• 特约评述 •
基于生物体-材料杂化体系的低碳生物合成的研究进展
郭心怡1, 郭树奇1,2, 李曙伟1, 焦子悦1, 费强1,2
- 1.西安交通大学,化学工程与技术学院,陕西 西安 710049
2.西安市一碳化合物生物转化技术重点实验室,陕西 西安 710049
-
收稿日期:2024-09-18修回日期:2024-12-26出版日期:2024-12-28 -
通讯作者:费强 -
作者简介:郭心怡 (1999—),女,在读博士研究生。研究方向为嗜甲烷菌细胞工厂构建及微生物-材料杂化体系转化甲烷合成化学品。E-mail:gxy9933@stu.xjtu.edu.cn费 强 (1980—),男,西安交通大学教授,博士生导师,陕西省杰青基金获得者、陕西高校青年创新团队负责人,一碳化合物生物转化技术西安市重点实验室主任,中国生物工程学会一碳生物技术专委会秘书长。近年承担国家重点研发计划、国家自然科学基金、陕西省重点研发计划等多项科研项目。以一碳气体的微生物固定及其高值化利用为研究目标,利用合成生物学和高密度发酵等技术改造和优化细胞工厂,实现食品、材料、化学品、能源等产品的生物制造。E-mail:feiqiang@xjtu.edu.cn -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(2217281)
Progress in Biological Entity-Material Hybrid System for Low-Carbon Biosynthesis
Xinyi GUO1, Shuqi GUO1,2, Shuwei LI1, Ziyue JIAO1, Qiang FEI1,2
- 1.School of Chemical Engineering and Technology,Xi’an Jiaotong University,Xi’an 710049,Shaanxi,China
2.Xi’an Key Laboratory of C1 Compound Bioconversion Technology,Xi’an 710049,Shaanxi,China
-
Received:2024-09-18Revised:2024-12-26Online:2024-12-28 -
Contact:Qiang FEI
摘要:
为推进绿色低碳生物经济发展,基于生物体(工业菌种或工业酶)的低碳生物合成技术由于在温室气体等低碳原料转化和利用中展现出的巨大应用潜力而广受关注。目前,低碳原料的生物转化过程仍面临能量利用效率低、转化速率慢等问题,这已成为制约其广泛应用的主要瓶颈之一。生物体与材料的杂化体系能够通过利用光能或电能等可再生能源驱动微生物细胞或工业酶将低碳原料转化为目标产物,这为低碳生物制造领域的发展提供了新的路径。本文基于可再生能源驱动生物体转化低碳原料的新兴技术,首先探讨了通过光催化材料或电极材料从可再生能源中捕获能量的相关技术进展。然后结合光/电驱动的生物体-材料杂化体系在转化温室气体原料合成各类化学品中的应用实例,深入分析了在不同材料和电子传递机制下,杂化体系对能量供给、底物转化和耗能产物合成等效率的影响作用和最新研究进展。最后,针对杂化体系在化学品低碳合成过程中遇到的挑战,展望了具有可行性的进一步解决方案及应用前景,为“双碳”目标的实现提供了技术支撑。
中图分类号:
引用本文
郭心怡, 郭树奇, 李曙伟, 焦子悦, 费强. 基于生物体-材料杂化体系的低碳生物合成的研究进展[J]. 合成生物学, DOI: 10.12211/2096-8280.2024-073.
Xinyi GUO, Shuqi GUO, Shuwei LI, Ziyue JIAO, Qiang FEI. Progress in Biological Entity-Material Hybrid System for Low-Carbon Biosynthesis[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2024-073.
