合成生物学 ›› 2020, Vol. 1 ›› Issue (6): 656-673.DOI: 10.12211/2096-8208.2020-050
微生物细胞工厂的设计构建:从诱变育种到全基因组定制化创制
袁姚梦1, 邢新会1,2, 张翀1
- 1.工业生物催化教育部重点实验室,清华大学化工系生物化工研究所,清华大学合成与系统生物学研究中心,北京 100084
2.清华大学深圳国际研究生院生物医药与健康工程研究院,广东 深圳 518055
-
收稿日期:2020-04-16修回日期:2020-09-26出版日期:2020-12-31发布日期:2021-01-15 -
通讯作者:张翀 -
作者简介:袁姚梦(1997—),女,博士研究生。主要研究方向为合成生物学、代谢工程。E-mail:2631825401@qq.com
张翀(1979—),男,博士,副教授,博士生导师。主要研究方向为合成生物学、代谢工程、生物化工、系统生物学。E-mail:chongzhang@mail.tsinghua.edu.cn -
基金资助:国家自然科学基金重点项目(2193000018);国家重点研发计划(2019YFA0904802)
Progress and prospective of engineering microbial cell factories: from random mutagenesis to customized design in genome scale
YUAN Yaomeng1, XING Xinhui1,2, ZHANG Chong1
- 1.MOE Key Laboratory for Industrial Biocatalysis,Institute of Biochemical Engineering,Department of Chemical Engineering,Center for Synthetic & Systems Biology,Tsinghua University,Beijing 100084,China
2.Institute of Biopharmaceutical and Health Engineering,Tsinghua Shenzhen International Graduate School,Shenzhen 518055,Guangdong,China
-
Received:2020-04-16Revised:2020-09-26Online:2020-12-31Published:2021-01-15 -
Contact:ZHANG Chong
摘要:
微生物细胞工厂(microbial cell factories,MCFs)被广泛用于生产丰富多样的化学品、食品、药品和能源,是绿色生物制造的核心环节。早期主要通过天然微生物的筛选和诱变育种的方式获得高产菌种,然而作为一种“以时间(人力)换水平”的非理性策略,其创制效率极低。随着分子生物学和基因工程研究方法的不断发展,对微生物系统认知和改造能力的进步促使代谢工程学科诞生。基于生物学知识的理性/半理性代谢工程设计和构建策略,目前已发展了从分子、途径到基因组层次不同的MCFs设计和工程化构建策略。本文结合实际案例对MCFs的设计及构建策略进行综述,首先回顾传统诱变育种和代谢工程指导的理性/半理性设计策略,探讨如何突破代谢工程经典框架的限制,实现全基因组水平定制化MCFs的快速构建,最后对这一新的构建范式的未来进行展望。
中图分类号:
引用本文
袁姚梦, 邢新会, 张翀. 微生物细胞工厂的设计构建:从诱变育种到全基因组定制化创制[J]. 合成生物学, 2020, 1(6): 656-673.
YUAN Yaomeng, XING Xinhui, ZHANG Chong. Progress and prospective of engineering microbial cell factories: from random mutagenesis to customized design in genome scale[J]. Synthetic Biology Journal, 2020, 1(6): 656-673.
| 分类 | 诱变技术/诱变剂 | 描述 | 参考文献 |
|---|---|---|---|
| 物理诱变 | 电离辐射(X射线、γ射线等) | 引起DNA双链或单链断裂,实现DNA的删除或结构改变 | [ |
| 非电离辐射(紫外线) | 使嘧啶形成二聚体,实现GC的删除、移码突变以及GC→AT的转换 | [ | |
| 化学诱变 | 烷化剂(烷基磺酸盐、芥子气等) | 使DNA碱基发生烷化,导致DNA复制时发生配对错误 | [ |
碱基类似物 (嘧啶类似物、嘌呤类似物) | 与DNA碱基结构类似,在DNA复制时掺入并引发配对错误 | [ | |
| 移码诱变剂(原黄素、吖啶橙等) | 与DNA结合导致碱基增添或缺失 | [ | |
| 脱氨剂(亚硝酸) | 引起A、C、G碱基的脱氨,实现GC与AT的相互转换;引起DNA交联作用,引发突变 | [ | |
| 羟化剂(羟胺) | 引起胞嘧啶脱氨,实现GC→AT的转换 | [ | |
| 生物诱变 | 噬菌体、质粒和DNA转座子 | 通过碱基取代和DNA链断裂实现碱基的删除、重复和插入 | [ |
| 原生质体融合 | 将两个亲株的原生质体进行融合,形成杂合二倍体,使两个亲株发生基因组重组 | [ | |
| DNA改组 | 对多个同源序列组成的文库进行随机片段化,再利用PCR使其发生随机的重组,实现多亲本的基因重组 | [ | |
| 基因组重排 | 先利用传统诱变手段获得多个表型改进的菌株,然后模拟DNA改组的反应条件,对原生质体进行多次递推式融合,实现正向突变的富集 | [ | |
