Drug discovery and natural products: end of an era or an endless frontier?
1
2009
... 植物次生代谢产物种类丰富,广泛参与植物生长、发育、防御等生理活动,是天然药物以及先导物的重要来源[1-6].许多植物次生代谢产物含有多个手性中心,结构复杂,虽然大部分可以通过化学全合成手段获得,但可以有效放大的并不多,多数仍依赖于从植物中分离提取.然而,植物生长周期长,组分复杂,从中提取往往面临着效率低、耗时长、成本高等问题,有些含量低的次生代谢产物更是难以获取,极大地制约了新型药物的研发.相较于植物,微生物生长周期短、遗传操作简单、易于培养,可结合代谢工程有效地富集目标分子,因此,通过在微生物宿主中重构植物代谢途径,以期形成经济、高效的植物次生代谢产物生产的替代途径,成为合成生物学研究领域的热点.近年来,植物代谢途径研究、酶工程以及生物技术的发展,促使植物次生代谢产物的微生物合成研究取得了多个突破性进展,最具代表性的例子就是通过酿酒酵母(Saccharomyces cerevisiae)生产抗疟药青蒿素的前体青蒿酸(artemisinin)[4],已进入工业化生产阶段,见证了微生物合成生产途径的发展潜力. ...
Natural products as a screening resource
0
2007
Opportunities in metabolic engineering to facilitate scalable alkaloid production
0
2009
Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development
1
2014
... 植物次生代谢产物种类丰富,广泛参与植物生长、发育、防御等生理活动,是天然药物以及先导物的重要来源[1-6].许多植物次生代谢产物含有多个手性中心,结构复杂,虽然大部分可以通过化学全合成手段获得,但可以有效放大的并不多,多数仍依赖于从植物中分离提取.然而,植物生长周期长,组分复杂,从中提取往往面临着效率低、耗时长、成本高等问题,有些含量低的次生代谢产物更是难以获取,极大地制约了新型药物的研发.相较于植物,微生物生长周期短、遗传操作简单、易于培养,可结合代谢工程有效地富集目标分子,因此,通过在微生物宿主中重构植物代谢途径,以期形成经济、高效的植物次生代谢产物生产的替代途径,成为合成生物学研究领域的热点.近年来,植物代谢途径研究、酶工程以及生物技术的发展,促使植物次生代谢产物的微生物合成研究取得了多个突破性进展,最具代表性的例子就是通过酿酒酵母(Saccharomyces cerevisiae)生产抗疟药青蒿素的前体青蒿酸(artemisinin)[4],已进入工业化生产阶段,见证了微生物合成生产途径的发展潜力. ...
Bisbenzylisoquinoline alkaloids
0
2019
Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world
3
2013
... 植物次生代谢产物种类丰富,广泛参与植物生长、发育、防御等生理活动,是天然药物以及先导物的重要来源[1-6].许多植物次生代谢产物含有多个手性中心,结构复杂,虽然大部分可以通过化学全合成手段获得,但可以有效放大的并不多,多数仍依赖于从植物中分离提取.然而,植物生长周期长,组分复杂,从中提取往往面临着效率低、耗时长、成本高等问题,有些含量低的次生代谢产物更是难以获取,极大地制约了新型药物的研发.相较于植物,微生物生长周期短、遗传操作简单、易于培养,可结合代谢工程有效地富集目标分子,因此,通过在微生物宿主中重构植物代谢途径,以期形成经济、高效的植物次生代谢产物生产的替代途径,成为合成生物学研究领域的热点.近年来,植物代谢途径研究、酶工程以及生物技术的发展,促使植物次生代谢产物的微生物合成研究取得了多个突破性进展,最具代表性的例子就是通过酿酒酵母(Saccharomyces cerevisiae)生产抗疟药青蒿素的前体青蒿酸(artemisinin)[4],已进入工业化生产阶段,见证了微生物合成生产途径的发展潜力. ...
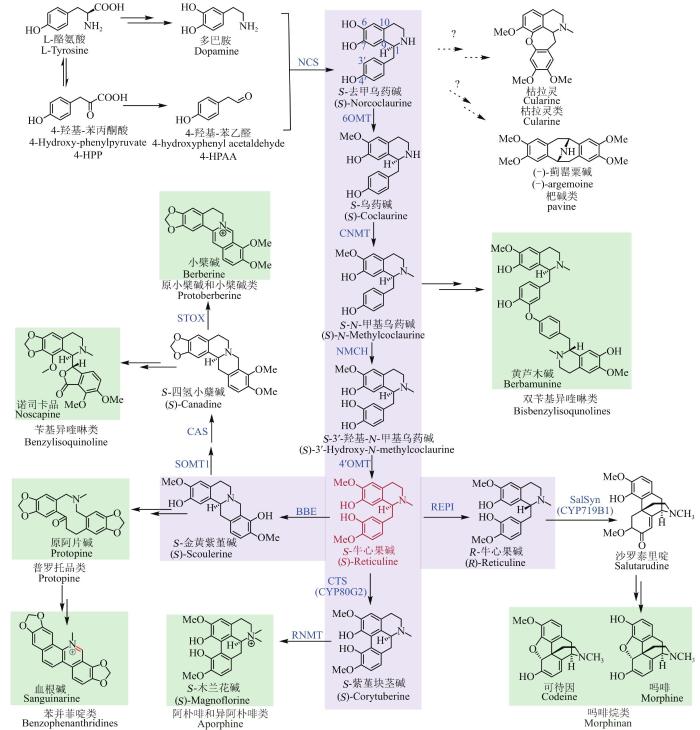
... 苄基异喹啉类生物碱(benzylisoquinoline alkaloid,BIA)是一类具有重要研究和药用价值的次生代谢产物,广泛分布于罂粟科、木兰科、芸香科、小檗科等植物,已被发现2500多种.该类生物碱具有抗癌、镇痛、止咳、降压、抗菌、抗炎、免疫抑制等多种生理活性[7-12],目前成功应用于临床的就有镇痛药吗啡(morphine)和可待因(codeine),止咳药海罂粟碱(glaucine)、诺司卡品(noscapine),肌肉松弛剂罂粟碱(papaverine)以及用于帕金森病治疗的阿扑吗啡(apomorphine)等[6].工业上生产BIA主要依赖于从植物中直接提取或提取前体,经简单的化学半合成获得.其中,最为人熟知的BIA植物供体就是罂粟(Opium poppy),已经被人们广泛种植,用于提供吗啡、可待因和阿扑吗啡前体等.除此之外,大部分含有BIA的植物属于野生资源,难以大规模种植,采集困难,受环境影响大.因此,研究人员致力于对BIA的生物合成途径进行解析,在此基础上通过模拟植物中BIA的合成途径在微生物里重构该类生物碱的合成途径,以期获得高效的BIA生产途径和更多的活性衍生物.到目前为止,研究者们已经打通了多种BIA的微生物合成途径,包括蒂巴因(thebaine)、木兰花碱(magnofluorine)等,为通过微生物工业化生产BIA提供了基础.然而,由于多数BIA结构复杂,代谢途径冗长,涉及到的催化酶对主代谢流途径上的BIA中间体具有宽泛的选择性,导致代谢流不可控、目的化合物产量低等问题.本文对BIA的微生物合成途径以及突破性成果进行总结,并结合相关酶体外催化特性的研究进展,指出在酶催化元件的选取和改进方面需要关注的问题和解决方案,希望能为后续推进BIA微生物合成途径的工业化应用提供参考. ...
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
Anticancer and reversing multidrug resistance activities of natural isoquinoline alkaloids and their structure-activity relationship
2
2018
... 苄基异喹啉类生物碱(benzylisoquinoline alkaloid,BIA)是一类具有重要研究和药用价值的次生代谢产物,广泛分布于罂粟科、木兰科、芸香科、小檗科等植物,已被发现2500多种.该类生物碱具有抗癌、镇痛、止咳、降压、抗菌、抗炎、免疫抑制等多种生理活性[7-12],目前成功应用于临床的就有镇痛药吗啡(morphine)和可待因(codeine),止咳药海罂粟碱(glaucine)、诺司卡品(noscapine),肌肉松弛剂罂粟碱(papaverine)以及用于帕金森病治疗的阿扑吗啡(apomorphine)等[6].工业上生产BIA主要依赖于从植物中直接提取或提取前体,经简单的化学半合成获得.其中,最为人熟知的BIA植物供体就是罂粟(Opium poppy),已经被人们广泛种植,用于提供吗啡、可待因和阿扑吗啡前体等.除此之外,大部分含有BIA的植物属于野生资源,难以大规模种植,采集困难,受环境影响大.因此,研究人员致力于对BIA的生物合成途径进行解析,在此基础上通过模拟植物中BIA的合成途径在微生物里重构该类生物碱的合成途径,以期获得高效的BIA生产途径和更多的活性衍生物.到目前为止,研究者们已经打通了多种BIA的微生物合成途径,包括蒂巴因(thebaine)、木兰花碱(magnofluorine)等,为通过微生物工业化生产BIA提供了基础.然而,由于多数BIA结构复杂,代谢途径冗长,涉及到的催化酶对主代谢流途径上的BIA中间体具有宽泛的选择性,导致代谢流不可控、目的化合物产量低等问题.本文对BIA的微生物合成途径以及突破性成果进行总结,并结合相关酶体外催化特性的研究进展,指出在酶催化元件的选取和改进方面需要关注的问题和解决方案,希望能为后续推进BIA微生物合成途径的工业化应用提供参考. ...