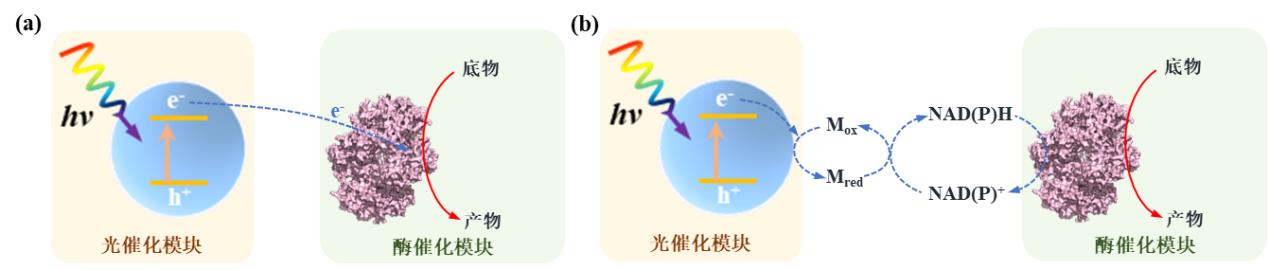
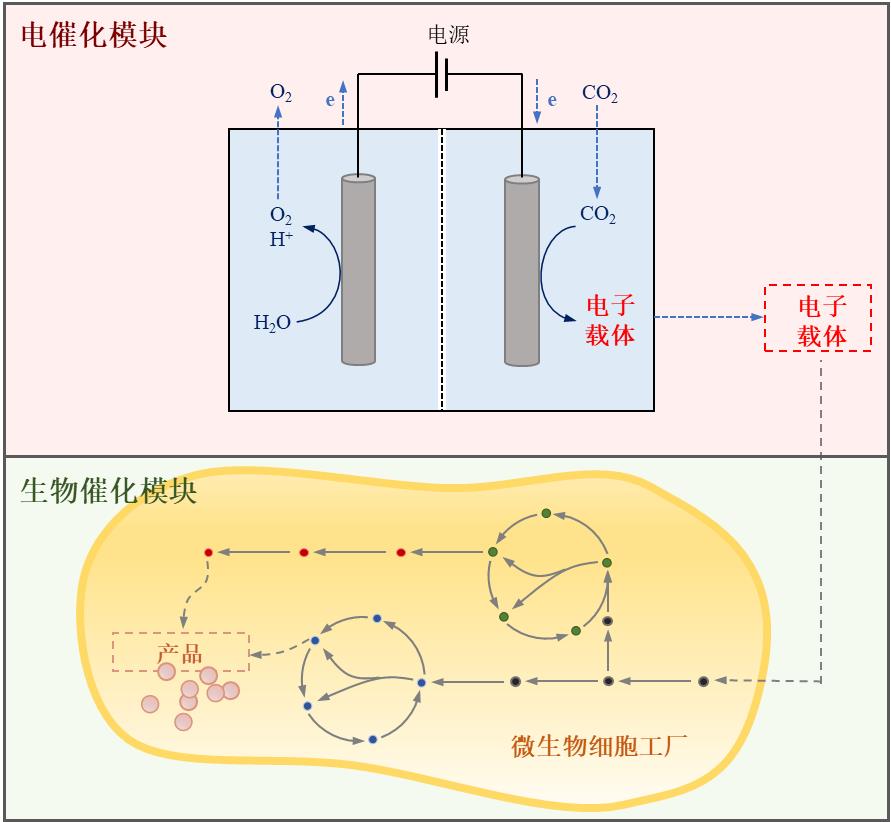
图1 光驱动酶-材料杂化体系中电子传递机制(a)直接电子转移模式;(b)间接电子转移模式。(Mox:氧化介质,Mred:还原介质)[22]
Fig. 1 Electron transport mechanism in light-driven material-enzyme hybrid system. (a) Direct electron transfer between cell and electrode; (b) Indirect electron transfer between cell and electrode. (Mox: oxidized mediator, Mred: reduced mediator)
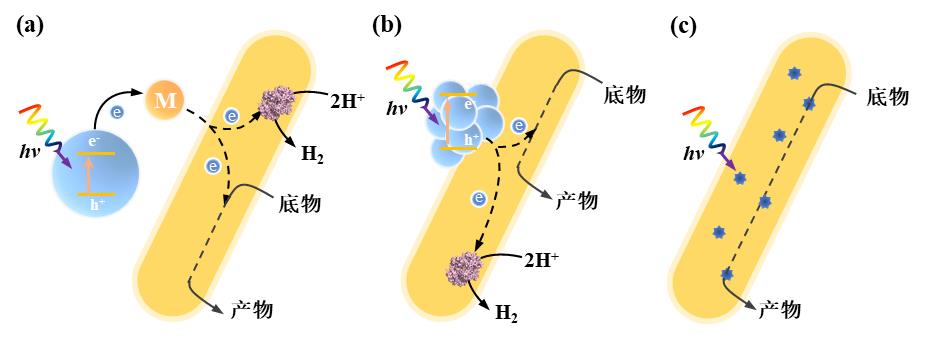
图2 光驱动微生物-材料杂化系统中材料-微生物杂化形式及相应电子转移机制。材料分别分散在细胞外部(a),结合在细胞表面(b)或分布在细胞内部(c)(M:电子介质)[26]
Fig. 2 Material-microbial hybrid forms and corresponding electron transfer mechanisms in light-driven material-microbial hybrid systems. Materials are distributed outside the cell (a), adsorbed on the cell surface (b) or distributed inside the cell (c). (M: electron mediator)
| 产品 种类 | 原料 | 产品 | 材料 | 菌种 | 产品产率 | 量子产率 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 有机酸 | CO2 | 乙酸 | CdS NPs | Moorella thermoacetica | 0.1 mM/h | 2.44 ± 0.62% | [ |
| CdS NPs | Clostridium autoethanogenum | 12.1 mM | - | [ | |||
| AuNCs | Moorella thermoacetica | 6.0 Mm/g/week | 2.86 ± 0.38% | [ | |||
| PFP/PDI | Moorella thermoacetica | 0.6 mM | 1.6% | [ | |||
| MOF | Moorella thermoacetica | - | - | [ | |||
| CdS NPs | Sporomusa ovata | 40.0 mM | 16.8 ± 9% | [ | |||
| CO2 | L-苹果酸 | CdS NPs | Escherichia coli | 1.5 mol/mol | - | [ | |
| 葡萄糖 | 莽草酸 | InP NPs | Saccharomyces cerevisiae | 48.5mg/L | - | [ | |
| 生物醇 | CO2 | 甲醇 | poly(allylamine hydrochloride) | 甲酸脱氢酶、甲醛脱氢酶、酵母醇脱氢酶 | 60.39 μM | - | [ |
| artificial thylakoid | 甲酸脱氢酶、甲醛脱氢酶、酵母醇脱氢酶 | 99 Μm/h | 0.66 ± 0.13% | [ | |||
| CdS-NPs | Clostridium ljungdahlii、 Acetobacterium woodii、 Moorella thermoacetica、 Pseudomonas aeruginosa | 0.4 g/L | - | [ | |||
| CH4 | 甲醇 | BM-TiO2 | Methylosinus trichosporium | 15761.0 μmol/g/h | [ | ||
| 萜类 | TY培养基 | 法尼烯 | meso-Al2O3 | Escherichia coli | 1816.0 mg/L | - | [ |
| CO2/葡萄糖 | 类胡萝卜素 | Au NPs | Chlorella zofingiensis | 10.7 mg/L | - | [ | |
| 聚合物 | CO2/果糖 | PHB | g-C3N4 | Ralstonia eutropha | 9.1 g/L | 29.72% ± 5.53% | [ |
| CO2/果糖 | PHB | CdS NPs | Cupriavidus necator | 0.6 mg/L | - | [ |
表1 光驱动生物体-材料杂化体系合成化学品
Tab. 1 Chemicals synthesized by light-driven material-microbial hybrid system
| 产品 种类 | 原料 | 产品 | 材料 | 菌种 | 产品产率 | 量子产率 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 有机酸 | CO2 | 乙酸 | CdS NPs | Moorella thermoacetica | 0.1 mM/h | 2.44 ± 0.62% | [ |
| CdS NPs | Clostridium autoethanogenum | 12.1 mM | - | [ | |||
| AuNCs | Moorella thermoacetica | 6.0 Mm/g/week | 2.86 ± 0.38% | [ | |||
| PFP/PDI | Moorella thermoacetica | 0.6 mM | 1.6% | [ | |||
| MOF | Moorella thermoacetica | - | - | [ | |||
| CdS NPs | Sporomusa ovata | 40.0 mM | 16.8 ± 9% | [ | |||
| CO2 | L-苹果酸 | CdS NPs | Escherichia coli | 1.5 mol/mol | - | [ | |
| 葡萄糖 | 莽草酸 | InP NPs | Saccharomyces cerevisiae | 48.5mg/L | - | [ | |
| 生物醇 | CO2 | 甲醇 | poly(allylamine hydrochloride) | 甲酸脱氢酶、甲醛脱氢酶、酵母醇脱氢酶 | 60.39 μM | - | [ |
| artificial thylakoid | 甲酸脱氢酶、甲醛脱氢酶、酵母醇脱氢酶 | 99 Μm/h | 0.66 ± 0.13% | [ | |||
| CdS-NPs | Clostridium ljungdahlii、 Acetobacterium woodii、 Moorella thermoacetica、 Pseudomonas aeruginosa | 0.4 g/L | - | [ | |||
| CH4 | 甲醇 | BM-TiO2 | Methylosinus trichosporium | 15761.0 μmol/g/h | [ | ||
| 萜类 | TY培养基 | 法尼烯 | meso-Al2O3 | Escherichia coli | 1816.0 mg/L | - | [ |