| 复合诱变 | 结合多种诱变方式提高诱变效率 | [ | |
| 新型诱变技术 | 离子注入诱变 | 离子注入细胞导致DNA损伤,细胞修复损伤的过程中出现突变 | [ |
| 等离子体诱变 | 等离子体作用于细胞造成DNA损伤,细胞修复损伤的过程中出现突变 | [ |
表1 常见物理、化学、生物诱变技术汇总
Tab. 1 Summary of mutagenesis technologies
| 分类 | 诱变技术/诱变剂 | 描述 | 参考文献 |
|---|---|---|---|
| 物理诱变 | 电离辐射(X射线、γ射线等) | 引起DNA双链或单链断裂,实现DNA的删除或结构改变 | [ |
| 非电离辐射(紫外线) | 使嘧啶形成二聚体,实现GC的删除、移码突变以及GC→AT的转换 | [ | |
| 化学诱变 | 烷化剂(烷基磺酸盐、芥子气等) | 使DNA碱基发生烷化,导致DNA复制时发生配对错误 | [ |
碱基类似物 (嘧啶类似物、嘌呤类似物) | 与DNA碱基结构类似,在DNA复制时掺入并引发配对错误 | [ | |
| 移码诱变剂(原黄素、吖啶橙等) | 与DNA结合导致碱基增添或缺失 | [ | |
| 脱氨剂(亚硝酸) | 引起A、C、G碱基的脱氨,实现GC与AT的相互转换;引起DNA交联作用,引发突变 | [ | |
| 羟化剂(羟胺) | 引起胞嘧啶脱氨,实现GC→AT的转换 | [ | |
| 生物诱变 | 噬菌体、质粒和DNA转座子 | 通过碱基取代和DNA链断裂实现碱基的删除、重复和插入 | [ |
| 原生质体融合 | 将两个亲株的原生质体进行融合,形成杂合二倍体,使两个亲株发生基因组重组 | [ | |
| DNA改组 | 对多个同源序列组成的文库进行随机片段化,再利用PCR使其发生随机的重组,实现多亲本的基因重组 | [ | |
| 基因组重排 | 先利用传统诱变手段获得多个表型改进的菌株,然后模拟DNA改组的反应条件,对原生质体进行多次递推式融合,实现正向突变的富集 | [ | |
| 复合诱变 | 结合多种诱变方式提高诱变效率 | [ | |
| 新型诱变技术 | 离子注入诱变 | 离子注入细胞导致DNA损伤,细胞修复损伤的过程中出现突变 | [ |
| 等离子体诱变 | 等离子体作用于细胞造成DNA损伤,细胞修复损伤的过程中出现突变 | [ |
| 类别 | 分析方法 | 描述 | 参考文献 |
|---|---|---|---|
| 动力学分析 | ODE&米氏方程 | 利用常微分方程(ODE)和米氏方程,能够描述胞内代谢物浓度随时间的变化趋势,从而建立代谢网络的动力学模型 | [ |
| 代谢控制分析(MCA) | MCA可用于估算动力学模型中各个通量的控制系数,从而确定代谢途径中需要过表达的目标基因,增加通过途径的通量 | [ | |
| 代谢网络分析 | 代谢通量分析(MFA) | MFA根据胞内代谢物的质量平衡确定线性方程组并求解,能够计算特定培养条件下细胞内的实际代谢通量分布。MFA需要依赖大量实验数据来增加可测量通量的数量,从而计算出不可测量通量向量 | [ |
| 通量平衡分析(FBA) | FBA可用于确定细胞代谢网络中每个反应的最佳通量,该方法基于凸分析,通过对系统施加最大化(最小化)的目标函数来确定代谢通量矢量 | [ | |
| 代谢途径分析(MPA) | MPA可用于识别代谢网络中存在的所有代谢通量向量,该方法仅以化学计量学和反应热力学为约束条件,不需要动力学参数进行计算(例如基元模式分析) | [ | |
| 整合了热力学的代谢网络分析 | 网络嵌入的热力学分析(NET) | 以热力学第二定律为依据,可用于检测代谢组学数据和假定的通量方向是否符合热力学一致性 | [ |
| 基于热力学的代谢通量分析(TMFA) | TMFA同时使用热力学方向性约束和质量守恒约束计算代谢通量分布 | [ | |
| 计算机应变优化算法 | 基于约束的重构与分析(COBRA) | COBRA采用基因组规模的计算机模拟,可用于代谢途径预测和优化,从而改善生产速率和产量 | [ |
| 最小化代谢调节(MOMA) | MOMA用于使野生型菌株和缺失突变体之间代谢通量分布的差别最小化,能够预测基因操作对代谢网络的影响 | [ | |
| 开/关最小化调节(ROOM) | 与MOMA的优化目标相同 | [ | |
| OptKnock | 以产品产率为优化目标的基因敲除分析工具 | [ | |
| 生化网络集成计算浏览器(BNICE) | 使用广义酶反应规则发现新代谢途径的计算工具 | [ |
表2 用于代谢网络设计的分析方法
Tab. 2 Analytical methods for metabolic network design
| 类别 | 分析方法 | 描述 | 参考文献 |
|---|---|---|---|
| 动力学分析 | ODE&米氏方程 | 利用常微分方程(ODE)和米氏方程,能够描述胞内代谢物浓度随时间的变化趋势,从而建立代谢网络的动力学模型 | [ |
| 代谢控制分析(MCA) | MCA可用于估算动力学模型中各个通量的控制系数,从而确定代谢途径中需要过表达的目标基因,增加通过途径的通量 | [ | |
| 代谢网络分析 | 代谢通量分析(MFA) | MFA根据胞内代谢物的质量平衡确定线性方程组并求解,能够计算特定培养条件下细胞内的实际代谢通量分布。MFA需要依赖大量实验数据来增加可测量通量的数量,从而计算出不可测量通量向量 | [ |
| 通量平衡分析(FBA) | FBA可用于确定细胞代谢网络中每个反应的最佳通量,该方法基于凸分析,通过对系统施加最大化(最小化)的目标函数来确定代谢通量矢量 | [ | |