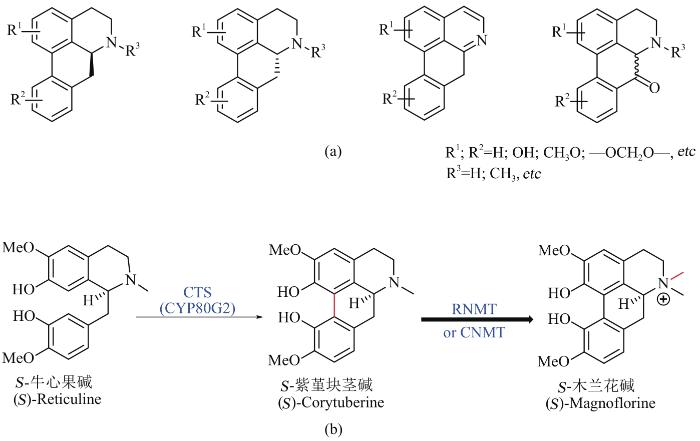
... 阿朴啡类生物碱含有特征性的联苯型四环结构骨架[图4(a)],广泛分布于自然界,具有抗癌、抗病毒、抗疟疾、抗炎等多种重要生理活性,目前已经在20个科100多种属植物中分离到了500多个化合物[7-11,70].阿朴啡结构的多样性很大程度依赖于骨架上多样的甲基化、亚二甲基化、羟化取代方式以及骨架氧化程度的差异,该类生物碱大部分生物合成途径没有被阐明,这也是限制该类生物碱在微生物底盘细胞中合成的主要原因.2008年,Sato等在C. japonica中发现了一个P450酶CYP80G2可以催化S-牛心果碱的8位和2′位发生偶联完成联苯型四环骨架的构筑,形成S-紫堇块茎碱(corytuberine)[25],该酶底物专一性较强,对于R-牛心果碱和类似物大部分不能识别,与上游途径中NMCH一起,可能会导致大量旁支产物不能回到主代谢流;S-紫堇块茎碱可以在RNMT催化下进一步转化成木兰花碱(magnoflorine)[71].基于此发现,该团队在建立了大肠杆菌牛心果碱合成途径的基础上,将CTS和CNMT(可替代RNMT行使氮上双甲基化功能)的编码基因转入酵母,通过共培养的方式完成了紫堇块茎碱和木兰花碱(7.2 mg/L)的微生物合成,成为首例微生物体系成功合成阿朴啡类生物碱[图4(b))[26].由于该类生物碱生物合成途径研究的欠缺,尤其是R型结构和一些关键后修饰生物合成途径的未知,导致目前只有紫堇块茎碱和木兰花碱得以在微生物体系中合成. ...
Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L
0
2010
Antimalarial activity and cytotoxicity of (-)-roemrefidine isolated from the stem bark of Sparattanthelium amazonum
0
1999
Three new alkaloids from Xylopia vielana and their antiinflammatory activities
0
2018
Antiplatelet actions of aporphinoids from Formosan plants
1
1999
... 阿朴啡类生物碱含有特征性的联苯型四环结构骨架[图4(a)],广泛分布于自然界,具有抗癌、抗病毒、抗疟疾、抗炎等多种重要生理活性,目前已经在20个科100多种属植物中分离到了500多个化合物[7-11,70].阿朴啡结构的多样性很大程度依赖于骨架上多样的甲基化、亚二甲基化、羟化取代方式以及骨架氧化程度的差异,该类生物碱大部分生物合成途径没有被阐明,这也是限制该类生物碱在微生物底盘细胞中合成的主要原因.2008年,Sato等在C. japonica中发现了一个P450酶CYP80G2可以催化S-牛心果碱的8位和2′位发生偶联完成联苯型四环骨架的构筑,形成S-紫堇块茎碱(corytuberine)[25],该酶底物专一性较强,对于R-牛心果碱和类似物大部分不能识别,与上游途径中NMCH一起,可能会导致大量旁支产物不能回到主代谢流;S-紫堇块茎碱可以在RNMT催化下进一步转化成木兰花碱(magnoflorine)[71].基于此发现,该团队在建立了大肠杆菌牛心果碱合成途径的基础上,将CTS和CNMT(可替代RNMT行使氮上双甲基化功能)的编码基因转入酵母,通过共培养的方式完成了紫堇块茎碱和木兰花碱(7.2 mg/L)的微生物合成,成为首例微生物体系成功合成阿朴啡类生物碱[图4(b))[26].由于该类生物碱生物合成途径研究的欠缺,尤其是R型结构和一些关键后修饰生物合成途径的未知,导致目前只有紫堇块茎碱和木兰花碱得以在微生物体系中合成. ...
Antiplatelet and vasorelaxing actions of some aporphinoids
1
1996
... 苄基异喹啉类生物碱(benzylisoquinoline alkaloid,BIA)是一类具有重要研究和药用价值的次生代谢产物,广泛分布于罂粟科、木兰科、芸香科、小檗科等植物,已被发现2500多种.该类生物碱具有抗癌、镇痛、止咳、降压、抗菌、抗炎、免疫抑制等多种生理活性[7-12],目前成功应用于临床的就有镇痛药吗啡(morphine)和可待因(codeine),止咳药海罂粟碱(glaucine)、诺司卡品(noscapine),肌肉松弛剂罂粟碱(papaverine)以及用于帕金森病治疗的阿扑吗啡(apomorphine)等[6].工业上生产BIA主要依赖于从植物中直接提取或提取前体,经简单的化学半合成获得.其中,最为人熟知的BIA植物供体就是罂粟(Opium poppy),已经被人们广泛种植,用于提供吗啡、可待因和阿扑吗啡前体等.除此之外,大部分含有BIA的植物属于野生资源,难以大规模种植,采集困难,受环境影响大.因此,研究人员致力于对BIA的生物合成途径进行解析,在此基础上通过模拟植物中BIA的合成途径在微生物里重构该类生物碱的合成途径,以期获得高效的BIA生产途径和更多的活性衍生物.到目前为止,研究者们已经打通了多种BIA的微生物合成途径,包括蒂巴因(thebaine)、木兰花碱(magnofluorine)等,为通过微生物工业化生产BIA提供了基础.然而,由于多数BIA结构复杂,代谢途径冗长,涉及到的催化酶对主代谢流途径上的BIA中间体具有宽泛的选择性,导致代谢流不可控、目的化合物产量低等问题.本文对BIA的微生物合成途径以及突破性成果进行总结,并结合相关酶体外催化特性的研究进展,指出在酶催化元件的选取和改进方面需要关注的问题和解决方案,希望能为后续推进BIA微生物合成途径的工业化应用提供参考. ...
Family portraits: the enzymes behind benzylisoquinoline alkaloid diversity
1
2018
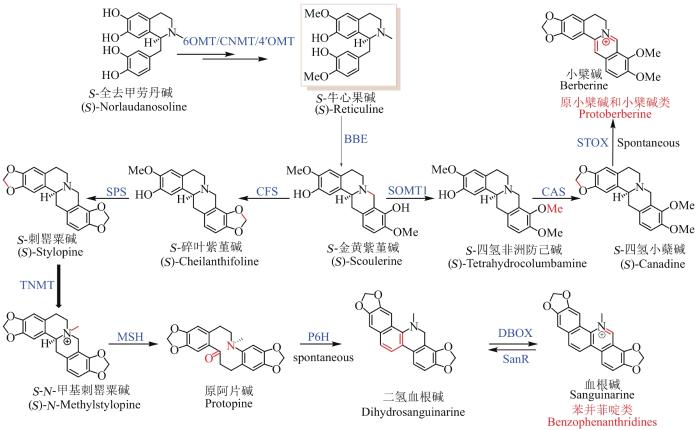
... 根据基本骨架类型,BIA可大致分为7类(图1):苄基异喹啉类(benzylisoquinoline)、双苄基异喹啉类(bisbenzylisoqunolines)、阿朴啡和异阿朴啡类(aporphines)、吗啡烷类(morphinane)、原小檗碱和小檗碱类(protoberberine)、普罗托品类(protopine)、苯并菲啶类(benzophenanthridines)[13-14].BIA虽然结构复杂多样,但是其生物合成途径的起始步骤却十分相似,大多是由酪氨酸的代谢产物多巴胺(dopamine)和4-羟基-苯乙醛(4-hydroxyphenyl acetaldehyde,4-HPAA)在去甲乌药碱合酶(NCS)的作用下,通过Pictet-Spengler反应先形成S-去甲乌药碱[(S)-norcoclaurine],随后经过3个甲基转移酶(6OMT,CNMT,4′OMT)和1个细胞色素P450氧化还原酶(NMCH)的作用形成关键的中间体S-牛心果碱[(S)-reticuline].以上步骤可统称为上游共性化合成途径(图1)[15-17]. ...
Microbial factories for the production of benzylisoquinoline alkaloids
2
2016
... 根据基本骨架类型,BIA可大致分为7类(图1):苄基异喹啉类(benzylisoquinoline)、双苄基异喹啉类(bisbenzylisoqunolines)、阿朴啡和异阿朴啡类(aporphines)、吗啡烷类(morphinane)、原小檗碱和小檗碱类(protoberberine)、普罗托品类(protopine)、苯并菲啶类(benzophenanthridines)[13-14].BIA虽然结构复杂多样,但是其生物合成途径的起始步骤却十分相似,大多是由酪氨酸的代谢产物多巴胺(dopamine)和4-羟基-苯乙醛(4-hydroxyphenyl acetaldehyde,4-HPAA)在去甲乌药碱合酶(NCS)的作用下,通过Pictet-Spengler反应先形成S-去甲乌药碱[(S)-norcoclaurine],随后经过3个甲基转移酶(6OMT,CNMT,4′OMT)和1个细胞色素P450氧化还原酶(NMCH)的作用形成关键的中间体S-牛心果碱[(S)-reticuline].以上步骤可统称为上游共性化合成途径(图1)[15-17]. ...
