合成生物学 ›› 2020, Vol. 1 ›› Issue (5): 516-527.DOI: 10.12211/2096-8280.2020-042
基于工程化盐单胞菌的下一代工业生物技术
陈江楠1, 陈潇宁2, 刘心怡3, 万薇4, 章义鑫1, 张自豪4, 郑逸飞1, 郑陶然1, 王宣1, 王子瑜1, 闫煦1, 张旭1, 吴赴清1( ), 陈国强1
), 陈国强1
- 1.清华大学生命科学学院,北京 100084
2.清华大学新雅书院,北京 100084
3.天津大学生命科学学院,天津 300072
4.山东大学生命科学学院,山东 济南 250100
-
收稿日期:2020-04-07修回日期:2020-05-06出版日期:2020-10-31发布日期:2020-12-03 -
通讯作者:吴赴清 -
基金资助:国家重点研发计划“合成生物学”重点专项(2018YFA0900200)
Engineering Halomonas spp. for next generation industrial biotechnology (NGIB)
CHEN Jiangnan1, CHEN Xiaoning2, LIU Xinyi3, WAN Wei4, ZHANG Yixin1, ZHANG Zihao4, ZHENG Yifei1, ZHENG Taoran1, WANG Xuan1, WANG Ziyu1, YAN Xu1, ZHANG Xu1, WU Fuqing1( ), CHEN Guoqiang1
), CHEN Guoqiang1
- 1.School of Life Sciences,Tsinghua University,Beijing 100084,China
2.XinYa College,Tsinghua University,Beijing 100084,China
3.School of Life Sciences,Tianjin University,Tianjin 300072,China
4.School of Life Science,Shandong University,Jinan 250100,Shandong,China
-
Received:2020-04-07Revised:2020-05-06Online:2020-10-31Published:2020-12-03 -
Contact:WU Fuqing
摘要:
我国是工业生物技术大国,拥有世界上最大的发酵产业,但传统发酵需要灭菌操作,发酵过程高耗能、耗淡水且不能连续,导致生产成本偏高,无法与化学工业竞争。因此,急需开发下一代工业生物技术(NGIB)来克服这些缺点。NGIB利用盐单胞菌等极端微生物作为底盘细胞,具有发酵不需灭菌、节能节水、设备投资少、产物终浓度高、分离过程简单等优点。本文围绕下一代工业生物技术的发展过程,系统介绍了近年来在技术优势强化,生物元件和工具开发如Porin启动子系统、CRISPRi系统、CRISPR-Cas9系统等,新产物合成如聚(3-羟基丁酸-co-3-羟基戊酸)(PHBV)、聚(3-羟基丁酸-co-4-羟基丁酸)(P3HB4HB)、表面活性剂蛋白等,以及PHA分离过程优化、发酵工艺放大、废水循环利用等方面取得的最新进展。随着合成生物学发展和应用,基于工程化盐单胞菌的下一代工业生物技术体系正在不断完善,优势也愈加明显。下一代工业生物技术将为大幅提升绿色生物制造的竞争力提供强力支撑。
中图分类号:
引用本文
陈江楠, 陈潇宁, 刘心怡, 万薇, 章义鑫, 张自豪, 郑逸飞, 郑陶然, 王宣, 王子瑜, 闫煦, 张旭, 吴赴清, 陈国强. 基于工程化盐单胞菌的下一代工业生物技术[J]. 合成生物学, 2020, 1(5): 516-527.
CHEN Jiangnan, CHEN Xiaoning, LIU Xinyi, WAN Wei, ZHANG Yixin, ZHANG Zihao, ZHENG Yifei, ZHENG Taoran, WANG Xuan, WANG Ziyu, YAN Xu, ZHANG Xu, WU Fuqing, CHEN Guoqiang. Engineering Halomonas spp. for next generation industrial biotechnology (NGIB)[J]. Synthetic Biology Journal, 2020, 1(5): 516-527.