| 代谢途径分析(MPA) | MPA可用于识别代谢网络中存在的所有代谢通量向量,该方法仅以化学计量学和反应热力学为约束条件,不需要动力学参数进行计算(例如基元模式分析) | [ | |
| 整合了热力学的代谢网络分析 | 网络嵌入的热力学分析(NET) | 以热力学第二定律为依据,可用于检测代谢组学数据和假定的通量方向是否符合热力学一致性 | [ |
| 基于热力学的代谢通量分析(TMFA) | TMFA同时使用热力学方向性约束和质量守恒约束计算代谢通量分布 | [ | |
| 计算机应变优化算法 | 基于约束的重构与分析(COBRA) | COBRA采用基因组规模的计算机模拟,可用于代谢途径预测和优化,从而改善生产速率和产量 | [ |
| 最小化代谢调节(MOMA) | MOMA用于使野生型菌株和缺失突变体之间代谢通量分布的差别最小化,能够预测基因操作对代谢网络的影响 | [ | |
| 开/关最小化调节(ROOM) | 与MOMA的优化目标相同 | [ | |
| OptKnock | 以产品产率为优化目标的基因敲除分析工具 | [ | |
| 生化网络集成计算浏览器(BNICE) | 使用广义酶反应规则发现新代谢途径的计算工具 | [ |
| 模型范围 | 模型名称 | 菌株 | 反应/代谢物 | 参考文献 |
|---|---|---|---|---|
| 核心代谢模型 | 未报道 | 大肠杆菌 | 95/94 | [ |
| 未报道 | 谷氨酸棒杆菌 | 未报道 | [ | |
| 未报道 | 酿酒酵母 | 70/83 | [ | |
| 未报道 | 酿酒酵母 | 78/98 | [ | |
| 未报道 | 酿酒酵母 | 37/27 | [ | |
| 全基因组代谢模型(GSMM) | iFF708 | 酿酒酵母 | 1145/825 +708基因 | [ |
| iND750 | 酿酒酵母 | 1149/646 +750基因 | [ | |
| Yeast 4.0 | 酿酒酵母 | 1865/1319 +932基因 | [ | |
| iJO1366 | 大肠杆菌 | 2583/1805 +1366基因 | [ | |
| 未报道 | 谷氨酸棒杆菌 | 495/408 +411基因 | [ | |
| 未报道 | 乳酸菌 | 621/509 +358基因 | [ | |
| 未报道 | 枯草芽孢杆菌 | 754/637 +614基因 | [ | |
全基因组代谢模型 +基因表达水平 | T. maritima | 海栖热袍菌 | 17535/18209 | [ |
| iOL1650-ME | 大肠杆菌 | 76414/56902 | [ | |
全基因组代谢模型 +蛋白质结构特性 | T. maritima | 海栖热袍菌 | 645/503 +478酶结构 | [ |
| E. coli GEM-PRO | 大肠杆菌 | 2583/1805 +1268酶结构 | [ | |
全基因组代谢模型 +转录调控 | iAF1260 PROM | 大肠杆菌 | 2583/1805 +1773转录调控作用 | [ |
| iMH805/837 | 酿酒酵母 | 1489/972 +805基因+837转录调控作用 | [ | |
| 全细胞模型 | ‘Whole-cell’ M. genitalium | 生殖支原体 | 28个细胞过程子模块 +525基因 | [ |
表3 常见的微生物代谢模型汇总
Tab. 3 Summary of microbial metabolism models
| 模型范围 | 模型名称 | 菌株 | 反应/代谢物 | 参考文献 |
|---|---|---|---|---|
| 核心代谢模型 | 未报道 | 大肠杆菌 | 95/94 | [ |
| 未报道 | 谷氨酸棒杆菌 | 未报道 | [ | |
| 未报道 | 酿酒酵母 | 70/83 | [ | |
| 未报道 | 酿酒酵母 | 78/98 | [ | |
| 未报道 | 酿酒酵母 | 37/27 | [ | |
| 全基因组代谢模型(GSMM) | iFF708 | 酿酒酵母 | 1145/825 +708基因 | [ |
| iND750 | 酿酒酵母 | 1149/646 +750基因 | [ | |
| Yeast 4.0 | 酿酒酵母 | 1865/1319 +932基因 | [ | |
| iJO1366 | 大肠杆菌 | 2583/1805 +1366基因 | [ | |
| 未报道 | 谷氨酸棒杆菌 | 495/408 +411基因 | [ | |
| 未报道 | 乳酸菌 | 621/509 +358基因 | [ | |
| 未报道 | 枯草芽孢杆菌 | 754/637 +614基因 | [ | |
全基因组代谢模型 +基因表达水平 | T. maritima | 海栖热袍菌 | 17535/18209 | [ |
| iOL1650-ME | 大肠杆菌 | 76414/56902 | [ | |
全基因组代谢模型 +蛋白质结构特性 | T. maritima | 海栖热袍菌 | 645/503 +478酶结构 | [ |
| E. coli GEM-PRO | 大肠杆菌 | 2583/1805 +1268酶结构 | [ | |
全基因组代谢模型 +转录调控 | iAF1260 PROM | 大肠杆菌 | 2583/1805 +1773转录调控作用 | [ |
| iMH805/837 | 酿酒酵母 | 1489/972 +805基因+837转录调控作用 | [ | |
| 全细胞模型 | ‘Whole-cell’ M. genitalium | 生殖支原体 | 28个细胞过程子模块 +525基因 | [ |