| CO2/葡萄糖 | 类胡萝卜素 | Au NPs | Chlorella zofingiensis | 10.7 mg/L | - | [ | |
| 聚合物 | CO2/果糖 | PHB | g-C3N4 | Ralstonia eutropha | 9.1 g/L | 29.72% ± 5.53% | [ |
| CO2/果糖 | PHB | CdS NPs | Cupriavidus necator | 0.6 mg/L | - | [ |
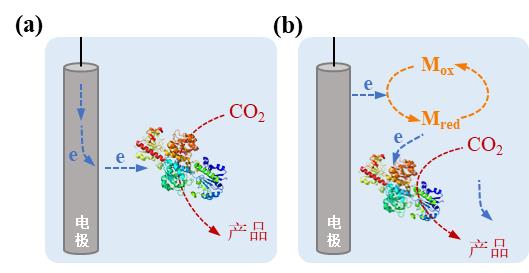
图3 电驱动酶-材料杂化体系中电极-微生物杂化形式及相应电子传递机制。(a)直接电子转移模式;(b)间接电子转移模式(Mox:氧化介质,Mred:还原介质)
Fig. 3 Enzyme-electrode hybrid forms and corresponding electron transport mechanisms in electrically driven material-microbial hybrid system. (a) Direct electron transfer between cell and electrode; (b) Indirect electron transfer between cell and electrode. (Mox: oxidized mediator, Mred: reduced mediator)
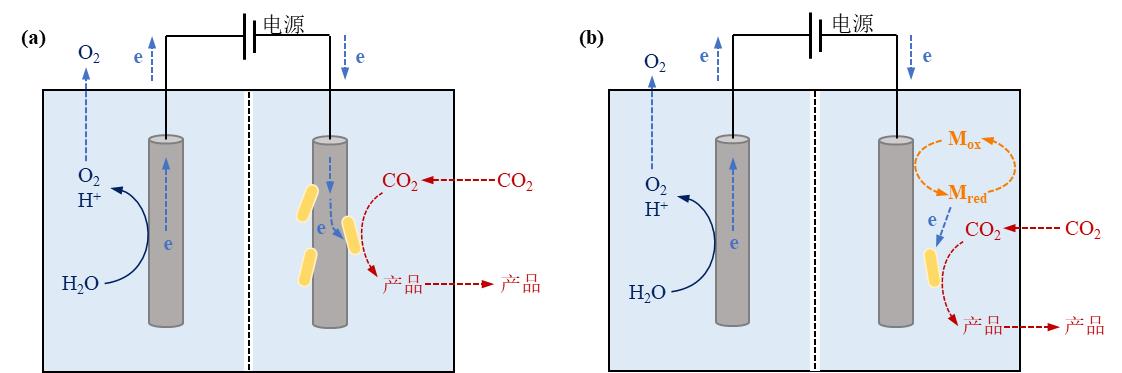
图4 电驱动微生物-材料杂化体系中电极-微生物杂化形式及相应电子传递机制。(a)直接电子转移模式;(b)间接电子转移模式(Mox:氧化介质,Mred:还原介质)
Fig. 4 Microbe-electrode hybrid forms and corresponding electron transport mechanisms in electrically driven material-microbial hybrid system. (a) Direct electron transfer between cell and electrode; (b) Indirect electron transfer between cell and electrode. (Mox: oxidized mediator, Mred: reduced mediator)
| 1 | Wen D, Fang W, Liu Y, et al. Valorization of carbon dioxide with alcohols[J]. Chinese Chemical Letters, 2024, 35(7): 109394. |
| 2 | Su H, Lin J. Biosynthesis pathways of expanding carbon chains for producing advanced biofuels[J]. Biotechnology for Biofuels and Bioproducts, 2023, 16(1): 109. |
| 3 | Zhang C, Ottenheim C, Weingarten M, et al. Microbial utilization of next-generation feedstocks for the biomanufacturing of value-added chemicals and food ingredients[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10. |
| 4 | Bo C, Liu J, Zhang Y, et al. Effective photocatalytic methane oxidation over the TiO2/methanotrophs system[J]. Nano Today, 2023, 52: 101938. |
| 5 | 伊晓峰, 吴见平, 王远鹏. 半导体纳米材料介导微生物固定CO2研究进展[J]. 应用与环境生物学报, 2023, 29(4): 795-802. |
| 6 | Li A, Cao X, Fu R, et al. Biocatalysis of CO2 and CH4: Key enzymes and challenges[J]. Biotechnology Advances, 2024, 72: 108347. |
| 7 | Fang Z, Tang Y J, Koffas M A. Harnessing electrical-to-biochemical conversion for microbial synthesis[J]. Current Opinion in Biotechnology, 2022, 75: 102687. |
| 8 | Li J, Tian Y, Zhou Y, et al. Abiotic-biological hybrid systems for CO2 conversion to value-added chemicals and fuels[J]. Transactions of Tianjin University, 2020, 26(4): 237-247. |
| 9 | Hamby H, Li B, Shinopoulos K E, et al. Light-driven carbon-carbon bond formation via CO2 reduction catalyzed by complexes of CdS nanorods and a 2-oxoacid oxidoreductase[J]. Proceedings of the National Academy of Sciences, 2020, 117(1): 135-140.. |
| 10 | Li J, Han H, Chang Y, et al. The material-microorganism interface in microbial hybrid electrocatalysis systems[J]. Nanoscale, 2023, 15(13): 6009-6024. |
| 11 | Proppe A H, Li Y C, Aspuru-Guzik A, et al. Bioinspiration in light harvesting and catalysis[J]. Nature Reviews Materials, 2020, 5(11): 828-846. |
| 12 | Zavafer A, Cheah M H, Hillier W, et al. Photodamage to the oxygen evolving complex of photosystem II by visible light[J]. Scientific Reports, 2015, 5(1): 16363. |
| 13 | Murata N, Nishiyama Y. ATP is a driving force in the repair of photosystem II during photoinhibition[J]. Plant, Cell & Environment, 2018, 41(2): 285-299. |