图2 盐单胞菌H. bluephagenesis中已开发的下一代分子生物技术工具(a)、方法(b)和合成的多种产物(c)[5]1—Porin启动子表达系统;2—T7-like诱导表达系统;3—CRISPRi基因编辑系统;4—CRISPR/Cas9基因编辑系统;5—提升氧气利用率;6—形态工程改造;7—高密度生长;8—大规模发酵;9—PHA产品类型(PHB、PHBV和P3HB4HB);10—其他产品类型(PhaP,PhaR,ALA和ectoine)gRNA—向导RNA;PHBV—聚3羟基丁酸-3羟基戊酸酯;RBS—核糖体结合位点;sgRNA—单链向导RNA;VHb—透明颤菌血红蛋白
Fig. 2 Engineering halophilic Halomonas spp. for NGIB tools (a), methods (b) and production of multiple products (c)1—Porin promoter expression system; 2—T7‐like expression system; 3—CRISPRi engineering system; 4—CRISPR/Cas9 engineering system; 5—increasing oxygen availability; 6—morphology engineering; 7—high‐cell‐density growth; 8—large‐scale fermentation; 9—PHA product types(PHB, PHBV, and P3HB4HB); 10—other products (PhaP, PhaR, ALA, and ectoine)gRNA—guide RNA; PHBV—poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate); RBS—ribosome binding site; sgRNA—single‐guide RNA; VHb—Vitreoscilla hemoglobin

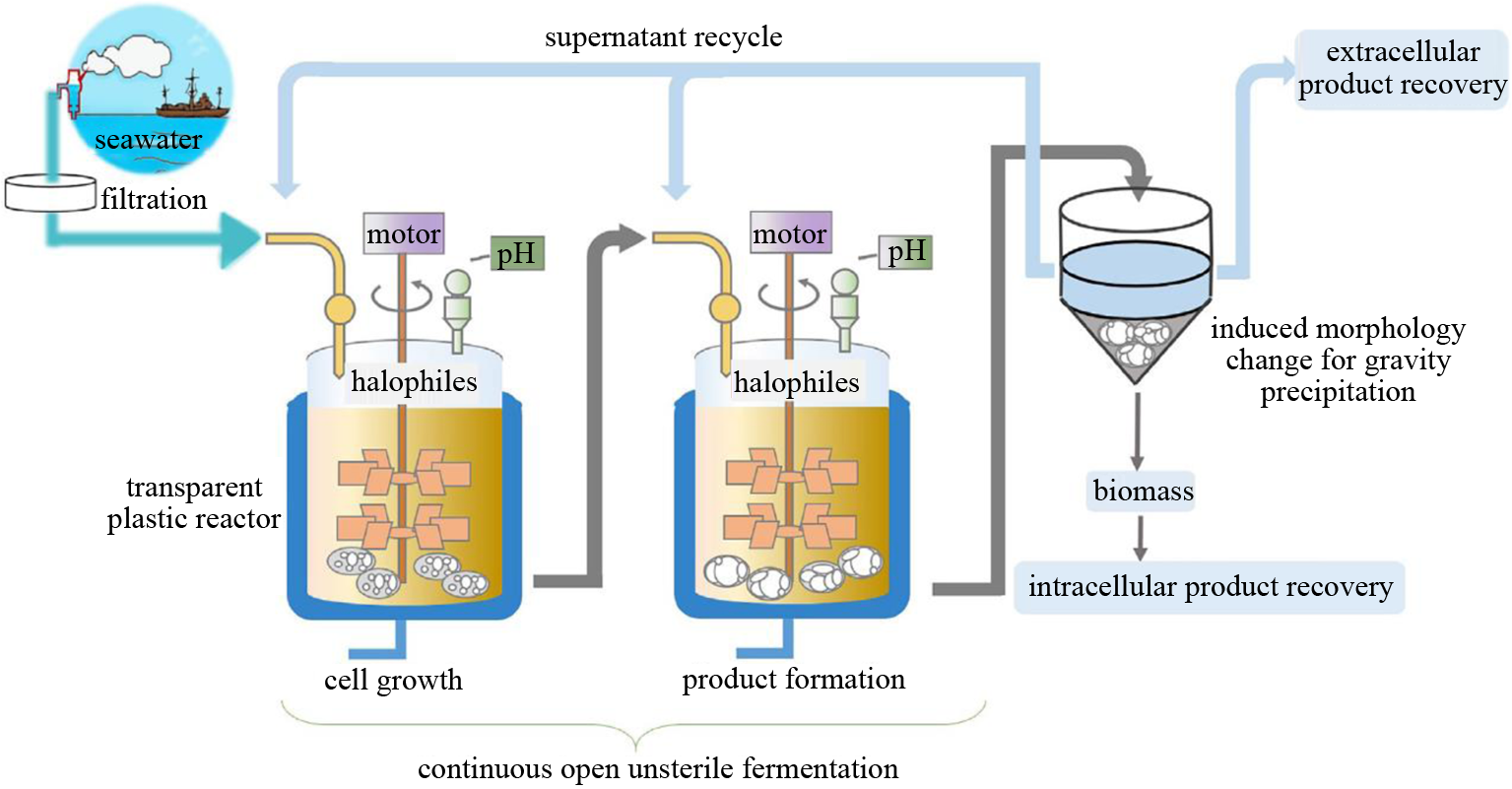
图3 利用盐单胞菌成功合成多种不同PHA材料[73](a)使用T7-like诱导表达系统高产PHB;(b)利用非相关碳源在H. bluephagenesis TD08(prpC基因敲除)菌株生产PHBV;(c)利用γ‐丁内酯和葡萄糖在H. bluephagenesis TD40中生产P3HB4HB;(d)在H. bluephagenesis TD(phaC敲除)菌株中生产PhaP蛋白;(e)在H. bluephagenesis TD(phaC敲除)菌株中生产PhaR蛋白;(f)在加大或者加长的H. campaniensis LS21中(mreB基因敲除或者ftsZ基因敲除)生产PHB;(g)第一个5000L不灭菌开放连续塑料发酵罐,Halomonas spp.在发酵罐中生长;(h)在H. bluephagenesis TD LTT22中生产ALA基因:phaC—编码PHA合酶;phaA—编码β‐酮硫解酶;phaB—编码NADPH依赖乙酰乙酰辅酶A还原酶;prpC—编码2-柠檬酸甲酯合酶;orfZ—编码2-柠檬酸甲酯合酶;mreB—编码细胞骨架蛋白;ftsZ—编码细胞分裂蛋白;△ftsZ::ftsZ‐gfp—gfp基因插入ftsZ基因的C端,使后者丧失功能;hem1—编码ALA合酶
Fig. 3 Engineering halophilic Halomonas spp. for production of multiple PHA products(a) Production of PHB using T7‐like expression system in H. bluephagenesis TD‐HIGH; (b) Production of PHBV in H. bluephagenesis TD08 (without prpC) in the absence of propionic acid; (c) Production of P34HB in H. bluephagenesis TD40 using γ‐butyrolactone and glucose as carbon sources; (d) Production of PhaP in H. bluephagenesis TD (without phaC); (e) Production of PhaR by H. bluephagenesis TD (without phaC); (f) Production of PHB by enlarged or elongated H. campaniensis LS21 without mreB and deficient ftsZ, respectively; (g) The first 5000L plastic fermentor used to grow Halomonas spp. under open unsterile and continuous conditions; (h) Production of ALA in H. bluephagenesis TD LTT22Genes: phaC—encoding PHA synthase; phaA—encoding β‐ketothiolase; phaB—encoding NADPH dependent acetoacetyl‐CoA reductase; prpC—encoding 2‐methylcitrate synthase; orfZ—encoding 2‐methylcitrate synthase; mreB—encoding cytoskeleton protein; ftsZ—encoding cell division protein; △ftsZ::ftsZ‐gfp—a gfp gene was inserted into the C‐terminal of ftsZ to destroy its function; hem1—encoding 5‐amino‐levulinic acid synthase
| 传统工业生物技术 | 下一代工业生物技术 | 实现途径 |
|---|---|---|
| 大量消耗淡水 | 对淡水依赖较低 | 海水或者循环用水 |
| 高能耗 | 低能耗 | 无灭菌开放式发酵、提高氧气利用率 |
| 易染菌 | 难染菌 | 筛选极端微生物 |
| 高成本投资 | 低成本投资 | 低成本设备 |
| 微生物生长条件苛刻 | 微生物生长条件灵活 | 筛选极端微生物 |