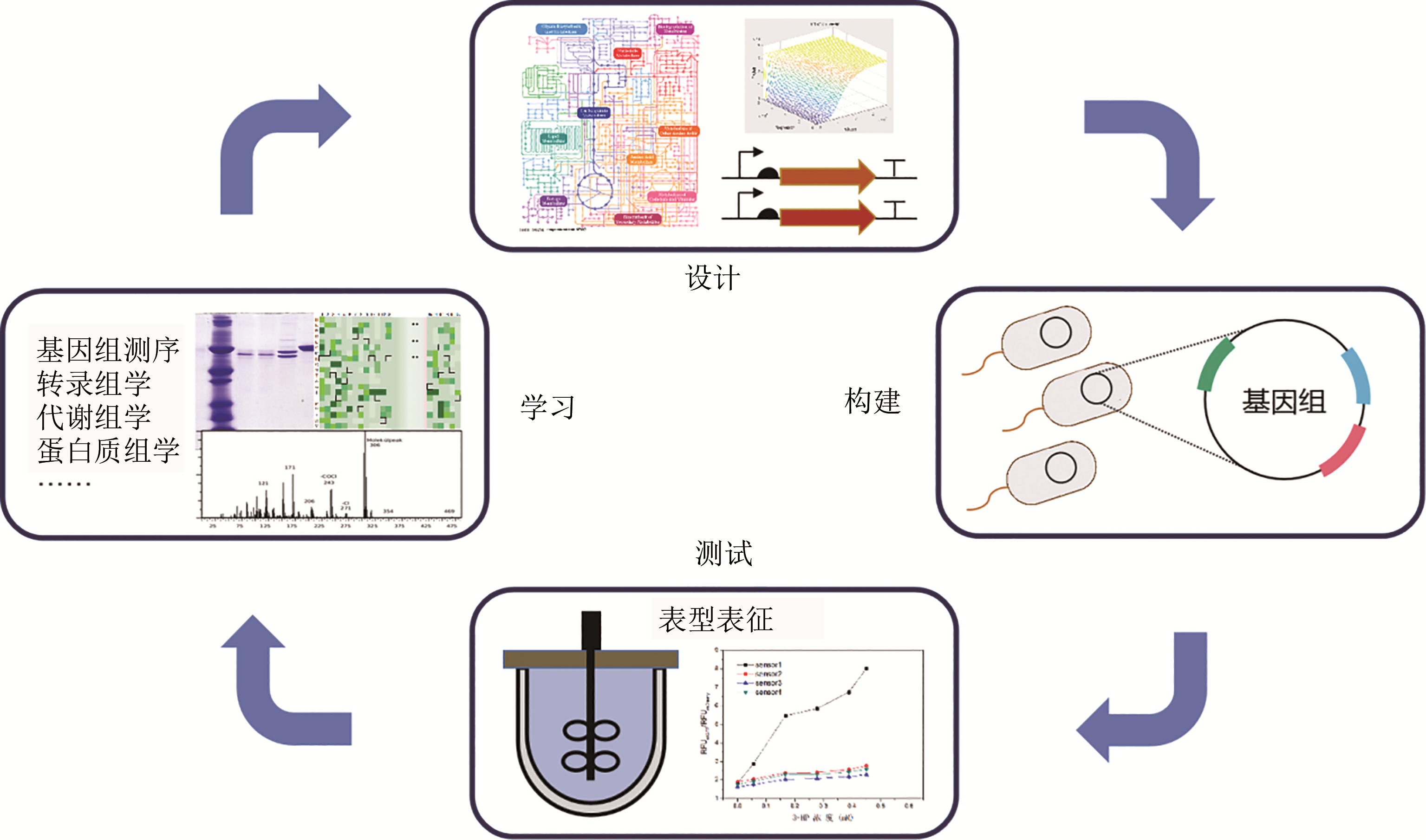
图2 菌株理性工程化的试错流程(The construction of MCFs based on systematic metabolic engineering relies on the ‘Design-Build-Test-Learn’ iterative cycle. Firstly, metabolic models are used to design the metabolic network of MCFs. Secondly, synthetic biology tools are used to build the target MCFs. Thirdly, the MCFs are characterized to evaluate performance. Finally, the results are analyzed and the metabolic model will be modified to further improve the performance)
Fig. 2 Iterative trial-and-error cycle of rational engineering of strains
| 产物 | 宿主 | 原料 | 公司 | 参考文献 |
|---|---|---|---|---|
| 琥珀酸 | 大肠杆菌 | 玉米糖 | BioAmber | [ |
大肠杆菌 克鲁斯假丝酵母 | 蔗糖 | Myriant(现名GC Innovation America) | ||
| 酿酒酵母 | 淀粉、糖类 | Reverdia | ||
| 巴斯夫产琥珀酸菌 | 甘油、糖类 | Succinity | ||
| 1,4-丁二醇 | 大肠杆菌 | 糖类 | Genomatica和DuPont Tate & Lyle | [ |
| 1,3-丙二醇 | 大肠杆菌 | 糖类 | DuPont Tate & Lyle | [ |
| 聚羟基链烷酸酯(PHA) | 大肠杆菌 | 糖类 | Metabolix(现名 Yeild10 science) | [ |
| 3-羟基丙酸 | 大肠杆菌 | 未报道 | OPXbio & Dow Chemical | [ |
| 未报道 | 未报道 | Perstorp | ||
| 乙醇 | 酿酒酵母 运动发酵单胞菌 马克斯克鲁维酵母 | 蔗糖、玉米糖、木质纤维素 | [ | |
| 异丁醇 | 酿酒酵母 | 糖类 | Gevo Butalco Butamax | [ |
| 法尼烯 | 酿酒酵母 | 未报道 | Amyris | [ |
| 青蒿素(半合成) | 酿酒酵母 | 未报道 | Amyris | [ |
表4 代谢工程指导的经典设计策略的商业化应用案例
Tab. 4 Commercial application of classic design strategies guided by metabolic engineering
| 产物 | 宿主 | 原料 | 公司 | 参考文献 |
|---|---|---|---|---|
| 琥珀酸 | 大肠杆菌 | 玉米糖 | BioAmber | [ |
大肠杆菌 克鲁斯假丝酵母 | 蔗糖 | Myriant(现名GC Innovation America) | ||
| 酿酒酵母 | 淀粉、糖类 | Reverdia | ||
| 巴斯夫产琥珀酸菌 | 甘油、糖类 | Succinity | ||
| 1,4-丁二醇 | 大肠杆菌 | 糖类 | Genomatica和DuPont Tate & Lyle | [ |
| 1,3-丙二醇 | 大肠杆菌 | 糖类 | DuPont Tate & Lyle | [ |
| 聚羟基链烷酸酯(PHA) | 大肠杆菌 | 糖类 | Metabolix(现名 Yeild10 science) | [ |
| 3-羟基丙酸 | 大肠杆菌 | 未报道 | OPXbio & Dow Chemical | [ |
| 未报道 | 未报道 | Perstorp | ||
| 乙醇 | 酿酒酵母 运动发酵单胞菌 马克斯克鲁维酵母 | 蔗糖、玉米糖、木质纤维素 | [ | |
| 异丁醇 | 酿酒酵母 | 糖类 | Gevo Butalco Butamax | [ |
| 法尼烯 | 酿酒酵母 | 未报道 | Amyris | [ |
| 青蒿素(半合成) | 酿酒酵母 | 未报道 | Amyris | [ |
| 分类 | 名称 | 描述 | 参考 文献 |
|---|---|---|---|
| 不可追踪技术 | MAGE | 基于重组的基因组编辑技术,可使用多个ssDNA同时对多个目标位点进行修饰。与其他基因编辑工具(如CRISPR/Cas9)联用可进一步提高基因编辑效率。主要用于原核基因组编辑 | [ |
| YOGE | 原理与MAGE类似,主要用于真核基因组编辑 | [ | |
| 可追踪技术 | TRMR | 基于同源重组的基因组编辑技术,能够同时对基因组上千个基因位点进行修饰 | [ |
| CREATE | 该技术基于同源重组和CRISPR/Cas9基因编辑技术,能够在全基因组范围内实现可追踪编辑 | [ | |
| Prime Editor | 使用融合了工程逆转录酶的催化活性受损的Cas9和pegRNA,以更高的效率、更低的脱靶率在全基因组范围内实现所有12种单碱基的自由转换以及多碱基的精准插入和删除 | [ | |
| Target AID | 激活诱导的胞嘧啶脱氨酶(AID)可实现C到T的突变,Target AID技术以核酸酶活性缺失的CRISPR/Cas9系统作为AID的DNA靶向模块,实现定点诱变 | [ | |