... 在过去十多年时间里,我们见证了苄基异喹啉类生物碱的微生物合成途径从无到有、产量从低到高的发展历程,每一步都离不开天然生物合成途径的解析,每一个突破都伴随着关键酶催化反应及其机理的阐明.因此,大部分BIA生物合成途径认知的缺失,如杷碱类(pavine)、枯拉灵类(cularine)以及骨架上的修饰途径等(图1),为未来发展BIA的微生物合成途径带来了巨大挑战.除此之外,BIA微生物合成途径中酶活性低和底物选择性差等导致的代谢流不可控、产率低等问题也是我们亟待解决的难题.所幸的是,基因测序技术和生物技术的发展大大加快了我们对天然产物生物合成途径解析的速度,也为发展BIA的微生物合成途径积累了越来越多可供筛选的酶学元件;与此同时,酶工程和晶体学的发展也极大地提高了酶催化性能的优化效率.研究者们也开发了多种方案去解决酶底物选择性差引起的代谢流不可控问题,包括适当地调整代谢流通量以及通过时间和空间的调整,阻止酶与有干扰的代谢中间体的接触,从而减少副产物的生成等[14].前者常用的调整方式有运用不同强度的启动子,控制基因的拷贝数等;后者则可通过将酶放在不同细胞器以及菌株中进行表达从而有效地隔离酶与有干扰的代谢中间体,以及通过调控酶在不同时间点表达,率先积累某一目标代谢中间体,再启动后续途径中酶的表达,从而促进代谢中间体的进一步转化,有效防止代谢旁流.此外,研究者们也采用选取底物识别宽泛的酶以及通过酶工程去拓展各个酶的底物识别能力,从而将旁支产物重新转化至主代谢流上的化合物,提高微生物合成BIA的产量. ...
Knockdown of berberine bridge enzyme by RNAi accumulates (S)-reticuline and activates a silent pathway in cultured California poppy cells
1
2007
... 根据基本骨架类型,BIA可大致分为7类(图1):苄基异喹啉类(benzylisoquinoline)、双苄基异喹啉类(bisbenzylisoqunolines)、阿朴啡和异阿朴啡类(aporphines)、吗啡烷类(morphinane)、原小檗碱和小檗碱类(protoberberine)、普罗托品类(protopine)、苯并菲啶类(benzophenanthridines)[13-14].BIA虽然结构复杂多样,但是其生物合成途径的起始步骤却十分相似,大多是由酪氨酸的代谢产物多巴胺(dopamine)和4-羟基-苯乙醛(4-hydroxyphenyl acetaldehyde,4-HPAA)在去甲乌药碱合酶(NCS)的作用下,通过Pictet-Spengler反应先形成S-去甲乌药碱[(S)-norcoclaurine],随后经过3个甲基转移酶(6OMT,CNMT,4′OMT)和1个细胞色素P450氧化还原酶(NMCH)的作用形成关键的中间体S-牛心果碱[(S)-reticuline].以上步骤可统称为上游共性化合成途径(图1)[15-17]. ...
Molecular cloning and characterization of tetrahydroprotoberberine cis-N-methyltransferase, an enzyme involved in alkaloid biosynthesis in opium poppy
0
2007
Molecular cloning and characterization of a cytochrome P450 in sanguinarine biosynthesis from Eschscholzia californica cells
1
2013
... 根据基本骨架类型,BIA可大致分为7类(图1):苄基异喹啉类(benzylisoquinoline)、双苄基异喹啉类(bisbenzylisoqunolines)、阿朴啡和异阿朴啡类(aporphines)、吗啡烷类(morphinane)、原小檗碱和小檗碱类(protoberberine)、普罗托品类(protopine)、苯并菲啶类(benzophenanthridines)[13-14].BIA虽然结构复杂多样,但是其生物合成途径的起始步骤却十分相似,大多是由酪氨酸的代谢产物多巴胺(dopamine)和4-羟基-苯乙醛(4-hydroxyphenyl acetaldehyde,4-HPAA)在去甲乌药碱合酶(NCS)的作用下,通过Pictet-Spengler反应先形成S-去甲乌药碱[(S)-norcoclaurine],随后经过3个甲基转移酶(6OMT,CNMT,4′OMT)和1个细胞色素P450氧化还原酶(NMCH)的作用形成关键的中间体S-牛心果碱[(S)-reticuline].以上步骤可统称为上游共性化合成途径(图1)[15-17]. ...
A concerted mechanism for berberine bridge enzyme
1
2008
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
Characterization of a flavoprotein oxidase from opium poppy catalyzing the final steps in sanguinarine and papaverine biosynthesis
1
2012
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
Purification and properties of (S)-tetrahydroprotoberberine oxidase from suspension-cultured cells of Berberis wilsoniae
1
1988
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
Complete biosynthesis of opioids in yeast
3
2015
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
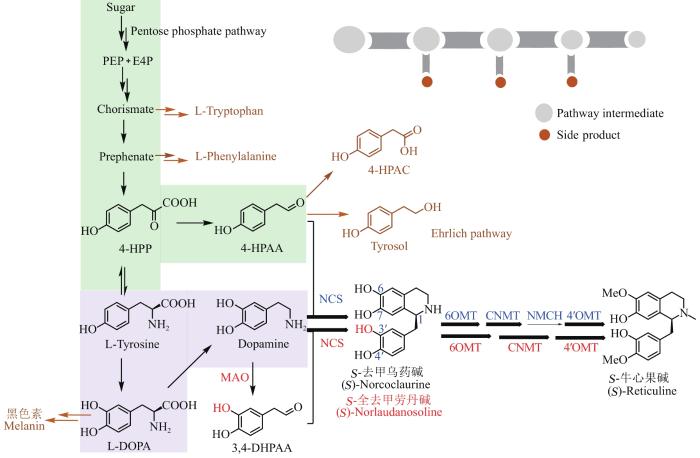
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
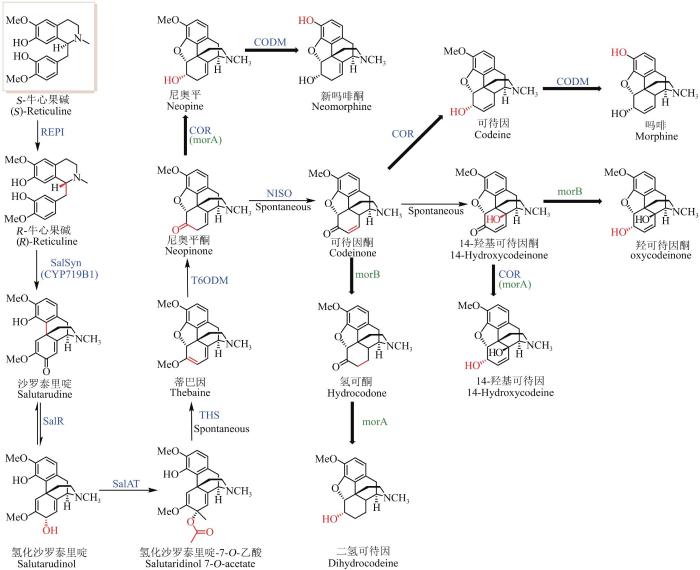
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
Plant science. Morphinan biosynthesis in opium poppy requires a P450-oxidoreductase fusion protein
1
2015
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
Stereochemical inversion of (S)-reticuline by a cytochrome P450 fusion in opium poppy
2
2015
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
Dioxygenases catalyze the O-demethylation steps of morphine biosynthesis in opium poppy
2
2010
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Molecular cloning and characterization of CYP80G2, a cytochrome P450 that catalyzes an intramolecular C-C phenol coupling of (S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cells
2
2008
... S-牛心果碱通过异构、偶联、重排、甲基化、去甲基化等反应,几乎可以形成所有骨架类型的BIA,如S-牛心果碱通过小檗碱桥酶(BBE)作用可形成S-金黄紫堇碱[(S)-scoulerine][18],是原小檗碱类和小檗碱类、苄基异喹啉类、苯并菲啶类等BIA生物碱的共同中间体,可在甲基转移酶(SOMT1)、P450酶CAS和黄素依赖的氧化酶(STOX)的催化下生成小檗碱(berberine)[19-20],或通过6步酶反应形成血根碱(sanguinarine).异构酶REPI则可催化S-牛心果碱形成R-牛心果碱,后者是吗啡烷类生物碱的共同中间体[21-23],可在P450酶SalSyn作用下偶联形成吗啡烷的基本骨架[24];S-牛心果碱也可以通过P450酶CTS作用形成阿朴啡类生物碱[25].虽然BIA结构多样,但是每一个大类特征骨架的形成途径相同,骨架上的取代修饰类型也很少,主要包括羟基、甲基、甲氧基和亚甲二氧基等,对应的修饰酶的类型也十分相似,比如负责骨架6位和7位羟基的甲基化都是由S-腺苷-L-甲硫氨酸[(S)-adenosyl-L-methionine,SAM]依赖的甲基化酶,负责6、7位和3′、4′位亚甲基二氧基形成的都是P450酶.后修饰酶性质以及底物的相似性,导致在微生物里重构植物中BIA的天然合成途径时,常常发生交叉反应和代谢旁流.掌握BIA生物合成途径中酶的催化特性,从而有目的地改进,将是在微生物中实现BIA高效合成途径的关键点之一. ...