| 分批发酵 | 连续发酵 | 选择不易染菌的底盘菌 |
| 细胞不易分离 | 分离难度小 | 通过形态学工程改变细胞尺寸 |
| 发酵周期长 | 发酵周期短 | 利用合成生物学加快细胞生长 |
| 一个菌种对应一种产品 | 多个产品的平台菌 | 在平台菌中构建多条代谢途径 |
| 产物在胞内或者培养基中 | 实现胞内和胞外产品共产 | 工程化获得多种产品的共产 |
| 单一碳源培养生长 | 混合碳源或者混合废料生长 | 筛选或构建消耗混合碳源的菌种 |
| 自动化程度低 | 自动化控制智能化 | 菌种适应多种生长环境 |
| 底物有效转化率低 | 底物有效转化率高 | 敲除或者减弱代谢旁路 |
| 获得胞内产物难度大 | 减弱细胞壁 | 工程化细胞壁合成机制 |
| 胞外产物产量低 | 胞外产物产量高 | 减弱外膜结构 |
表1 下一代工业生物技术与当前工业生物技术对比[9]
Tab. 1 Next generation industrial biotechnology (NGIB) compared with current industrial biotechnology
| 传统工业生物技术 | 下一代工业生物技术 | 实现途径 |
|---|---|---|
| 大量消耗淡水 | 对淡水依赖较低 | 海水或者循环用水 |
| 高能耗 | 低能耗 | 无灭菌开放式发酵、提高氧气利用率 |
| 易染菌 | 难染菌 | 筛选极端微生物 |
| 高成本投资 | 低成本投资 | 低成本设备 |
| 微生物生长条件苛刻 | 微生物生长条件灵活 | 筛选极端微生物 |
| 分批发酵 | 连续发酵 | 选择不易染菌的底盘菌 |
| 细胞不易分离 | 分离难度小 | 通过形态学工程改变细胞尺寸 |
| 发酵周期长 | 发酵周期短 | 利用合成生物学加快细胞生长 |
| 一个菌种对应一种产品 | 多个产品的平台菌 | 在平台菌中构建多条代谢途径 |
| 产物在胞内或者培养基中 | 实现胞内和胞外产品共产 | 工程化获得多种产品的共产 |
| 单一碳源培养生长 | 混合碳源或者混合废料生长 | 筛选或构建消耗混合碳源的菌种 |
| 自动化程度低 | 自动化控制智能化 | 菌种适应多种生长环境 |
| 底物有效转化率低 | 底物有效转化率高 | 敲除或者减弱代谢旁路 |
| 获得胞内产物难度大 | 减弱细胞壁 | 工程化细胞壁合成机制 |
| 胞外产物产量低 | 胞外产物产量高 | 减弱外膜结构 |
| 42 | LI Teng, CHEN Xiangbin, CHEN Jinchun, et al. Open and continuous fermentation: products, conditions and bioprocess economy [J]. Biotechnology Journal, 2014, 9(12): 1503-1511. |
| 43 | LING Chen, QIAO Guanqing, SHUAI Bowen, et al. Engineering self-flocculating Halomonas campaniensis for wastewaterless open and continuous fermentation [J]. Biotechnology and Bioengineering, 2018, 116: 805-815. |
| 44 | 邓姗, 任元元, 周远洁. 浅谈工业化发酵产品成本工艺控制关键[J]. 食品与发酵科技, 2019, 55(2): 78-80. |
| DENG Shan, REN Yuanyuan, ZHOU Yuanjie. Key points of cost control in industrial fermentation products [J]. Food and Fermentation Science & Technology, 2019, 55(2): 78-80. | |
| 45 | TAN Dan, WU Qiong, CHEN Jinchun, et al. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates [J]. Metabolic Engineering, 2014, 26: 34-47. |
| 46 | SABAPATHY P C, DEVARAJ S, PARTHIPAN A, et al. Polyhydroxyalkanoate production from statistically optimized media using rice mill effluent as sustainable substrate with an analysis on the biopolymer's degradation potential [J]. International Journal of Biological Macromolecules, 2019, 126: 977-986. |
| 47 | AL-SHORGANI N K N, AL-TABIB A I, KADIER A, et al. Continuous butanol fermentation of dilute acid-pretreated de-oiled rice bran by Clostridium acetobutylicum YM1 [J]. Scientific Reports, 2019, 9(1): 4622. |
| 48 | FU X Z, TAN D, AIBAIDULA G, et al. Development of Halomonas TD01 as a host for open production of chemicals [J]. Metabolic Engineering, 2014, 23: 78-91. |
| 49 | YIN Jin, FU Xiaozhi, WU Qiong, et al. Development of an enhanced chromosomal expression system based on porin synthesis operon for halophile Halomonas sp. [J]. Applied Microbiology and Biotechnology, 2014, 98(21): 8987-8997. |