| TAM | 该系统将AID-P182X与dCas9蛋白融合,可将G或C突变为另外三个碱基,从而在目标位点处产生大量突变 | [ | |
| CRISPR-X | 使用dCas9募集更高活性的AIDΔ和MS2修饰的sgRNA变体,能以较低的脱靶率同时实现多个靶基因的特异性突变 | [ | |
| EVOLVR | 该系统由一个CRISPR引导的切口酶和一个易错DNA聚合酶组成,可在靶位点处可调窗口长度内实现所有核苷酸的突变 | [ | |
| CRISPRi | 使用dCas9蛋白及其sgRNA阻断靶基因转录,实现基因表达水平的下调 | [ | |
| CRISPRa | 使用与转录激活因子融合的dCas9蛋白实现靶基因表达水平的上调 | [ |
表5 基因组高通量编辑技术
Tab. 5 High-throughput genotype construction technologies
| 分类 | 名称 | 描述 | 参考 文献 |
|---|---|---|---|
| 不可追踪技术 | MAGE | 基于重组的基因组编辑技术,可使用多个ssDNA同时对多个目标位点进行修饰。与其他基因编辑工具(如CRISPR/Cas9)联用可进一步提高基因编辑效率。主要用于原核基因组编辑 | [ |
| YOGE | 原理与MAGE类似,主要用于真核基因组编辑 | [ | |
| 可追踪技术 | TRMR | 基于同源重组的基因组编辑技术,能够同时对基因组上千个基因位点进行修饰 | [ |
| CREATE | 该技术基于同源重组和CRISPR/Cas9基因编辑技术,能够在全基因组范围内实现可追踪编辑 | [ | |
| Prime Editor | 使用融合了工程逆转录酶的催化活性受损的Cas9和pegRNA,以更高的效率、更低的脱靶率在全基因组范围内实现所有12种单碱基的自由转换以及多碱基的精准插入和删除 | [ | |
| Target AID | 激活诱导的胞嘧啶脱氨酶(AID)可实现C到T的突变,Target AID技术以核酸酶活性缺失的CRISPR/Cas9系统作为AID的DNA靶向模块,实现定点诱变 | [ | |
| TAM | 该系统将AID-P182X与dCas9蛋白融合,可将G或C突变为另外三个碱基,从而在目标位点处产生大量突变 | [ | |
| CRISPR-X | 使用dCas9募集更高活性的AIDΔ和MS2修饰的sgRNA变体,能以较低的脱靶率同时实现多个靶基因的特异性突变 | [ | |
| EVOLVR | 该系统由一个CRISPR引导的切口酶和一个易错DNA聚合酶组成,可在靶位点处可调窗口长度内实现所有核苷酸的突变 | [ | |
| CRISPRi | 使用dCas9蛋白及其sgRNA阻断靶基因转录,实现基因表达水平的下调 | [ | |
| CRISPRa | 使用与转录激活因子融合的dCas9蛋白实现靶基因表达水平的上调 | [ |
| 技术 | 通量 | 功能 | 案例 | 参考文献 |
|---|---|---|---|---|
| FACS | 约105细胞/s | 分选 | 利用色氨酸传感器,实现紫色杆菌素高产大肠杆菌筛选 | [ |
| 利用甲羟戊酸细胞传感器,实现甲羟戊酸高产甲基杆菌筛选 | [ | |||
| 利用赖氨酸传感器,实现赖氨酸高产谷氨酸棒杆菌筛选 | [ | |||
| FADS | 约104液滴/s | 培养+分选 | 将胞外重组酶活性与液滴荧光强度耦合,实现酶活测定及胞外重组酶高产菌株筛选 | [ |
| 将传感器菌与MCF菌株共培养,实现对香豆酸高产酵母的筛选 | [ | |||
| 将β-半乳糖苷酶产量与液滴荧光强度耦合,实现β-半乳糖苷酶高产大肠杆菌筛选 | [ | |||
| Droplet-FACS | 培养+分选 | 通过检测核黄素的荧光,实现核黄素高产解脂耶氏酵母筛选 | [ | |
| Gel FACS | 约3000液滴/s | 培养+分选 | 将胞外木聚糖酶的产量与凝胶液滴的荧光强度耦合,实现胞外木聚糖酶高产毕赤酵母筛选 | [ |
| RADS | 260细胞/min | 培养+分选 | 利用虾青素的拉曼光谱,实现虾青素高产雨生红球藻的筛选 | [ |
表6 微生物代谢物高通量表征/筛选技术
Tab. 6 High-throughput selection/screening technologies in single-cell level
| 技术 | 通量 | 功能 | 案例 | 参考文献 |
|---|---|---|---|---|
| FACS | 约105细胞/s | 分选 | 利用色氨酸传感器,实现紫色杆菌素高产大肠杆菌筛选 | [ |
| 利用甲羟戊酸细胞传感器,实现甲羟戊酸高产甲基杆菌筛选 | [ | |||
| 利用赖氨酸传感器,实现赖氨酸高产谷氨酸棒杆菌筛选 | [ | |||
| FADS | 约104液滴/s | 培养+分选 | 将胞外重组酶活性与液滴荧光强度耦合,实现酶活测定及胞外重组酶高产菌株筛选 | [ |
| 将传感器菌与MCF菌株共培养,实现对香豆酸高产酵母的筛选 | [ | |||
| 将β-半乳糖苷酶产量与液滴荧光强度耦合,实现β-半乳糖苷酶高产大肠杆菌筛选 | [ | |||
| Droplet-FACS | 培养+分选 | 通过检测核黄素的荧光,实现核黄素高产解脂耶氏酵母筛选 | [ | |
| Gel FACS | 约3000液滴/s | 培养+分选 | 将胞外木聚糖酶的产量与凝胶液滴的荧光强度耦合,实现胞外木聚糖酶高产毕赤酵母筛选 | [ |
| RADS | 260细胞/min | 培养+分选 | 利用虾青素的拉曼光谱,实现虾青素高产雨生红球藻的筛选 | [ |
