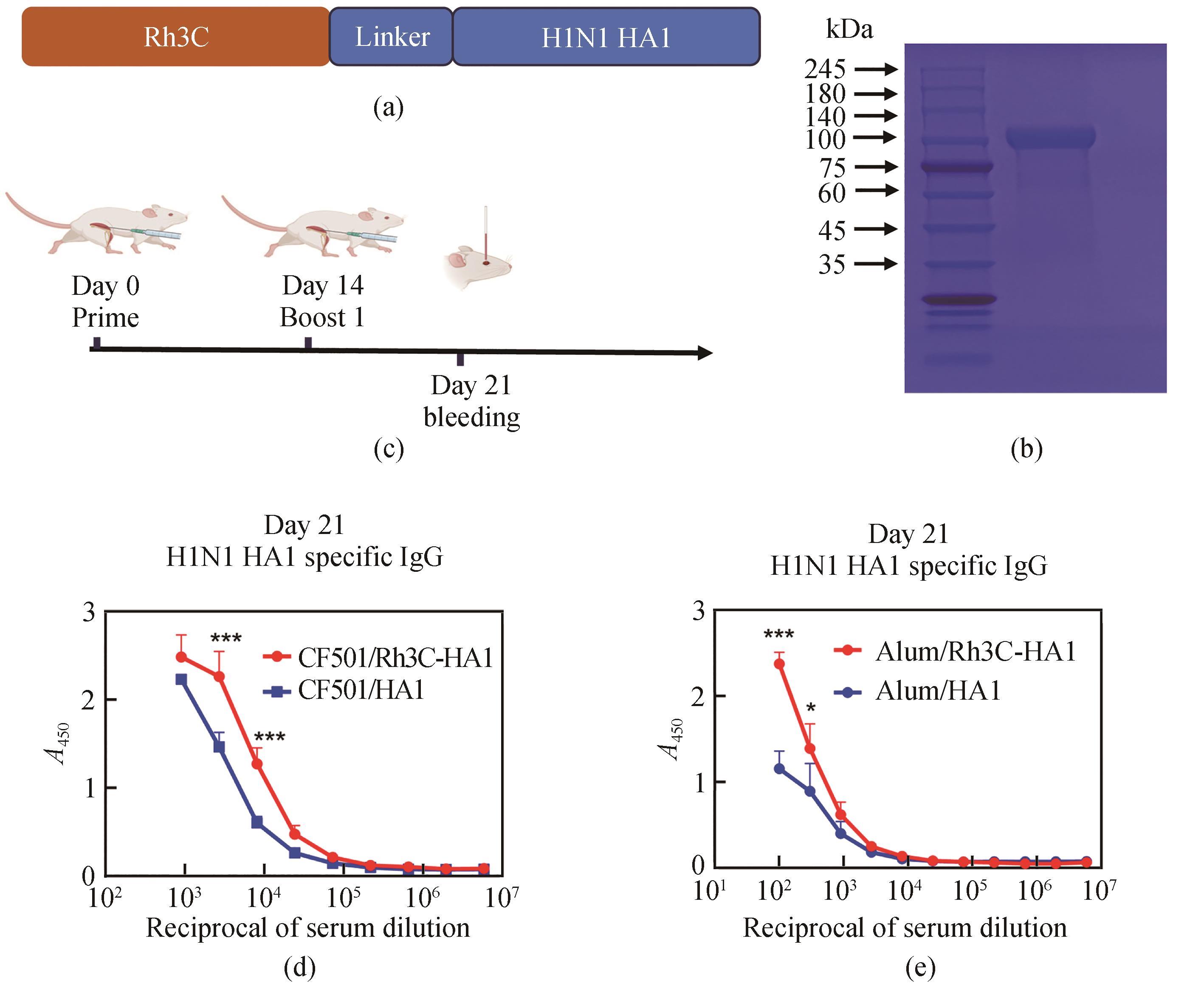
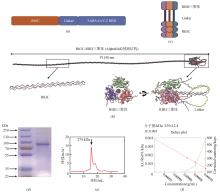
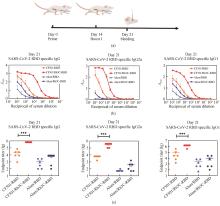
Fig.4
Design and construction of the Rh3C-conjugated influenza virus HA1 (Rh3C-HA1) and evaluation of its immunogenicity
[(a) Design of the Rh3C-conjugated influenza virus HA1 (Rh3C-HA1); (b) SDS-PAGE analysis for the expressed Rh3C-HA1 protein; (c) Overview of the immunization protocol. Mice were vaccinated at day 0 and day 14, with serum collection at day 21; (d and e) ELISA analyses of the HA1-specific IgG in the serum collected at day 21 from mice immunized with CF501/RBD or CF501/Rh3C-RBD and Alum/RBD or Alum/Rh3C-RBD. Statistical analysis was performed using the two-way ANOWA. *** and * indicating the statistical significance at P<0.0001 and P<0.05, respectively.]
Extracts from the Article
为了评价该Rh3C是否可作为一种增加抗原免疫原性的通用元件,进一步将该Rh3C与流感病毒H1N1 HA1蛋白进行融合表达[图4(a)]。经过SDS-PAGE鉴定后,发现Rh3C-HA1成功被表达,并具有较高的纯度[图4(b)]。进一步将Rh3C-HA1和HA1分别与佐剂CF501或Alum联用免疫Balb/c小鼠[图4(c)]。免疫两次后利用ELISA检测HA1特异性的IgG抗体水平。结果如图4(d)、(e)所示。无论是利用CF501佐剂还是铝佐剂,Rh3C-HA1所诱导的HA1特异性IgG抗体滴度要显著性地高于HA1抗原诱导的IgG抗体水平。该结果提示,Rh3C三聚化策略不仅可提高SARS-CoV-2 RBD抗原诱发特异性结合抗体和中和抗体,还可提高流感HA1诱发的特异性结合抗体。有望成为一种通用的增强抗原免疫原性的三聚化策略。
Other Images/Table from this Article
|