| 50 | LI Tingting, LI Teng, JI Weiyue, et al. Engineering of core promoter regions enables the construction of constitutive and inducible promoters in Halomonas sp. [J]. Biotechnology Journal, 2016, 11(2): 219-227. |
| 51 | SHEN Rui, YIN Jin, YE Jianwen, et al. Promoter engineering for enhanced P(3HB-co-4HB) production by Halomonas bluephagenesis [J]. ACS Synthetic Biology, 2018, 7(8): 1897-1906. |
| 52 | TAO Wei, LV Li, CHEN Guoqiang. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi [J]. Microbial Cell Factories, 2017, 16(1): 48. |
| 53 | ZHAO Han, ZHANG Haoqian M, CHEN Xiangbin, et al. Novel T7-like expression systems used for Halomonas [J]. Metabolic Engineering, 2017, 39: 128-140. |
| 54 | QIN Qin, LING Chen, ZHAO Yiqing, et al. CRISPR/Cas9 editing genome of extremophile Halomonas spp. [J]. Metabolic Engineering, 2018, 47: 219-229. |
| 55 | LING Chen, QIAO Guanqing, SHUAI Bowen, et al. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA) [J]. Metabolic Engineering, 2018, 49: 275-286. |
| 56 | CHEN Xiangbin, YIN Jin, YE Jianwen, et al. Engineering Halomonas bluephagenesis TD01 for non-sterile production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [J]. Bioresource Technology, 2017, 244: 534-541. |
| 57 | PENA C, CASTILLO T, GARCIA A, et al. Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): a review of recent research work [J]. Microbial Biotechnology, 2014, 7(4): 278-293. |
| 58 | RIVERA-BRISO A L, SERRANO-AROCA A. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): enhancement strategies for advanced applications [J]. Polymers (Basel), 2018, 10(7): 732. |
| 59 | JIANG Xiaoran, YAO Zhihao, CHEN Guoqiang. Controlling cell volume for efficient PHB production by Halomonas [J]. Metabolic Engineering, 2017, 44: 30-37. |
| 60 | 车雪梅, 司徒卫, 余柳松, 等. 聚羟基脂肪酸酯的应用展望[J]. 生物工程学报, 2018, 34(10): 1531-1542. |
| CHE Xuemei, SITU Wei, YU Liusong, et al. Application perspectives of polyhydroxyalkanoates [J]. Chinese Journal of Biotechnology, 2018, 34(10): 1531-1542. | |
| 61 | HAJNAL I, CHEN X B, CHEN G Q. A novel cell autolysis system for cost-competitive downstream processing [J]. Applied Microbiology and Biotechnology, 2016, 100(21): 9103-9110. |
| 62 | YE Jianwen, HUANG Wuzhe, WANG Dongsheng, et al. Pilot scale-up of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) production by Halomonas bluephagenesis via cell growth adapted optimization process [J]. Biotechnology Journal, 2018, 13(5): e1800074. |
| 63 | ZHANG C R, BOOP F A, RUGE J. The use of 5-aminolevulinic acid in resection of pediatric brain tumors: a critical review [J]. Journal of Neuro-Oncology, 2019, 141(3): 567-573. |
| 1 | 李雪静. 新形势下炼油工业发展新动向及新挑战[J]. 石化技术与应用, 2019, 37(4): 225-229. |
| LI Xuejing. New trends and challenges of refining industry in the new situation [J]. Petrochemical Technology & Application, 2019, 37(4): 225-229. | |
| 2 | 余春浩. 石油泄漏对土壤的污染[J]. 当代化工, 2019, 48(10): 2385-2387. |
| YU Chunhao. Review of soil pollution in petrochemical industry [J]. Contemporary Chemical Industry, 2019, 48(10): 2385-2387. | |