... 阿朴啡类生物碱含有特征性的联苯型四环结构骨架[图4(a)],广泛分布于自然界,具有抗癌、抗病毒、抗疟疾、抗炎等多种重要生理活性,目前已经在20个科100多种属植物中分离到了500多个化合物[7-11,70].阿朴啡结构的多样性很大程度依赖于骨架上多样的甲基化、亚二甲基化、羟化取代方式以及骨架氧化程度的差异,该类生物碱大部分生物合成途径没有被阐明,这也是限制该类生物碱在微生物底盘细胞中合成的主要原因.2008年,Sato等在C. japonica中发现了一个P450酶CYP80G2可以催化S-牛心果碱的8位和2′位发生偶联完成联苯型四环骨架的构筑,形成S-紫堇块茎碱(corytuberine)[25],该酶底物专一性较强,对于R-牛心果碱和类似物大部分不能识别,与上游途径中NMCH一起,可能会导致大量旁支产物不能回到主代谢流;S-紫堇块茎碱可以在RNMT催化下进一步转化成木兰花碱(magnoflorine)[71].基于此发现,该团队在建立了大肠杆菌牛心果碱合成途径的基础上,将CTS和CNMT(可替代RNMT行使氮上双甲基化功能)的编码基因转入酵母,通过共培养的方式完成了紫堇块茎碱和木兰花碱(7.2 mg/L)的微生物合成,成为首例微生物体系成功合成阿朴啡类生物碱[图4(b))[26].由于该类生物碱生物合成途径研究的欠缺,尤其是R型结构和一些关键后修饰生物合成途径的未知,导致目前只有紫堇块茎碱和木兰花碱得以在微生物体系中合成. ...
Microbial production of plant benzylisoquinoline alkaloids
4
2008
... S-牛心果碱在植物中的生物合成途径起始于多巴胺和4-羟基苯乙醛(4-HPAA),两个底物在NCS的作用下,通过Pictet-Spengler反应形成S-去甲乌药碱,随后经6OMT催化6位羟基甲基化形成S-乌药碱[(S)-coclaurine],再经CNMT催化氮上甲基化形成S型N-甲基乌药碱[(S)-N-methylcoclaurine],最后经NMCH和4′OMT依次催化4′位发生羟化和羟基的甲基化形成S-牛心果碱(图1).由于细胞色素P450酶NMCH是膜蛋白,在大肠杆菌中表达难度大,是最初采用大肠杆菌合成牛心果碱的主要瓶颈.为了克服该瓶颈,2008年,Minami和Sato等通过在大肠杆菌中引入多巴胺的单胺氧化酶(MAO)来替代NMCH,串联NCS、6OMT、CNMT和4′OMT的编码基因,以多巴胺为前体,成功通过大肠杆菌合成了外消旋的牛心果碱(2 mg/L,1.3%)(图2)[26].随后,上述团队通过提高体系中NCS的表达水平以及稳定中间体,将牛心果碱的产量和产率分别提升至54 mg/L和13%[27];同时,成功通过提高大肠杆菌中酪氨酸的产量,完成从简单的甘油前体到S-牛心果碱的合成,产量约为46 mg/L[28-30]. ...
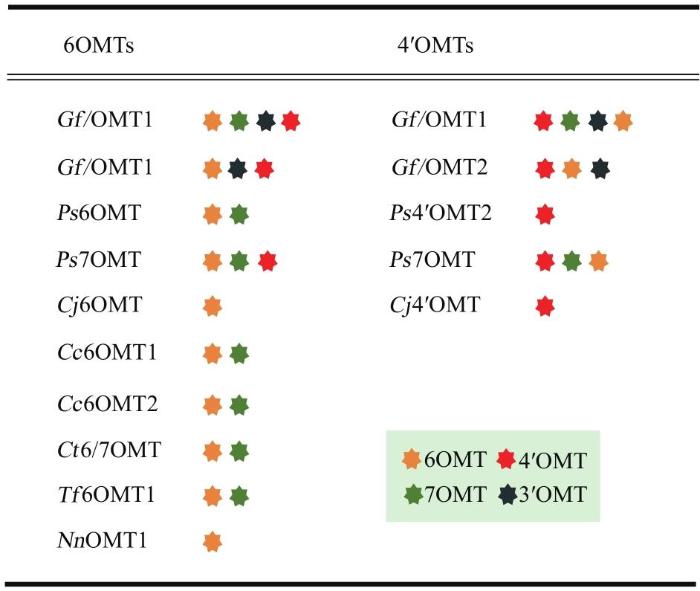
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... 阿朴啡类生物碱含有特征性的联苯型四环结构骨架[图4(a)],广泛分布于自然界,具有抗癌、抗病毒、抗疟疾、抗炎等多种重要生理活性,目前已经在20个科100多种属植物中分离到了500多个化合物[7-11,70].阿朴啡结构的多样性很大程度依赖于骨架上多样的甲基化、亚二甲基化、羟化取代方式以及骨架氧化程度的差异,该类生物碱大部分生物合成途径没有被阐明,这也是限制该类生物碱在微生物底盘细胞中合成的主要原因.2008年,Sato等在C. japonica中发现了一个P450酶CYP80G2可以催化S-牛心果碱的8位和2′位发生偶联完成联苯型四环骨架的构筑,形成S-紫堇块茎碱(corytuberine)[25],该酶底物专一性较强,对于R-牛心果碱和类似物大部分不能识别,与上游途径中NMCH一起,可能会导致大量旁支产物不能回到主代谢流;S-紫堇块茎碱可以在RNMT催化下进一步转化成木兰花碱(magnoflorine)[71].基于此发现,该团队在建立了大肠杆菌牛心果碱合成途径的基础上,将CTS和CNMT(可替代RNMT行使氮上双甲基化功能)的编码基因转入酵母,通过共培养的方式完成了紫堇块茎碱和木兰花碱(7.2 mg/L)的微生物合成,成为首例微生物体系成功合成阿朴啡类生物碱[图4(b))[26].由于该类生物碱生物合成途径研究的欠缺,尤其是R型结构和一些关键后修饰生物合成途径的未知,导致目前只有紫堇块茎碱和木兰花碱得以在微生物体系中合成. ...
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Improvement of reticuline productivity from dopamine by using engineered Escherichia coli
1
2013
... S-牛心果碱在植物中的生物合成途径起始于多巴胺和4-羟基苯乙醛(4-HPAA),两个底物在NCS的作用下,通过Pictet-Spengler反应形成S-去甲乌药碱,随后经6OMT催化6位羟基甲基化形成S-乌药碱[(S)-coclaurine],再经CNMT催化氮上甲基化形成S型N-甲基乌药碱[(S)-N-methylcoclaurine],最后经NMCH和4′OMT依次催化4′位发生羟化和羟基的甲基化形成S-牛心果碱(图1).由于细胞色素P450酶NMCH是膜蛋白,在大肠杆菌中表达难度大,是最初采用大肠杆菌合成牛心果碱的主要瓶颈.为了克服该瓶颈,2008年,Minami和Sato等通过在大肠杆菌中引入多巴胺的单胺氧化酶(MAO)来替代NMCH,串联NCS、6OMT、CNMT和4′OMT的编码基因,以多巴胺为前体,成功通过大肠杆菌合成了外消旋的牛心果碱(2 mg/L,1.3%)(图2)[26].随后,上述团队通过提高体系中NCS的表达水平以及稳定中间体,将牛心果碱的产量和产率分别提升至54 mg/L和13%[27];同时,成功通过提高大肠杆菌中酪氨酸的产量,完成从简单的甘油前体到S-牛心果碱的合成,产量约为46 mg/L[28-30]. ...
A bacterial platform for fermentative production of plant alkaloids
2
2011
... S-牛心果碱在植物中的生物合成途径起始于多巴胺和4-羟基苯乙醛(4-HPAA),两个底物在NCS的作用下,通过Pictet-Spengler反应形成S-去甲乌药碱,随后经6OMT催化6位羟基甲基化形成S-乌药碱[(S)-coclaurine],再经CNMT催化氮上甲基化形成S型N-甲基乌药碱[(S)-N-methylcoclaurine],最后经NMCH和4′OMT依次催化4′位发生羟化和羟基的甲基化形成S-牛心果碱(图1).由于细胞色素P450酶NMCH是膜蛋白,在大肠杆菌中表达难度大,是最初采用大肠杆菌合成牛心果碱的主要瓶颈.为了克服该瓶颈,2008年,Minami和Sato等通过在大肠杆菌中引入多巴胺的单胺氧化酶(MAO)来替代NMCH,串联NCS、6OMT、CNMT和4′OMT的编码基因,以多巴胺为前体,成功通过大肠杆菌合成了外消旋的牛心果碱(2 mg/L,1.3%)(图2)[26].随后,上述团队通过提高体系中NCS的表达水平以及稳定中间体,将牛心果碱的产量和产率分别提升至54 mg/L和13%[27];同时,成功通过提高大肠杆菌中酪氨酸的产量,完成从简单的甘油前体到S-牛心果碱的合成,产量约为46 mg/L[28-30]. ...