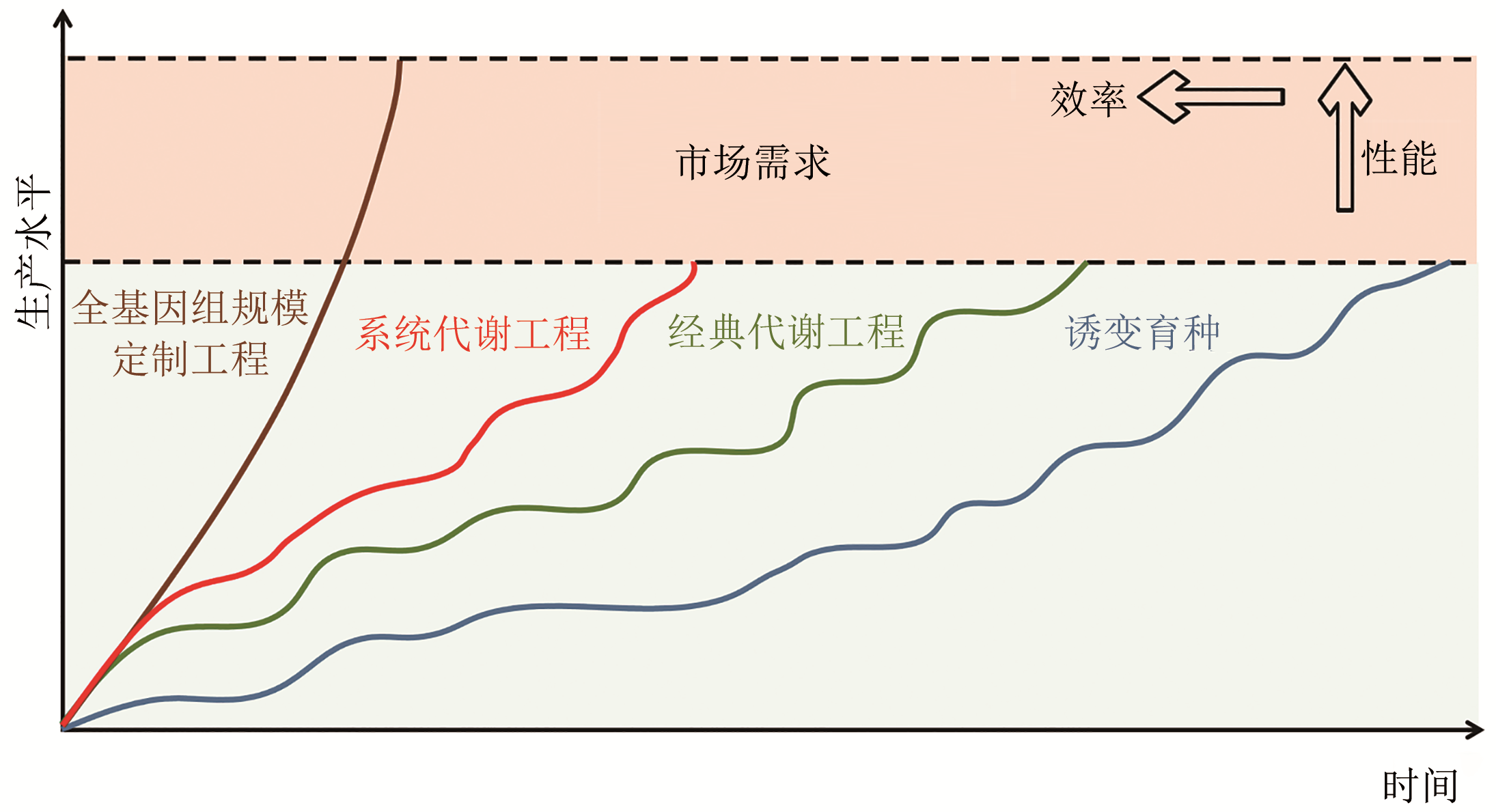
图3 微生物细胞工厂设计和构建策略效率以及性能对比(Random mutagenesis of natural microbes is firstly developed but time-consuming for MCFs contruction. Taking the DBTL cycle as basic process, metabolic engineering enables rational/semi-rational design of metabolic pathways which can accelerate the construction of MCFs. The development of systematic metabolic engineering further enhances the construction efficiency of MCFs. However, these strategies are difficult to meet the growing demand for phenotypic ‘high ground’ in industral production. With the development of high-throughput technology, data-driven genome-wide customized engineering is expected to overcome these problems)
Fig. 3 Comparison of MCFs construction efficiency and performance in different stages
| 1 | NIELSEN J. Cell factory engineering for improved production of natural products[J]. Natural Product Reports, 2019, 36(9): 1233-1236. |
| 2 | SANO C. History of glutamate production[J]. The American Journal of Clinical Nutrition, 2009, 90(3), 728S-732S. |
| 3 | OTERO J M, NIELSEN J. Industrial systems biology[J]. Biotechnology and Bioengineering, 2010, 105(3), 439-460. |
| 4 | Presidential green chemistry challenge awards[EB/OL]. . |
| 5 | ATSUMI S, HIGASHIDE W, LIAO J C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde[J]. Nature Biotechnology, 2009, 27(12), 1177-1180. |
| 6 | GALANIE S, THODEY K, TRENCHARD I J, et al. Complete biosynthesis of opioids in yeast[J]. Science, 2015, 349(6252): 1095-1100. |
| 7 | PARK J H, LEE K H, KIM T Y, et al. Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(19): 7797-7802. |
| 8 | XIA X X, QIAN Z G, KI C S, et al. Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(32):14059-14063. |
| 9 | STEEN E J, KANG Y, BOKINSKY G, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass[J]. Nature, 2010, 463(7280): 559-562. |
| 10 | LEE G, NA J. Future of microbial polyesters[J]. Microbial Cell Factories, 2013, 12(1): 54. |
| 11 | BROWN S W, OLIVER S G. Isolation of ethanol-tolerant mutants of yeast by continuous selection[J]. European Journal of Applied Microbiology and Biotechnology, 1982, 16(2/3): 119-122. |
| 12 | MEADOWS L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production[J]. Nature, 2016, 537(7622): 694-697. |
| 13 | WEIKERT C, SAUER U, BAILEY J. Use of a glycerol-limited, long-term chemostat for isolation of Escherichia coli mutants with improved physiological properties[J]. Microbiology, 1997, 143(5): 1567-1574. |