| 3 | VENKATESH A, POSEN I D, MACLEAN H L, et al. Environmental aspects of biotechnology [J]. Advances in Biochemical Engineering Biotechnology, 2020, 173: 77-119. |
| 4 | OTERO J M, NIELSEN J. Industrial systems biology [J]. Biotechnology and Bioengineering, 2010, 105(3): 439-460. |
| 5 | YU Linping, WU Fuqing, CHEN Guoqiang. Next-generation industrial biotechnology - transforming the current industrial biotechnology into competitive processes [J]. Biotechnology Journal, 2019, 14(9): e1800437. |
| 6 | CHEN Zhu, WAN Caixia. Non-sterile fermentations for the economical biochemical conversion of renewable feedstocks [J]. Biotechnology Letters, 2017, 39(12): 1765-1777. |
| 7 | PHILP J C, RITCHIE R J, ALLAN J E. Biobased chemicals: the convergence of green chemistry with industrial biotechnology [J]. Trends in Biotechnology, 2013, 31(4): 219-222. |
| 8 | RADDADI N, CHERIF A, DAFFONCHIO D, et al. Biotechnological applications of extremophiles, extremozymes and extremolytes [J]. Applied Microbiology and Biotechnology, 2015, 99(19): 7907-7913. |
| 9 | CHEN Guoqiang, JIANG Xiaoran. Next generation industrial biotechnology based on extremophilic bacteria [J]. Current Opinion in Biotechnology, 2018, 50: 94-100. |
| 10 | YIN Jin, CHEN Jinchun, WU Qiong, et al. Halophiles, coming stars for industrial biotechnology [J]. Biotechnology Advances, 2015, 33(7): 1433-1442. |
| 64 | WAINWRIGHT J V, ENDO T, COOPER J B, et al. The role of 5-aminolevulinic acid in spinal tumor surgery: a review [J]. Journal of Neuro-Oncology, 2019, 141(3): 575-584. |
| 65 | KANG Zhen, DING Wenwen, GONG Xu, et al. Recent advances in production of 5-aminolevulinic acid using biological strategies [J]. World Journal of Microbiology and Biotechnology, 2017, 33(11): 200. |
| 66 | BISSON A W, YEN-PANG HSU, SQUYRES G R, et al. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division [J]. Science, 2017, 355(6326): 739-743. |
| 67 | HUSSAIN S, WIVAGG C N, SZWEDZIAK P, et al. MreB filaments align along greatest principal membrane curvature to orient cell wall synthesis [J]. eLife, 2018, 7: e32471. |
| 68 | SHI H D, BRATTON B P, GITAI Z, et al. How to build a bacterial cell: MreB as the foreman of E. coli construction [J]. Cell, 2018, 172(6): 1294-1305. |
| 69 | LIU Yunqi, YIN Shengli, WANG Yujie, et al. Effect of high porosity on biodegradation of poly (4-hydroxybutyrate) in vivo [J]. Journal of Biomaterials Applications, 2014, 28(7): 1105-1112. |
| 70 | JIANG Xiaoran, WANG Huan, SHEN Rui, et al. Engineering the bacterial shapes for enhanced inclusion bodies accumulation [J]. Metabolic Engineering, 2015, 29: 227-237. |
| 71 | ELHADI D, LV L, JIANG X R, et al. CRISPRi engineering E. coli for morphology diversification [J]. Metabolic Engineering, 2016, 38: 358-369. |