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
(R,S)-tetrahydropapaveroline production by stepwise fermentation using engineered Escherichia coli
0
2014
L-Tyrosine production by deregulated strains of Escherichia coli
1
2007
... S-牛心果碱在植物中的生物合成途径起始于多巴胺和4-羟基苯乙醛(4-HPAA),两个底物在NCS的作用下,通过Pictet-Spengler反应形成S-去甲乌药碱,随后经6OMT催化6位羟基甲基化形成S-乌药碱[(S)-coclaurine],再经CNMT催化氮上甲基化形成S型N-甲基乌药碱[(S)-N-methylcoclaurine],最后经NMCH和4′OMT依次催化4′位发生羟化和羟基的甲基化形成S-牛心果碱(图1).由于细胞色素P450酶NMCH是膜蛋白,在大肠杆菌中表达难度大,是最初采用大肠杆菌合成牛心果碱的主要瓶颈.为了克服该瓶颈,2008年,Minami和Sato等通过在大肠杆菌中引入多巴胺的单胺氧化酶(MAO)来替代NMCH,串联NCS、6OMT、CNMT和4′OMT的编码基因,以多巴胺为前体,成功通过大肠杆菌合成了外消旋的牛心果碱(2 mg/L,1.3%)(图2)[26].随后,上述团队通过提高体系中NCS的表达水平以及稳定中间体,将牛心果碱的产量和产率分别提升至54 mg/L和13%[27];同时,成功通过提高大肠杆菌中酪氨酸的产量,完成从简单的甘油前体到S-牛心果碱的合成,产量约为46 mg/L[28-30]. ...
Dopamine-first" mechanism enables the rational engineering of the norcoclaurine synthase aldehyde activity profile
3
2015
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
... [31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae
2
2008
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Reconstitution of a 10-gene pathway for synthesis of the plant alkaloid dihydrosanguinarine in Saccharomyces cerevisiae
2
2014
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
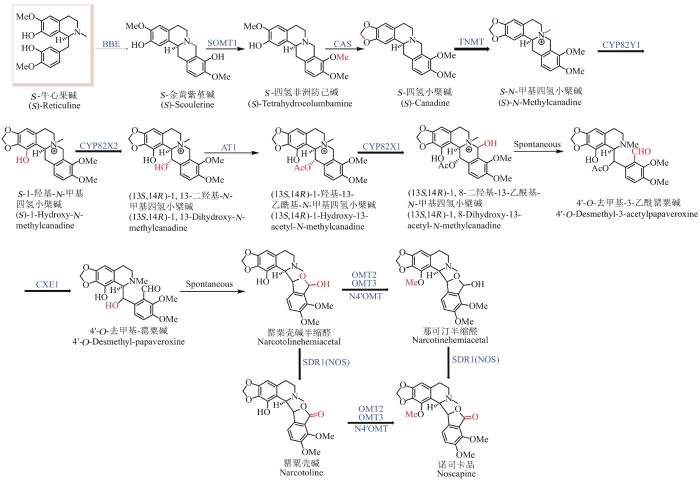
... 对于血根碱的微生物合成,2014年,Martin团队将血根碱生物合成途径上6OMT、CNMT、4′OMT以及CFS、SPS、TNMT、MSH、P6H的编码基因导入酿酒酵母,以外消旋的全去甲劳丹碱为前体,成功通过酿酒酵母合成了二氢血根碱(dihydrosanguinarine)及其氧化衍生物血根碱[33](图5).该过程还产生了副产物N-甲基金黄紫堇碱(N-methylscoulerine)和N-甲基碎叶紫堇碱(N-methylcheilanthifoline),可能是TNMT底物识别宽泛导致,因此该研究推测TNMT的底物宽泛性以及膜蛋白的表达活性可能是影响产量的主要原因.隔年,Smolke团队也基于相似的策略,通过对途径上左旋华紫堇碱[(S)-cheilanthifoline]的产量进行优化,从而获得了血根碱及代谢通路上的中间体,包括刺罂粟碱(stylopine,676 μg/L)、cis-N-甲基刺罂粟碱(cis-N-methylstylopine,548 μg/L)、原阿片碱(protopine,252 μg/L)和血根碱(80 μg/L)[82].紧接着,Sato团队也通过将BBE、CFS和 SPS的编码基因导入酿酒酵母,通过喂养外消旋的牛心果碱获得了血根碱的前体刺罂粟碱[83].然而到目前为止,还未能通过微生物体系建立起原小檗碱和小檗碱类以及苯并菲啶类BIA的从头合成途径. ...
Tetrahydropterin-dependent amino acid hydroxylases
1
1999
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
Bacterial tyrosinases
1
2006
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications
1
2006
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose
1
2015
... 通过酿酒酵母合成牛心果碱也是在2008年首次实现,由于当时还不能在酿酒酵母中表达出有活性的NCS[31],因此,Hawkins和Smolke等直接以外消旋的全去甲劳丹碱(norlaudanosoline)为前体,通过将6OMT、CNMT和4′OMT的编码基因导入酿酒酵母,获得了外消旋的牛心果碱(150 mg/L,10%),绕开了NCS和NMCH负责的两步催化[32-33].除了NCS的低活性,在酵母中从头合成牛心果碱还主要受限于L-酪氨酸3位无法羟化,导致多巴胺合成受阻(图2,紫色部分).虽然在动物和植物里发现了酪氨酸3位单加氧酶(tyrosine 3-monooxygenase;EC 1.14.16.2)可以催化L-酪氨酸3位发生羟化,但该反应需要辅因子四氢生物蝶呤(tetrahydrobiopterin)的参与,而酵母不能合成该辅因子[34].另一个可催化L-酪氨酸3位发生羟化的酶为酪氨酸酶(tyrosinase;EC 1.14.18.1),已经成功用于大肠杆菌合成牛心果碱,然而,该酪氨酸酶会催化L-3,4-二羟基-苯丙氨酸(L-3,4-dihydroxyphenylalanine,L-DOPA)进一步氧化生成大量的副产物黑色素(melanin),且不能在酵母中表达出活性蛋白[28, 35-36].上述瓶颈的突破是在2015年,由两个团队分别采用了两种策略完成.其中,Dueber和Martin团队从甜菜(Beta vulgaris)中筛选到了一个可以在酵母中发挥活性的酪氨酸羟化酶,该酶野生型不需要四氢生物蝶呤辅因子,但会继续氧化L-DOPA形成L-多巴醌(L-dopaquinone),导致副产物黑色素的形成.对此,该团队巧妙地利用了一个可以将L-DOPA转化成黄色荧光色素甜菜黄素(betaxanthin)的生物传感器,加快了随机突变优化的筛选速度,获得了L-DOPA氧化活性受到抑制的酪氨酸羟化酶突变体,将L-DOPA的产量提高了2.8倍[37].而Smolke团队则通过在酵母中表达褐家鼠(Rattus norvegicus)来源的酪氨酸合酶三突变体TyrHR37E/R38E/W166Y——突变后可减少L-酪氨酸和儿茶酚胺类化合物对酶的抑制,并在酵母中重构褐家鼠中四氢生物蝶呤的合成途径来完成L-酪氨酸3位的羟化;同时,该团队也对初级代谢产物磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)和赤藓糖-4-磷酸(erythrose-4-phosphate,E4P)到4-羟基-苯丙酮酸(4-hydroxy-phenylpyruvate,4-HPP)转化路径上的四个相关酶进行过表达和突变优化,提高了L-酪氨酸和4-HPAA的产量[21].在此基础上,上述两个团队均将各自建立的L-DOPA酵母合成途径,串联了L-DOPA脱羧酶、NCS、6OMT、CNMT、NMCH和4′OMT的编码基因,在酵母中实现了从葡萄糖到S-牛心果碱的合成.虽然每升产量都在微克级别,与达到工业化生产水平差距甚大,但在实验水平上证实了酵母从头合成牛心果碱的可行性,对后续研究起到了极大的推动作用. ...
A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids
2
2020
... 2020年,Martin团队在前期研究基础上,继续对酵母中莽草酸途径(shikimate pathway)、埃利希途径(Ehrlich pathway)和L-酪氨酸的代谢途径进行了连续20多次的迭代优化,包括过表达莽草酸途径上分支酸合酶、预苯酸脱氢酶、苯丙酮酸脱羧酶和敲除旁支产物L-苯丙氨酸和L-色氨酸合成相关的预苯酸脱水酶、吲哚-3-甘油磷酸合酶来积累4-HPP;对埃利希途径上7个相关的还原酶和氧化酶的编码基因进行敲除,从而阻止4-HPAA被还原和氧化等,结合酶突变抑制反馈调节以及发酵工艺的优化,将通过酵母从头合成S-牛心果碱的产量提高了57 000倍,提升至4.6 g/L[38].该研究成果标志着微生物合成S-牛心果碱已经达到了工业化生产水平,体现了微生物体系生产植物次生代谢产物的巨大潜力. ...