| 14 | SILMAN N J, CARVER M A, JONES C W. Physiology of amidase production by methylophilus methylotrophus: isolation of hyperactive strains using continuous culture[J]. Microbiology, 1989, 135(11): 3153-3164. |
| 15 | CORNISH A, GREENWOOD J A, JONES C W. Binding-protein-dependent sugar transport by Agrobacterium radiobacter and A. tumefaciens grown in continuous culture[J]. Microbiology, 1989, 135(11): 3001-3013. |
| 16 | BAILEY J. Toward a science of metabolic engineering[J]. Science, 1991, 252(5013): 1668-1675. |
| 17 | STEPHANOPOULOS G, VALLINO J. Network rigidity and metabolic engineering in metabolite overproduction[J]. Science, 1991, 252(5013): 1675-1681. |
| 18 | STEPHANOPOULOS G, SINSKEY A J. Metabolic engineering-methodologies and future prospects[J]. Trends in Biotechnology, 1993, 11(9): 392-396. |
| 19 | FURUSAWA C, HORINOUCHI T, HIRSAWA T, et al. Systems metabolic engineering: the creation of microbial cell factories by rational metabolic design and evolution[J]. Advances in Biochemical Engineering/Biotechnology, 2012, 131(3): 1-23. |
| 20 | NIELSEN J. Production of bio-pharmaceutical proteins by yeast: advances through metabolic engineering[J]. Bioengineered, 2012, 4(4): 207-211. |
| 21 | HONG K K, NIELSEN J. Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries[J]. Cellular and Molecular Life Sciences, 2012, 69(16),2671-2690. |
| 22 | CHEN Z, RAPPERT S, SUN J, et al. Integrating molecular dynamics and co-evolutionary analysis for reliable target prediction and deregulation of the allosteric inhibition of aspartokinase for amino acid production[J]. Journal of Biotechnology, 2011, 154(4): 248-254. |
| 23 | CHO B K, FEDEROWICZ S, PARK Y S, et al. Deciphering the transcriptional regulatory logic of amino acid metabolism[J]. Nature Chemical Biology, 2011, 8(1): 65-71. |
| 24 | JIN Y S, STEPHANOPOULOS G. Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli [J]. Metabolic Engineering, 2007, 9(4): 337-347. |
| 25 | ÖZAYDIN B, BURD H, LEE T S, et al. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production[J]. Metabolic Engineering, 2013, 15: 174-183. |
| 26 | ZHANG Y X, PERRY K, VINCI V A, et al. Genome shuffling leads to rapid phenotypic improvement in bacteria[J]. Nature, 2002, 415(6872), 644-646. |
| 27 | ZHANG X, ZHANG X F, LI H P, et al. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool[J]. Applied Microbiology and Biotechnology, 2014, 98(12): 5387-5396. |