| 72 | SHEN Rui, NING Zhiyu, LAN Yuxuan, et al. Manipulation of polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis [J]. Metabolic Engineering, 2019, 54: 117-126. |
| 73 | ZHANG Xu, LIN Yina, CHEN Guoqiang. Halophiles as chassis for bioproduction [J]. Advanced Biosystems, 2018, 2: 1-12. |
| 74 | 陈则立,李彤,朱华静. 阳离子交换膜在高盐废水处理中的应用[J]. 膜科学与技术, 2019, 39(5): 136-142. |
| CHEN Zeli, LI Tong, ZHU Huajing. Application of cation exchange membrane in the treatment of high-salt wastewater [J]. Membrane Science and Technology, 2019, 39(5): 136-142. | |
| 11 | CAI Lei, TAN Dan, AIBAIDULA G, et al. Comparative genomics study of polyhydroxyalkanoates (PHA) and ectoine relevant genes from Halomonas sp. TD01 revealed extensive horizontal gene transfer events and co-evolutionary relationships [J]. Microbial Cell Factories, 2011, 10: 88. |
| 12 | KAWATA Y, KAWASAKI K, SHIGERI Y. Draft genome sequence of Halomonas sp. strain KM-1, a moderately halophilic bacterium that produces the bioplastic poly(3-hydroxybutyrate) [J]. Journal of Bacteriology, 2012, 194(10): 2738-2739. |
| 13 | LIN Yanbing, FAN Haoxin, HAO Xiuli, et al. Draft genome sequence of Halomonas sp. strain HAL1, a moderately halophilic arsenite-oxidizing bacterium isolated from gold-mine soil [J]. Journal of Bacteriology, 2011, 194(1): 199-200. |
| 14 | PHUNG LE T, SILVER S, TRIMBLE W L, et al. Draft genome of Halomonas species strain GFAJ-1 (ATCC BAA-2256) [J]. Journal of Bacteriology, 2012, 194(7): 1835-1836. |
| 15 | SCHWIBBERT K, MARIN-SANGUINO A, BAGYAN I, et al. A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581T [J]. Environmental Microbiology, 2011, 13(8): 1973-1994. |
| 16 | ZHENG Yang, CHEN Jinchun, MA Yiming, et al. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction [J]. Metabolic Engineering, 2020, 58: 82-93. |
| 17 | CHEN Yong, CHEN Xinyu, DU Hetong, et al. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV) [J]. Metabolic Engineering, 2019, 54: 69-82. |
| 18 | YE Jianwen, HU Dingkai, CHE Xuemei, et al. Engineering of Halomonas bluephagenesis for low cost production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose [J]. Metabolic Engineering, 2018, 47: 143-152. |
| 19 | LAN Luhong, ZHAO Han, CHEN Jinchun, et al. Engineering Halomonas spp. as a low-cost production host for production of bio-surfactant protein PhaP [J]. Biotechnology Journal, 2016, 11(12): 1595-1604. |
| 20 | LI Tian, GUO Yingying, QIAO Guanqing, et al. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate [J]. ACS Synthetic Biology, 2016, 5(11): 1264-1274. |
| 21 | 杨博, 南昊. 我国水资源现状及其安全对策研究[J]. 太原学院学报(自然科学版), 2016, 34(1): 9-12. |
| YANG Bo, Hao NAN. Research on the present situation of China's water resources and its security countermeasures [J]. Journal of Taiyuan University (Natural Science Edition), 2016, 34(1): 9-12. | |
| 22 | 姜祖岩. 我国海水利用产业发展形势与存在问题分析[J]. 海洋经济, 2019, 9(1): 20-28. |