| 14 | Liu H, Cheng M, Liu Y, et al. Single atoms meet metal-organic frameworks: collaborative efforts for efficient photocatalysis[J]. Energy & Environmental Science, 2022, 15(9): 3722-3749. |
| 15 | Sun X, Huang H, Zhao Q, et al. Thin-layered photocatalysts[J]. Advanced Functional Materials, 2020, 30(22): 1910005. |
| 16 | Hawkins A S, Mcternan P M, Lian H, et al. Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals[J]. Current Opinion in Biotechnology, 2013, 24(3): 376-384. |
| 17 | Yang N, Tian Y, Zhang M, et al. Photocatalyst-enzyme hybrid systems for light-driven biotransformation[J]. Biotechnology Advances, 2022, 54: 107808. |
| 18 | Chen K, Arnold F H. Engineering new catalytic activities in enzymes[J]. Nature Catalysis, 2020, 3(3): 203-213. |
| 19 | Noji T, Jin T, Nango M, et al. CO2 photoreduction by formate dehydrogenase and a Ru-complex in a nanoporous glass reactor[J]. ACS Applied Materials & Interfaces, 2017, 9(4): 3260-3265. |
| 20 | Chaudhary Y S, Woolerton T W, Allen C S, et al. Visible light-driven CO2 reduction by enzyme coupled CdS nanocrystals[J]. Chemical Communications, 2011, 48(1): 58-60. |
| 21 | Woolerton T W, Sheard S, Reisner E, et al. Efficient and clean photoreduction of CO2 to CO by enzyme-modified TiO2 nanoparticles using visible light[J]. Journal of the American Chemical Society, 2010, 132(7): 2132-2133. |
| 22 | Li S, Shi J, Liu S, et al. Molecule-electron-proton transfer in enzyme-photo-coupled catalytic system[J]. Chinese Journal of Catalysis, 2023, 44: 96-110. |
| 23 | Kornienko N, Zhang J Z, Sakimoto K K, et al. Interfacing nature's catalytic machinery with synthetic materials for semi-artificial photosynthesis[J]. Nature Nanotechnology, 2018, 13(10): 890-899. |
| 24 | Cestellos-Blanco S, Zhang H, Kim J M, et al. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis[J]. Nature Catalysis, 2020, 3(3): 245-255. |
| 25 | Sakimoto K K, Wong A B, Yang P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 26 | Song J, Lin H, Zhao G, et al. Photocatalytic material-microorganism hybrid system and its application—a review[J]. Micromachines, 2022, 13(6): 861. |
| 27 | Honda Y, Watanabe M, Hagiwara H, et al. Inorganic/whole-cell biohybrid photocatalyst for highly efficient hydrogen production from water[J]. Applied Catalysis B: Environmental, 2017, 210: 400-406. |
| 28 | Zhang H, Liu H, Tian Z, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nature Nanotechnology, 2018, 13(10): 900-905. |
| 29 | Jiang Z, Wang B, Yu J C, et al. AglnS2/In2S3 heterostructure sensitization of Escherichia coli for sustainable hydrogen production[J]. Nano Energy, 2018, 46: 234-240. |
| 30 | Wang J, Chen N, Bian G, et al. Solar-driven overproduction of biofuels in microorganisms[J]. Angewandte Chemie International Edition, 2022, 61(32): e202207132. |
| 31 | Ding Y, Bertram J R, Eckert C, et al. Nanorg microbial factories: light-driven renewable biochemical synthesis using quantum dot-bacteria nanobiohybrids[J]. Journal of the American Chemical Society, 2019, 141(26): 10272-10282. |
| 32 | Kumaravel V, Imam M D, Badreldin A, et al. Photocatalytic hydrogen production: Role of sacrificial reagents on the activity of oxide, carbon, and sulfide catalysts[J]. Catalysts, 2019, 9(3): 276. |