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
A highly characterized synthetic landing pad system for precise multicopy gene integration in yeast
1
2018
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Complete biosynthesis of noscapine and halogenated alkaloids in yeast
1
2018
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
Asymmetric synthesis of tetrahydroisoquinolines by enzymatic Pictet-Spengler reaction
1
2014
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
The catalytic potential of Coptis japonica NCS2 revealed-development and utilisation of a fluorescamine-based assay
2
2012
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
... ,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Functional analysis of norcoclaurine synthase in Coptis japonica
0
2007
Pictet-Spenglerases in alkaloid biosynthesis: future applications in biocatalysis
1
2020
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Structural basis of enzymatic (S)-norcoclaurine biosynthesis
1
2009
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Norcoclaurine synthase-mediated stereoselective synthesis of 1,1′-disubstituted, spiro- and bis-tetrahydroisoquinoline alkaloids
0
2021
A novel enzymatic strategy for the synthesis of substituted tetrahydroisoquinolines
0
2016
One-pot triangular chemoenzymatic cascades for the syntheses of chiral alkaloids from dopamine
0
2015
Acceptance and kinetic resolution of α-methyl-substituted aldehydes by norcoclaurine synthases
0
2019
Enzyme catalysed Pictet-Spengler formation of chiral 1,1'-disubstituted- and spiro-tetrahydroisoquinolines
0
2017
One-pot chemoenzymatic synthesis of trolline and tetrahydroisoquinoline analogues
1
2018
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Peroxisome compartmentalization of a toxic enzyme improves alkaloid production
1
2021
... 除去初级代谢相关途径,目前微生物合成S-牛心果碱途径中所涉及到的酶包括NCS、6OMT、CNMT、NMCT和4′OMT等,主要来源于罂粟(Papaver somniferum)、花菱草(Eschscholzia californica)、日本黄连(Coptis japonica)、黄唐松草(Thalictrum flavum)和南天竹(Nandina domestica)等植物.体外酶活测试研究表明野生型的NCS催化活性低,立体选择性受底物自发反应影响,可能是BIA合成途径中的限速步骤[31];对NCS的氮端进行截短可以有效提高其在微生物体内的活性[39-41].NCS采用的酶催化机理是先结合多巴胺,再结合4-HPAA.其中,多巴胺结构基本包含在酶活性口袋中,导致NCS对胺类底物的识别性较窄;4-HPAA大部分结构在活性口袋之外,导致NCS对醛类底物的识别性十分宽泛[31-42].比如C. japonica来源的CjNCS可以识别苯乙醛类、3-吲哚乙醛类、烷基醛类等底物类似物[38,42-45];T. flavum来源的TfNCS除了能识别醛类底物类似物外,还可以识别惰性更强的酮类底物类似物,包括环酮、苯基酮以及烷基酮等[44-51].这两类NCS也是构筑BIA微生物合成途径的常用酶,根据其对醛和酮类底物的杂泛性,预示着控制微生物体内相关类似物的合成,可能有助于提高BIAs的产量.值得一提的是,近期有报道称NCS对酵母具有一定的毒性[52],因此加大对该酶优化和挖掘的研究力度,可能是提高牛心果碱微生物合成产量的关键之一. ...
Molecular origins of functional diversity in benzylisoquinoline alkaloid methyltransferases
2
2019
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... [53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Molecular characterization of the S-adenosyl-L-methionine:3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase involved in isoquinoline alkaloid biosynthesis in Coptis japonica
2
2000
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... [54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
(R,S)-Reticuline 7-O-methyltransferase and (R,S)-norcoclaurine 6-O-methyltransferase of Papaver somniferum-cDNA cloning and characterization of methyl transfer enzymes of alkaloid biosynthesis in opium poppy
0
2003
Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy
2
2003
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... -56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Cell type-specific localization of transcripts encoding nine consecutive enzymes involved in protoberberine alkaloid biosynthesis
1
2005
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Overexpression of Coptis japonica norcoclaurine 6-O-methyltransferase overcomes the rate-limiting step in benzylisoquinoline alkaloid biosynthesis in cultured Eschscholzia californica
0
2007
Systematic silencing of benzylisoquinoline alkaloid biosynthetic genes reveals the major route to papaverine in opium poppy
2
2012
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... ,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Isolation and characterization of O-methyltransferases involved in the biosynthesis of glaucine in Glaucium flavum
1
2015
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Crystal structure of norcoclaurine-6-O-methyltransferase, a key rate-limiting step in the synthesis of benzylisoquinoline alkaloids
0
2016
The opium poppy genome and morphinan production
0
2018
Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera)
1
2020
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Molecular cloning and characterization of coclaurine N-methyltransferase from cultured cells of Coptis japonica
2
2002
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... [64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Targeted metabolite and transcript profiling for elucidating enzyme function: isolation of novel N-methyltransferases from three benzylisoquinoline alkaloid-producing species
1
2009
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants
2
2015
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
... ,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Comparative macroarray analysis of morphine containing Papaver somniferum and eight morphine free Papaver species identifies an O-methyltransferase involved in benzylisoquinoline biosynthesis
1
2005
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Molecular cloning and functional heterologous expression of two alleles encoding (S)-N-methylcoclaurine 3'-hydroxylase (CYP80B1), a new methyl jasmonate-inducible cytochrome P-450-dependent mono-oxygenase of benzylisoquinoline alkaloid biosynthesis
1
1998
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Distribution of morphinan and benzo[c]phenanthridine alkaloid gene transcript accumulation in Papaver somniferum
1
2000
... 6OMT、4′OMT和CNMT分别负责BIA骨架6位羟基、4′位羟基和氮原子上的甲基化,均属于SAM依赖的甲基化酶.自然界中,赋予BIA结构多样性的除了基本骨架的重排、偶联导致骨架类型的不同之外,很大程度还依赖于甲基化酶杂泛性和专一性的随机交叉作用,比如全去甲劳丹碱,在上述三个简单的甲基化酶的作用下,就可能可以形成20多种不同的BIA结构[53],因此,对这些酶的底物杂泛性和区域选择性进行解析,选取选择性高的酶元件,对于确保微生物合成BIA代谢流的方向、减少旁支产物十分重要.到目前为止已报道了10个6OMT,分别来自于7个种属[54-63];8个CNMT,分别来自4个种属[26,56-57,59,64-66];5个4′OMT,分别来自3个种属[54-56,59-60,66-67].多数6OMT和4′OMT对3′、4′、6或7位羟基存在交叉的区域选择性(图3)[53],其中,P. somniferum 和T. flavum来源的6OMT和4′OMT区域选择性较为专一,也是目前微生物合成牛心果碱最常用的甲基化酶元件.CNMT虽然区域选择性较好,但是具有宽泛的底物识别性,对于S型和R型苄基异喹啉类底物都能识别,且不能很好地控制氮上甲基化的数量[64-65],在复杂BIA的微生物合成过程中,可能会导致多种氮上单甲基化和双甲基化的旁支产物.NMCH为P450膜蛋白,难以在大肠杆菌中异源表达,因此以大肠杆菌作为宿主构筑牛心果碱时常用MAO代替,但其可被用于酵母体系.对于NMCH,目前只报道了2个,分别来自E. californica(CYP80B1)和P. somniferum(CYP80B3),底物专一性都很强,只识别S型N-甲基乌药碱,对于R型以及氮上未甲基化底物都不能识别[68-69],这可能会使微生物合成牛心果碱途径中其他酶元件所产生的旁支产物不能被NMCH催化回到主代谢流,导致整体产率的下降. ...
Aporphinoid alkaloids, V
1
1994
... 阿朴啡类生物碱含有特征性的联苯型四环结构骨架[图4(a)],广泛分布于自然界,具有抗癌、抗病毒、抗疟疾、抗炎等多种重要生理活性,目前已经在20个科100多种属植物中分离到了500多个化合物[7-11,70].阿朴啡结构的多样性很大程度依赖于骨架上多样的甲基化、亚二甲基化、羟化取代方式以及骨架氧化程度的差异,该类生物碱大部分生物合成途径没有被阐明,这也是限制该类生物碱在微生物底盘细胞中合成的主要原因.2008年,Sato等在C. japonica中发现了一个P450酶CYP80G2可以催化S-牛心果碱的8位和2′位发生偶联完成联苯型四环骨架的构筑,形成S-紫堇块茎碱(corytuberine)[25],该酶底物专一性较强,对于R-牛心果碱和类似物大部分不能识别,与上游途径中NMCH一起,可能会导致大量旁支产物不能回到主代谢流;S-紫堇块茎碱可以在RNMT催化下进一步转化成木兰花碱(magnoflorine)[71].基于此发现,该团队在建立了大肠杆菌牛心果碱合成途径的基础上,将CTS和CNMT(可替代RNMT行使氮上双甲基化功能)的编码基因转入酵母,通过共培养的方式完成了紫堇块茎碱和木兰花碱(7.2 mg/L)的微生物合成,成为首例微生物体系成功合成阿朴啡类生物碱[图4(b))[26].由于该类生物碱生物合成途径研究的欠缺,尤其是R型结构和一些关键后修饰生物合成途径的未知,导致目前只有紫堇块茎碱和木兰花碱得以在微生物体系中合成. ...