| JIANG Zuyan. Analysis on the development situation and existing problems of China's seawater utilization industry [J]. Marine Economy, 2019, 9(1): 20-28. | |
| 23 | CHEN Guoqiang. New challenges and opportunities for industrial biotechnology [J]. Microbial Cell Factories, 2012, 11: 111. |
| 24 | JIANG Xiaoran, YIN Jin, CHEN Xiangbin, et al. Halomonas and pathway engineering for bioplastics production [J]. Methods in Enzymology, 2018, 608: 309-328. |
| 25 | TOHME S, HACIOSMANOGLU G G, EROGLU M S, et al. Halomonas smyrnensis as a cell factory for co-production of PHB and levan [J]. International Journal of Biological Macromolecules, 2018, 118(A): 1238-1246. |
| 26 | JOULAK I, FINORE I, NICOLAUS B, et al. Evaluation of the production of exopolysaccharides by newly isolated Halomonas strains from Tunisian hypersaline environments [J]. International Journal of Biological Macromolecules, 2019, 138: 658-666. |
| 27 | ZHAO Qi, LI Shannan, Peiwen LÜ, et al. High ectoine production by an engineered Halomonas hydrothermalis Y2 in a reduced salinity medium [J]. Microbial Cell Factories, 2019, 18(1): 184. |
| 28 | ADIMOOLAM S R, NANJAN E S, SUBRAMANIAN M, et al. Metabolic heat coherent growth of Halomonas variabilis (HV) for enhanced production of extracellular polymeric substances (EPS) in a bio reaction calorimeter (BioRC) [J]. Preparative Biochemistry & Biotechnology, 2020, 50(1): 56-65. |
| 29 | TAN D, XUE Y S, AIBAIDULA G, et al. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01 [J]. Bioresource Technology, 2011, 102(17): 8130-8136. |
| 30 | YUE Haitao, LING Chen, YANG Tao, et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates [J]. Biotechnology for Biofuels, 2014, 7: 108. |
| 31 | KREYENSCHULTE D, KRULL R, MARGARITIS A. Recent advances in microbial biopolymer production and purification [J]. Critical Reviews in Biotechnology, 2014, 34: 1-15. |
| 32 | DON T M, CHEN C W, CHAN T H. Preparation and characterization of poly(hydroxyalkanoate) from the fermentation of Haloferax mediterranei [J]. Journal of Biomaterials Science, Polymer Edition, 2006, 1425-1438. |
| 33 | JOHNSON K, JIANG Yang, KLEEREBEZEM R, et al. Enrichment of a mixed bacterial culture with a high polyhydroxyalkanoate storage capacity [J]. Biomacromolecules, 2009, 10(4): 670-676. |
| 75 | 曹美玲, 李海, 刘佛财, 等. 高盐有机废水的处理与研究进展[J]. 有色金属科学与工程, 2019, 10(3): 92-98. |
| CAO Meiling, LI Hai, LIU Focai, et al. Recent evelopment in the treatment process for high salt organic wastewater [J]. Nonfenous Metals Science and Engineering, 2019, 10(3): 92-98. | |
| 76 | 李俊虎, 周珉, 王乔, 等. 高盐废水处理工艺最新研究进展[J]. 环境科技, 2018, 31(4): 74. |
| LI Junhu, ZHOU Min, WANG Qiao, et al. The latest progress of treatment technology for high salinity wastewater [J]. Environmental Science and Technology, 2018, 31(4): 74. | |
| 34 | CARCAMO M, SAA P A, TORRES J, et al. Effective dissolved oxygen control strategy for high-cell-density cultures [J]. IEEE Latin America Transactions, 2014, 12(3): 389-394. |