| 33 | Gai P, Yu W, Zhao H, et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid[J]. Angewandte Chemie International Edition, 2020, 59(18): 7224-7229. |
| 34 | Zhou X, Zeng Y, Tang Y Y, et al. Artificial regulation of state transition for augmenting plant photosynthesis using synthetic light-harvesting polymer materials[J]. Science Advances, 2020, 6(35): eabc5237. |
| 35 | Qi R, Zhao H, Zhou X, et al. In situ synthesis of photoactive polymers on a living cell surface via bio-palladium catalysis for modulating biological functions[J]. Angewandte Chemie International Edition, 2021, 60(11): 5759-5765. |
| 36 | Tremblay P L, Xu M, Chen Y, et al. Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction[J]. iScience, 2020, 23(1): 100784. |
| 37 | Jin S, Jeon Y, Jeon M S, et al. Acetogenic bacteria utilize light-driven electrons as an energy source for autotrophic growth[J]. Proceedings of the National Academy of Sciences, 2021, 118(9): e2020552118. |
| 38 | Ji Z, Zhang H, Liu H, et al. Cytoprotective metal-organic frameworks for anaerobic bacteria[J]. Proceedings of the National Academy of Sciences, 2018, 115(42): 10582-10587. |
| 39 | He Y, Wang S, Han X, et al. Photosynthesis of acetate by Sporomusa ovata–CdS biohybrid system[J]. ACS Applied Materials & Interfaces, 2022, 14(20): 23364-23374. |
| 40 | HU G, LI Z, MA D, et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals[J]. Nature Catalysis, 2021, 4(5): 395-406. |
| 41 | Guo J L, Suástegui M, Sakimoto K K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362(6416): 813-816. |
| 42 | Ji X, Su Z, Wang P, et al. Integration of artificial photosynthesis system for enhanced electronic energy-transfer efficacy: A case study for solar-energy driven bioconversion of carbon dioxide to methanol[J]. Small, 2016, 12(34): 4753-4762. |
| 43 | Zhang S, Shi J, Sun Y, et al. Artificial thylakoid for the coordinated photoenzymatic reduction of carbon dioxide[J]. ACS Catalysis, 2019, 9(5): 3913-3925. |
| 44 | Kumar M, Sahoo P C, Srikanth S, et al. Photosensitization of electro-active microbes for solar assisted carbon dioxide transformation[J]. Bioresource Technology, 2019, 272: 300-307. |
| 45 | Li X, Sun H, Mao X, et al. Enhanced photosynthesis of carotenoids in microalgae driven by light-harvesting gold nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(20): 7600-7608. |
| 46 | Xu M, Tremblay P L, Ding R, et al. Photo-augmented PHB production from CO2 or fructose by Cupriavidus necator and shape-optimized CdS nanorods[J]. Science of The Total Environment, 2021, 753: 142050. |
| 47 | Sakimoto K K, Zhang S J, Yang P. Cysteine-cystine photoregeneration for oxygenic photosynthesis of acetic acid from CO2 by a tandem inorganic-biological hybrid system[J]. Nano Letters, 2016, 16(9): 5883-5887. |
| 48 | Cestellos-Blanco S, Zhang H, Yang P. Solar-driven carbon dioxide fixation using photosynthetic semiconductor bio-hybrids[J]. Faraday Discussions, 2019, 215(0): 54-65. |
| 49 | Zhang H, Wang L, Pérez-Fortes M, et al. Techno-economic optimization of biomass-to-methanol with solid-oxide electrolyzer[J]. Applied Energy, 2020, 258: 114071. |
| 50 | Sheng Y, Guo F, Guo B, et al. Light-driven CO2 reduction with a surface-displayed enzyme cascade–C3N4 Hybrid[J]. ACS Synthetic Biology, 2023, 12(9): 2715-2724. |