Isolation and characterization of reticuline N-methyltransferase involved in biosynthesis of the aporphine alkaloid magnoflorine in opium poppy
1
2016
... 阿朴啡类生物碱含有特征性的联苯型四环结构骨架[图4(a)],广泛分布于自然界,具有抗癌、抗病毒、抗疟疾、抗炎等多种重要生理活性,目前已经在20个科100多种属植物中分离到了500多个化合物[7-11,70].阿朴啡结构的多样性很大程度依赖于骨架上多样的甲基化、亚二甲基化、羟化取代方式以及骨架氧化程度的差异,该类生物碱大部分生物合成途径没有被阐明,这也是限制该类生物碱在微生物底盘细胞中合成的主要原因.2008年,Sato等在C. japonica中发现了一个P450酶CYP80G2可以催化S-牛心果碱的8位和2′位发生偶联完成联苯型四环骨架的构筑,形成S-紫堇块茎碱(corytuberine)[25],该酶底物专一性较强,对于R-牛心果碱和类似物大部分不能识别,与上游途径中NMCH一起,可能会导致大量旁支产物不能回到主代谢流;S-紫堇块茎碱可以在RNMT催化下进一步转化成木兰花碱(magnoflorine)[71].基于此发现,该团队在建立了大肠杆菌牛心果碱合成途径的基础上,将CTS和CNMT(可替代RNMT行使氮上双甲基化功能)的编码基因转入酵母,通过共培养的方式完成了紫堇块茎碱和木兰花碱(7.2 mg/L)的微生物合成,成为首例微生物体系成功合成阿朴啡类生物碱[图4(b))[26].由于该类生物碱生物合成途径研究的欠缺,尤其是R型结构和一些关键后修饰生物合成途径的未知,导致目前只有紫堇块茎碱和木兰花碱得以在微生物体系中合成. ...
Quaternary protoberberine alkaloids
1
2007
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Biochemical evidence that berberine bridge enzyme belongs to a novel family of flavoproteins containing a bi-covalently attached FAD cofactor
1
2006
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Characterization and mechanism of the berberine bridge enzyme, a covalently flavinylated oxidase of benzophenanthridine alkaloid biosynthesis in plants
1
1995
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Berberine bridge enzyme catalyzes the six electron oxidation of (S)-reticuline to dehydroscoulerine
1
2009
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Characterization of three O-methyltransferases involved in noscapine biosynthesis in opium poppy
2
2012
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
Molecular cloning and characterization of CYP719, a methylenedioxy bridge-forming enzyme that belongs to a novel P450 family, from cultured Coptis japonica cells
1
2003
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Characterization of two methylenedioxy bridge-forming cytochrome P450-dependent enzymes of alkaloid formation in the Mexican prickly poppy Argemone mexicana
1
2011
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Heterologous expression of two FAD-dependent oxidases with (S)-tetrahydroprotoberberine oxidase activity from Arge mone mexicana and Berberis wilsoniae in insect cells
1
2011
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Isolation and characterization of a cDNA encoding (S)-cis-N-methylstylopine 14-hydroxylase from opium poppy, a key enzyme in sanguinarine biosynthesis
1
2013
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Optimization of yeast-based production of medicinal protoberberine alkaloids
1
2015
... 原小檗碱和小檗碱类以及苯并菲啶类属于BIA七大骨架类型中的两大类,具有抗微生物、降压、镇静、抗白血病和抗肿瘤等多种生理活性[72],大部分天然合成途径仍然未知.目前成功通过微生物合成的主要为小檗碱(berberine,原小檗碱和小檗碱类)和血根碱(sanguinarine,苯并菲啶类),两者的生物合成途径均是先由S-牛心果碱在黄素氧化酶BBE的作用下形成共同中间体S-金黄紫堇碱[(S)-Scoulerine].该酶对底物识别范围较窄,对R-牛心果碱以及大部分底物类似物都不能识别[73-74],但会催化S-牛心果碱发生六电子氧化形成脱氢金黄紫堇碱(dehydroscouierine)[75].随后,S-金黄紫堇碱经甲基化酶SOMT1、P450酶CAS和氧化还原酶STOX作用可以形成小檗碱[76-79];经四个P450酶CFS、SPS、MSH和P6H,一个甲基化酶TNMT和一个氧化还原酶DBOX的作用下可以生成血根碱[77-78, 80].由于小檗碱和血根碱下游途径中涉及到多个膜蛋白,包括BBE、多个P450酶等,因此常选用能表达膜蛋白的酿酒酵母体系作为异源合成的宿主.虽然在2008年,通过将C. japonica中编码BBE的基因导入酿酒酵母,和产牛心果碱的大肠杆菌工程菌共培养,就已经实现通过微生物将多巴胺转化成S-金黄紫堇碱(8.3 mg/L,2.2%)[26];也可以通过将P. somniferum 来源的BBE编码基因和T. flavum 来源的SOMT和CAS的编码基因依次导入产牛心果碱的酿酒酵母工程菌,以外消旋的全去甲劳丹碱为前体,依次获得小檗碱合成途径中的中间体四氢非洲防己胺[(S)-tetrahydrocolumbamine](约60 mg/L)和四氢小檗碱[(S)-canadine](约30 mg/L,转化率1%~2%)[32],后续研究报道四氢小檗碱可以自发形成小檗碱,但可能受限于当时的检测条件,并未报道有小檗碱的产生.直至2015年,Smolke等通过优化后者代谢通路上CAS的表达量,结合密码子优化、酶元件的筛选以及发酵条件的优化,将四氢小檗碱产量提高了70多倍,最终实现了小檗碱(39 μg/L)的微生物合成[81].值得一提的是,该途径会积累较多的中间代谢产物,导入或缺失Berberis wilsonae 来源的STOX编码基因并不会对小檗碱的最终产量造成影响,表明该STOX可能并没有在酵母中行使功能.在酵母中异源表达出活性更好的STOX,可能有助于提高小檗碱的产量. ...
Engineering strategies for the fermentative production of plant alkaloids in yeast
1
2015
... 对于血根碱的微生物合成,2014年,Martin团队将血根碱生物合成途径上6OMT、CNMT、4′OMT以及CFS、SPS、TNMT、MSH、P6H的编码基因导入酿酒酵母,以外消旋的全去甲劳丹碱为前体,成功通过酿酒酵母合成了二氢血根碱(dihydrosanguinarine)及其氧化衍生物血根碱[33](图5).该过程还产生了副产物N-甲基金黄紫堇碱(N-methylscoulerine)和N-甲基碎叶紫堇碱(N-methylcheilanthifoline),可能是TNMT底物识别宽泛导致,因此该研究推测TNMT的底物宽泛性以及膜蛋白的表达活性可能是影响产量的主要原因.隔年,Smolke团队也基于相似的策略,通过对途径上左旋华紫堇碱[(S)-cheilanthifoline]的产量进行优化,从而获得了血根碱及代谢通路上的中间体,包括刺罂粟碱(stylopine,676 μg/L)、cis-N-甲基刺罂粟碱(cis-N-methylstylopine,548 μg/L)、原阿片碱(protopine,252 μg/L)和血根碱(80 μg/L)[82].紧接着,Sato团队也通过将BBE、CFS和 SPS的编码基因导入酿酒酵母,通过喂养外消旋的牛心果碱获得了血根碱的前体刺罂粟碱[83].然而到目前为止,还未能通过微生物体系建立起原小檗碱和小檗碱类以及苯并菲啶类BIA的从头合成途径. ...
Efficient microbial production of stylopine using a Pichia pastoris expression system
1
2016
... 对于血根碱的微生物合成,2014年,Martin团队将血根碱生物合成途径上6OMT、CNMT、4′OMT以及CFS、SPS、TNMT、MSH、P6H的编码基因导入酿酒酵母,以外消旋的全去甲劳丹碱为前体,成功通过酿酒酵母合成了二氢血根碱(dihydrosanguinarine)及其氧化衍生物血根碱[33](图5).该过程还产生了副产物N-甲基金黄紫堇碱(N-methylscoulerine)和N-甲基碎叶紫堇碱(N-methylcheilanthifoline),可能是TNMT底物识别宽泛导致,因此该研究推测TNMT的底物宽泛性以及膜蛋白的表达活性可能是影响产量的主要原因.隔年,Smolke团队也基于相似的策略,通过对途径上左旋华紫堇碱[(S)-cheilanthifoline]的产量进行优化,从而获得了血根碱及代谢通路上的中间体,包括刺罂粟碱(stylopine,676 μg/L)、cis-N-甲基刺罂粟碱(cis-N-methylstylopine,548 μg/L)、原阿片碱(protopine,252 μg/L)和血根碱(80 μg/L)[82].紧接着,Sato团队也通过将BBE、CFS和 SPS的编码基因导入酿酒酵母,通过喂养外消旋的牛心果碱获得了血根碱的前体刺罂粟碱[83].然而到目前为止,还未能通过微生物体系建立起原小檗碱和小檗碱类以及苯并菲啶类BIA的从头合成途径. ...