| 35 | XUE Sijia, JIANG Hong, CHEN Lu, et al. Over-expression of Vitreoscilla hemoglobin (VHb) and flavohemoglobin (FHb) genes greatly enhances pullulan production [J]. International Journal of Biological Macromolecules, 2019, 132: 701-709. |
| 36 | MEJIA A, LUNA D, FERNANDEZ F J, et al. Improving rifamycin production in Amycolatopsis mediterranei by expressing a Vitreoscilla hemoglobin (vhb) gene fused to a cytochrome P450 monooxygenase domain [J]. 3 Biotechnology, 2018, 8(11): 456. |
| 37 | ZHANG Shumeng, WANG Jieping, WEI Yale, et al. Heterologous expression of VHb can improve the yield and quality of biocontrol fungus Paecilomyces lilacinus, during submerged fermentation [J]. Journal of Biotechnology, 2014, 187: 147-153. |
| 38 | OUYANG Pengfei, WANG Huan, HAJNAL I, et al. Increasing oxygen availability for improving poly(3-hydroxybutyrate) production by Halomonas [J]. Metabolic Engineering, 2018, 45: 20-31. |
| 39 | WU Hong, WANG Huan, CHEN Jinchun, et al. Effects of cascaded vgb promoters on poly(hydroxybutyrate) (PHB) synthesis by recombinant Escherichia coli grown micro-aerobically [J]. Applied Microbiology and Biotechnology, 2014, 98(24): 10013-10021. |
| 40 | 朱晁谊, 朱牧孜, 李爽. 微生物实验室进化的研究进展[J]. 生物加工过程, 2019, 17(1): 8-14. |
| ZHU Chaoyi, ZHU Muzi, LI Shuang. Research progress in microbial laboratory evolution [J]. Chinese Journal of Bioprocess Engineering, 2019, 17(1): 8-14. | |
| 41 | 贝泓涵, 张立卫, 孙菁. 微生物连续发酵过程线性反馈最优控制[J]. 大连理工大学学报, 2018, 58(3): 324-330. |
| BEI Honghan, ZHANG Liwei, SUN Jing. Linear optimal feedback control in continuous culture of microbial fermentation [J]. Journal of Dalian University of Technology, 2018, 58(3): 324-330. |
| [1] | 应汉杰, 柳东, 王振宇, 沈涛, 庄伟, 朱晨杰. 工业生物制造与“碳中和”目标探讨[J]. 合成生物学, 2025, 6(1): 1-7. |
| [2] | 张以恒, 陈雪梅, 石婷. 生物制造的市本率(PC值):定义与应用[J]. 合成生物学, 2025, 6(1): 8-17. |
| [3] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [4] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [5] | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6): 1231-1241. |
| [6] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [7] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [8] | 刘建明, 张炽坚, 张冰, 曾安平. 巴氏梭菌作为工业底盘细胞高效生产1,3-丙二醇——从代谢工程和菌种进化到过程工程和产品分离[J]. 合成生物学, 2024, 5(6): 1386-1403. |
| [9] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [10] | 陈国强, 谭丹. 重编程微生物底盘用于PHA材料的定制化低成本生物合成[J]. 合成生物学, 2024, 5(5): 1211-1226. |
| [11] | 张晨悦, 马英群, 王兴, 傅容湛, 黄技伟, 花秀夫, 范代娣, 费强. 全碳素生物转化沼气制备生物航煤制造路线研究进展[J]. 合成生物学, 2023, 4(6): 1246-1258. |
| [12] | 孙绘梨, 崔金玉, 栾国栋, 吕雪峰. 面向高效光驱固碳产醇的蓝细菌合成生物技术研究进展[J]. 合成生物学, 2023, 4(6): 1161-1177. |
| [13] | 孙美莉, 王凯峰, 陆然, 纪晓俊. 解脂耶氏酵母底盘细胞的工程改造及应用[J]. 合成生物学, 2023, 4(4): 779-807. |
| [14] | 张璨, 施李杨, 戴建武. 细胞培养肉用生物材料的设计[J]. 合成生物学, 2022, 3(4): 676-689. |
| [15] | 王倩, 祁庆生. 聚羟基脂肪酸酯的低碳生物制造:基于碳转化率的分析与应用[J]. 合成生物学, 2022, 3(4): 748-762. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||