| 51 | Wei W, Sun P, Li Z, et al. A surface-display biohybrid approach to light-driven hydrogen production in air[J]. Science Advances, 2018, 4(2): eaap9253. |
| 52 | Rowe S F, Le Gall G, Ainsworth E V, et al. Light-driven H2 evolution and C=C or C=O bond hydrogenation by Shewanella oneidensis: A versatile strategy for photocatalysis by nonphotosynthetic microorganisms[J]. ACS Catalysis, 2017, 7(11): 7558-7566. |
| 53 | Liu K, Wang F Q, Liu K, et al. Light-driven progesterone production by InP-(M. neoaurum) biohybrid system[J]. Bioresources and Bioprocessing, 2022, 9(1): 93. |
| 54 | Zhao Q, Li Y, Shen B, et al. UiO-66-mediated light-driven regeneration of intracellular NADH in Clostridium tyrobutyricum to strengthen butyrate production[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(8): 3405-3415. |
| 55 | Xiao K, Tsang T H, Sun D, et al. Interfacing iodine-doped hydrothermally carbonized carbon with Escherichia coli through an "add-on" mode for enhanced light-driven hydrogen production[J]. Advanced Energy Materials, 2021, 11(21): 2100291. |
| 56 | Wang B, Jiang Z, Yu J C, et al. Enhanced CO2 reduction and valuable C2+ chemical production by a CdS-photosynthetic hybrid system[J]. Nanoscale, 2019, 11(19): 9296-9301. |
| 57 | Ye J, Yu J, Zhang Y, et al. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid[J]. Applied Catalysis B: Environmental, 2019, 257: 117916. |
| 58 | Zhou Z, Li Y, Li M, et al. Efficient removal for multiple pollutants via Ag2O/BiOBr heterojunction: A promoted photocatalytic process by valid electron transfer pathway[J]. Chinese Chemical Letters, 2020, 31(10): 2698-2704. |
| 59 | Liang J, Xiao K, Wang X, et al. Revisiting solar energy flow in nanomaterial-microorganism hybrid systems[J]. Chemical Reviews, 2024. |
| 60 | Overa S, Ko B H, Zhao Y, et al. Electrochemical approaches for CO2 conversion to chemicals: A journey toward practical applications[J]. Accounts of Chemical Research, 2022, 55(5): 638-648. |
| 61 | Chiranjeevi P, Bulut M, Breugelmans T, et al. Current trends in enzymatic electrosynthesis for CO2 reduction[J]. Current Opinion in Green and Sustainable Chemistry, 2019, 16: 65-70. |
| 62 | Lu Y C, Song W, An D, et al. Designing compartmentalized hydrogel microparticles for cell encapsulation and scalable 3D cell culture[J]. Journal of Materials Chemistry B, 2014, 3(3): 353-360. |
| 63 | Long C J, He Y H, Guan Z. Emerging strategies for asymmetric synthesis: Combining enzyme promiscuity and photo-/electro-redox catalysis[J]. Asian Journal of Organic Chemistry, 2023, 12(2): e202200685. |
| 64 | Cadoux C, Milton R D. Recent enzymatic electrochemistry for reductive reactions[J]. ChemElectroChem, 2020, 7(9): 1974-1986. |
| 65 | Logan B E, Rossi R, Ragab A, et al. Electroactive microorganisms in bioelectrochemical systems[J]. Nature Reviews Microbiology, 2019, 17(5): 307-319. |
| 66 | 焦子悦, 黄小涵, 郭树奇, 等. 微生物固碳的电子供给策略研究进展[J]. 生物工程学报, 2022, 38(7): 2396-2409. |
| 67 | Bian B, Bajracharya S, Xu J, et al. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy[J]. Bioresource Technology, 2020, 302: 122863. |
| 68 | Combining metal-microbe and microbe-microbe dual direct electron transfer on Fe( 0)-cathode of bio-electrochemical system to enhance anaerobic digestion of cellulose wastewater[J]. Chinese Chemical Letters, 2022, 33(6): 3106-3112. |