[The effect of narcotine on cough reflex and on bronchial musculature
1
1954
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
Pharmacokinetics of oral noscapine
0
1990
Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells
0
1998
Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses
0
2000
Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine
0
2002
The anti-cancer activity of noscapine: a review
1
2009
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
A Papaver somniferum 10-gene cluster for synthesis of the anticancer alkaloid noscapine
1
2012
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
Acetylation serves as a protective group in noscapine biosynthesis in opium poppy
0
2015
CYP82Y1 is N-methylcanadine 1-hydroxylase, a key noscapine biosynthetic enzyme in opium poppy
1
2014
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
Engineering biosynthesis of the anticancer alkaloid noscapine in yeast
1
2016
... 苄基异喹啉类是BIA七大骨架类型之一,其中最具代表性的就是诺司卡品(noscapine).诺司卡品最初由法国化学家Robiquet在1817年从P. somniferum中分离鉴定,已长期作为止咳药物应用于临床,生物安全性高,并在1998年后陆续被报道具有抗肿瘤、抗癌等活性[84-89].与小檗碱和血根碱相同,诺司卡品的生源途径也经由中间体S-金黄紫堇碱,随后在S9OMT(SOMT1)、CAS作用下形成小檗碱的前体四氢小檗碱,所需的酶与小檗碱合成途径相同;最后四氢小檗碱在TNMT、CYP82Y1、CYP82X2、AT1、CYP82X1、CXE1、SDR1和N4′OMT等8个酶作用下形成诺司卡品[76,90-92].2016年,Smolke团队[93]报道了N4′OMT是由两个基因编码的异源二聚体酶(OMT2/OMT3),突破了在微生物体内合成诺司卡品的最后一个瓶颈.通过在酵母中表达16个诺司卡品生物合成的下游基因,以全去甲劳丹碱为前体,成功合成了诺斯卡品[(1.64±0.38)µmol/L].催化四氢小檗碱到诺司卡品合成的多个酶均具有较为宽泛的底物选择性,会产生多种旁支产物,然而也因为没有底物专一性酶的限制,所以大部分旁支产物最终都会重新汇聚到主代谢流,形成诺司卡品(图6).随后在2018年,Smolke团队将来自植物、细菌和动物的30多个基因导入酿酒酵母中,其中包括7个植物内质网定位酶基因;通过对异源途径各个酶表达水平的协调,优化发酵条件,成功通过酵母从头合成了诺司卡品(约2.8 mg/L),将产量提高了18 000倍.对此优化后的诺司卡品产生菌喂养卤素取代的酪氨酸类似物,可以得到对应卤素取代的苄基异喹啉类生物碱,表明了微生物合成在创造多种新颖BIA衍生物的潜在应用价值[40]. ...
Biosynthesis of the morphine alkaloids
1
1967
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
A microbial biomanufacturing platform for natural and semisynthetic opioids
3
2014
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
... [95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Synthesis of morphinan alkaloids in Saccharomyces cerevisiae
1
2015
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli
1
2016
... 吗啡烷类生物碱是目前临床上用于镇痛的主要药物之一,自1806年吗啡被分离到后,其生物合成途径也逐渐被破解[94],该类生物碱的基本骨架是由R-牛心果碱偶联形成[6].最初通过微生物合成吗啡烷类生物碱的主要瓶颈在于R-牛心果碱的生物合成途径未知,只能以一些R型或外消旋的中间体为前体,通过微生物转化而得,比如将P. somniferum来源的T6ODM、COR、CODM编码基因导入酿酒酵母,以蒂巴因(thebaine)为喂养前体,获得一系列吗啡烷类产物[95-96].直到2015年,吗啡生物合成途径中负责将S-牛心果碱转化成R-牛心果碱的差向易构酶(REPI)被解析[22-23],才使得通过微生物从头合成吗啡烷类生物碱得以实现(图7).REPI是由N端的细胞色素P450结构域和C端的醛酮还原酶(aldo-keto reductase)结构域融合而成,也被简写成STORR.Smolke团队和其他两个课题组共同报道了REPI的发现.通过在酵母中导入来源于植物、动物、细菌以及酵母的21条和23条相关合成基因,Smolke团队分别在酵母中实现了从糖到蒂巴因和氢可酮(hydrocodone)的合成.虽然产量上只有7.8 µg/L和0.3 µg/L,距离工业应用还需要大幅度的优化,但该工作为吗啡的微生物合成的可行性提供了有利的证据[21].隔年,Minami团队以大肠杆菌为宿主,由于REPI在大肠杆菌中表达活性不佳,该团队通过自发Pictet-Spengler反应提供R-牛心果碱和四步阶段发酵方式,从甘油合成了蒂巴因,是酵母合成体系产量的300倍;在此基础上继续引入T6ODM和MorB编码基因,利用尼奥平酮(neopinone)到可待因酮(codeinone)的自发反应,合成了氢可酮[97]. ...
CYP719B1 is salutaridine synthase, the C-C phenol-coupling enzyme of morphine biosynthesis in opium poppy
1
2009
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Comparative transcript and alkaloid profiling in Papaver species identifies a short chain dehydrogenase/reductase involved in morphine biosynthesis
0
2006
Acetyl coenzyme A:salutaridinol-7-O-acetyltransferase from Papaver somniferum plant cell cultures. The enzyme catalyzing the formation of thebaine in morphine biosynthesis
0
1995
Molecular characterization of the salutaridinol 7-O-acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum
1
2001
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Neodihydrothebaine and bractazonine, two dibenz[d,f]azonine alkaloids of Papaver bracteatum
1
1984
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
A pathogenesis-related 10 protein catalyzes the final step in thebaine biosynthesis
1
2018
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Dioxygenases catalyze O-demethylation and O,O-demethylenation with widespread roles in benzylisoquinoline alkaloid metabolism in opium poppy
2
2013
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
... ,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Molecular cloning and functional expression of codeinone reductase: the penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum
2
1999
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
... -105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Neopinone isomerase is involved in codeine and morphine biosynthesis in opium poppy
1
2019
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Codeinone reductase isoforms with differential stability, efficiency and product selectivity in opium poppy
1
2018
... R-牛心果碱在SalSyn、SalR和SalAT的作用下形成蒂巴因的前体氢化沙罗泰里啶-7-O-乙酸(salutaridinol 7-O-acetate)[98-101],该前体在前期研究中被认为是自发转化成蒂巴因,且受pH的影响,在pH=8~9时有利于转化为蒂巴因,在pH=6~7时,会自发转化成副产物[102].直至2018年在罂粟中发现可以催化该步转化发生的蒂巴因合酶THS,以全去甲劳丹碱为前体,在合成蒂巴因的酵母工程菌加入THS,蒂巴因产量是缺失THS的24倍,表明在植物体内该步反应是有酶参与的[103].蒂巴因接着在T6ODM作用下转化成尼奥平酮[104],可自发异构形成可待因酮(codeinone),继续在COR和CODM的作用下依次形成可待因(codeine)和吗啡(morphine)[105].由于COR和CODM底物识别十分宽泛,因此在通过微生物合成吗啡烷类生物碱时很难控制代谢流的方向,获取单一组分,早期合成吗啡烷类生物碱的酵母工程菌产生的生物碱超过50%是副产物[24,95,104-105].近期,在罂粟中又发现一个异构酶NISO可以催化尼奥平酮到可待因酮的转化,在酵母工程菌中导入CODM、T6ODM、COR和NISO编码基因可以获得7.6 µg/(L·OD)的可待因,产量是未导入NISO编码基因的42倍;继续导入Pseudomonas putida M10来源的morB可获得35 µg/(L·OD)氢可酮(hydrocodone),是前期不加NISO编码基因的30倍,表明NISO在植物体作用可能是加速尼奥平酮向可待因酮的转换,促进可待因和吗啡等生物碱的积累,减少旁支产物的生成[95,106-107].吗啡烷类生物碱的微生物合成途径的研究进展,深刻体现了BIA植物次生代谢产物生物合成途径的解析对构建微生物合成途径的重要性. ...
Enantioselective synthesis of 1-aryl-substituted tetrahydroisoquinolines employing imine reductase
1
2017
... 目前,微生物合成BIA的途径设计基本都是模拟其在植物体中的天然生物合成途径,因此,代谢途径冗长、酶元件的选择也十分局限,且依赖于生物合成途径的解析.由于植物遗传操作不便,大多基因不成簇以及存在内含子等因素,造成了植物生物合成途径解析较为困难.随着酶催化反应知识的不断增长和可获得的基因序列的积累,研究者们对BIA微生物合成途径的设计不再仅局限于传统上效仿自然的设计思路,逐渐开始创造全新的人工微生物合成途径,如通过对亚胺还原酶进行筛选和改造,获得了可立体专一性地还原1-苄基二氢异喹啉类化合物形成1-苄基四氢异喹啉类化合物的亚胺还原酶库,经CNMT催化可进一步形成牛心果碱类似物,从而建立了一条便捷高效的牛心果碱类似物的微生物合成途径[108-109],可作为多种BIA微生物合成的中间体,比如阿朴啡类生物碱、小檗碱等.这些全新的BIA人工微生物合成途径的设计,为发展该类生物碱的生产途径提供了更加广泛的启示,也是突破和超越现有BIA微生物合成途径研究成果的机遇. ...
Biosynthesis of plant tetrahydroisoquinoline alkaloids through an imine reductase route
1
2020
... 目前,微生物合成BIA的途径设计基本都是模拟其在植物体中的天然生物合成途径,因此,代谢途径冗长、酶元件的选择也十分局限,且依赖于生物合成途径的解析.由于植物遗传操作不便,大多基因不成簇以及存在内含子等因素,造成了植物生物合成途径解析较为困难.随着酶催化反应知识的不断增长和可获得的基因序列的积累,研究者们对BIA微生物合成途径的设计不再仅局限于传统上效仿自然的设计思路,逐渐开始创造全新的人工微生物合成途径,如通过对亚胺还原酶进行筛选和改造,获得了可立体专一性地还原1-苄基二氢异喹啉类化合物形成1-苄基四氢异喹啉类化合物的亚胺还原酶库,经CNMT催化可进一步形成牛心果碱类似物,从而建立了一条便捷高效的牛心果碱类似物的微生物合成途径[108-109],可作为多种BIA微生物合成的中间体,比如阿朴啡类生物碱、小檗碱等.这些全新的BIA人工微生物合成途径的设计,为发展该类生物碱的生产途径提供了更加广泛的启示,也是突破和超越现有BIA微生物合成途径研究成果的机遇. ...