| 69 | Omar B, Abou-Shanab R, El-Gammal M, et al. Simultaneous biogas upgrading and biochemicals production using anaerobic bacterial mixed cultures[J]. Water Research, 2018, 142: 86-95. |
| 70 | Lovley D R. Powering microbes with electricity: direct electron transfer from electrodes to microbes[J]. Environmental Microbiology Reports, 2011, 3(1): 27-35. |
| 71 | Koch C, Harnisch F. What is the essence of microbial electroactivity?[J]. Frontiers in Microbiology, 2016, 7. |
| 72 | Bond D R, Lovley D R. Electricity production by Geobacter sulfurreducens attached to electrodes[J]. Applied and Environmental Microbiology, 2003, 69(3): 1548-1555. |
| 73 | Marsili E, Baron D B, Shikhare I D, et al. Shewanella secretes flavins that mediate extracellular electron transfer[J]. Proceedings of the National Academy of Sciences, 2008, 105(10): 3968-3973. |
| 74 | Harrington T D, Mohamed A, Tran V N, et al. Neutral red-mediated microbial electrosynthesis by Escherichia coli, Klebsiella pneumoniae, and Zymomonas mobilis [J]. Bioresource Technology, 2015, 195: 57-65. |
| 75 | Lai B. Burning questions: Exploring the limits of microbial electrochemical technology for industrial biotechnological applications[J]. Microbial Biotechnology, 2024, 17(1). |
| 76 | Claassens N J, Cotton C A R, Kopljar D, et al. Making quantitative sense of electromicrobial production[J]. Nature Catalysis, 2019, 2(5): 437-447. |
| 77 | Zheng T, Xia C. Electrifying biosynthesis for CO2 upcycling[J]. Trends in Chemistry, 2023, 5(1): 7-10. |
| 78 | Zheng T, Zhang M, Wu L, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| 79 | Tremblay P L, Zhang T. Electrifying microbes for the production of chemicals[J]. Frontiers in Microbiology, 2015, 6(MAR). |
| 80 | Kang N K, Chau T H T, Lee E Y. Engineered methane biocatalysis: strategies to assimilate methane for chemical production[J]. Current Opinion in Biotechnology, 2024, 85: 103031. |
| 81 | 郭树奇, 焦子悦, 费强. 基于化学品生物合成的嗜甲烷菌人工细胞构建及应用进展[J]. 合成生物学, 2021, 2(6): 1017-1029. |
| 82 | Kracke F, Deutzmann J S, Gu W, et al. In situ electrochemical H2 production for efficient and stable power-to-gas electromethanogenesis[J]. Green Chemistry, 2020, 22(18): 6194-6203. |
| 83 | Bai Y, Zhou L, Irfan M, et al. Bioelectrochemical methane production from CO2 by Methanosarcina barkeri via direct and H2-mediated indirect electron transfer[J]. Energy, 2020, 210: 118445. |
| 84 | Fu Q, Kuramochi Y, Fukushima N, et al. Bioelectrochemical analyses of the development of a Thermophilic Biocathode catalyzing electromethanogenesis[J]. Environmental Science & Technology, 2015, 49(2): 1225-1232. |
| 85 | Fixen K R, Zheng Y, Harris D F, et al. Light-driven carbon dioxide reduction to methane by nitrogenase in a photosynthetic bacterium[J]. Proceedings of the National Academy of Sciences, 2016, 113(36): 10163-10167. |
| 86 | Bajracharya S, Srikanth S, Mohanakrishna G, et al. Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects[J]. Journal of Power Sources, 2017, 356: 256-273. |
| 87 | Flexer V, Chen J, Donose B C, et al. The nanostructure of three-dimensional scaffolds enhances the current density of microbial bioelectrochemical systems[J]. Energy & Environmental Science, 2013, 6(4): 1291-1298. |
| 88 | Katuri K P, Kamireddy S, Kavanagh P, et al. Electroactive biofilms on surface functionalized anodes: The anode respiring behavior of a novel electroactive bacterium, Desulfuromonas acetexigens [J]. Water Research, 2020, 185: 116284. |
| 89 | Wang X, Li X, Zhu Q. Electrocatalytic nanomaterials improve microbial extracellular electron transfer: A review[J]. Applied Sciences, 2024, 14(15): 6733. |
| 90 | Hou X, Huang L, Zhou P. Synergetic interaction of magnetic field and loaded magnetite for enhanced acetate production in biocathode of microbial electrosynthesis system[J]. International Journal of Hydrogen Energy, 2021, 46(10): 7183-7194. |
| 91 | Zhang T, Nie H, Bain T S, et al. Improved cathode materials for microbial electrosynthesis[J]. Energy & Environmental Science, 2012, 6(1): 217-224. |
| 92 | Tremblay P L, Höglund D, Koza A, et al. Adaptation of the autotrophic acetogen Sporomusa ovata to methanol accelerates the conversion of CO2 to organic products[J]. Scientific Reports, 2015, 5(1): 16168. |
| 93 | Liu C, Colón B C, Ziesack M, et al. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis[J]. Science, 2016, 352(6290): 1210-1213. |
| 94 | Treece T R, Pattanayak S, Matson M M, et al. Electrical-biological hybrid system for carbon efficient isobutanol production[J]. Metabolic Engineering, 2023, 80: 142-150. |
| 95 | Lim J, Choi S Y, Lee J W, et al. Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2 [J]. Proceedings of the National Academy of Sciences, 2023, 120(14): e2221438120. |
| 96 | Liu G, Zhong Y, Liu Z, et al. Solar-driven sugar production directly from CO2 via a customizable electrocatalytic–biocatalytic flow system[J]. Nature Communications, 2024, 15(1): 2636. |
| 97 | Zhang Y, Feng T, Zhou X, et al. Photoelectrocatalytic-microbial biohybrid for nitrogen reduction[J]. Advanced Materials, n/a(n/a): 2407239. |
| 98 | Su Y, Cestellos-Blanco S, Kim J M, et al. Close-packed nanowire-bacteria hybrids for efficient solar-driven CO2 fixation[J]. Joule, 2020, 4(4): 800-811. |
| 99 | Cestellos-Blanco S, Chan R R, Shen Y X, et al. Photosynthetic biohybrid coculture for tandem and tunable CO2 and N2 fixation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(26): e2122364119. |
| 100 | Liu C, Gallagher J J, Sakimoto K K, et al. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals[J]. Nano letters, 2015, 15(5): 3634. |
| [1] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [2] | 付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049. |
| [3] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [4] | 杨璐, 瞿旭东. 亚胺还原酶在手性胺合成中的应用[J]. 合成生物学, 2022, 3(3): 516-529. |
| [5] | 王汇滨, 车昌丽, 游松. Fe/α-酮戊二酸依赖型卤化酶在绿色卤化反应中的研究进展[J]. 合成生物学, 2022, 3(3): 545-566. |
| [6] | 楼玉姣, 徐鉴, 吴起. 生物催化惰性碳氢键的氘代反应研究进展[J]. 合成生物学, 2022, 3(3): 530-544. |
| [7] | 任杰, 曾安平. 基于二氧化碳的生物制造:从基础研究到工业应用的挑战[J]. 合成生物学, 2021, 2(6): 854-862. |
| [8] | 熊亮斌, 宋璐, 赵云秋, 刘坤, 刘勇军, 王风清, 魏东芝. 甾体化合物绿色生物制造:从生物转化到微生物从头合成[J]. 合成生物学, 2021, 2(6): 942-963. |
| [9] | 张发光, 曲戈, 孙周通, 马军安. 从化学合成到生物合成——天然产物全合成新趋势[J]. 合成生物学, 2021, 2(5): 674-696. |
| [10] | 汤恒, 韩鑫, 邹树平, 郑裕国. 多酶催化体系在医药化学品合成中的应用[J]. 合成生物学, 2021, 2(4): 559-576. |
| [11] | 吴淑可, 周颐, 王文, 张巍, 高鹏飞, 李智. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558. |
| [12] | 王俊婷, 郭潇佳, 李青, 万里, 赵宗保. 创制非天然辅酶偏好型甲醇脱氢酶[J]. 合成生物学, 2021, 2(4): 651-661. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||