| 28 | NICOLAOU S A, GAIDA S M, PAPOUTSAKIS E T. Coexisting/Coexpressing genomic libraries (CoGeL) identify interactions among distantly located genetic loci for developing complex microbial phenotypes[J]. Nucleic Acids Research, 2011, 39(22): e152. |
| 29 | WANG T, CHANG G G, JIA H G, et al. Pooled CRISPR interference screening enables genome-scale functional genomics study in bacteria with superior performance[J]. Nature Communications, 2018, 9(1):2475. |
| 30 | 李翔. 微生物诱变育种技术[J]. 现代商贸工业, 2017(34): 185-187. |
| LI X. Microbial mutation breeding technology[J]. Modern Business Trade Industry, 2017(34): 185-187. | |
| 31 | DEMAIN A L. From natural products discovery to commercialization: a success story[J]. Journal of Industrial Microbiology & Biotechnology, 2006, 33(7), 486-495. |
| 32 | 季宇彬, 朱兴杰, 徐昶儒, 等. 微生物诱变育种方法的研究进展[C]// 中国毒理学会环境与生态毒理学专业委员会成立大会. 2008. |
| JI Y B, ZHU X J, XU C R, et al. Research progress of microbial mutation breeding methods [C] // The Founding Conference of the Professional Committee of Environmental and Ecotoxicology of the Chinese Society of Toxicology. 2008. | |
| 33 | 微生物诱变育种编写组.微生物诱变育种[M].北京:科学出版社, 1973: 25-32. |
| Microbiology Mutation Breeding Compilation Group. Microbial mutation breeding [M]. Beijing: Science Press, 1973: 25-32. | |
| 34 | NESS J E, WELCH M, GIVER L, et al. DNA shuffling of subgenomic sequences of subtilisin[J]. Nature Biotechnology, 1999, 17(9): 893-896. |
| 35 | 施巧琴, 吴松钢. 工业微生物育种[M]. 福州: 福建科学技术出版社, 1991: 54-55. |
| SHI Q Q, WU S G. Breeding of industrial microbes[M]. Fuzhou: Fujian Science and Technology Publishing House, 1991: 54-55. | |
| 36 | CVIJOVIC M, BORDEL S, NIELSEN J. Mathematical models of cell factories: moving towards the core of industrial biotechnology[J]. Microbial Biotechnology, 2010, 4(5): 572-584. |
| 37 | FELL D A. Metabolic control analysis: a survey of its theoretical and experimental development[J]. Biochemical Journal, 1992, 286(2): 313-330. |
| 38 | WIECHERT W. 13C metabolic flux analysis[J]. Metabolic Engineering, 2001, 3(3): 195-206. |
| 39 | STEPHANOPOULOS G. Metabolic fluxes and metabolic engineering[J]. Metabolic Engineering, 1999, 1(1): 1-11. |
| 40 | VARMA A, PALSSON B O. Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110[J]. Applied and Environmental Microbiology, 1994, 60 (10): 3724-3731. |
| 41 | PFEIFFER T, SANCHEZ-VALDENEBRO I, NUNO J, et al. METATOOL: for studying metabolic networks[J]. Bioinformatics, 1999, 15(3): 251-257. |
| 42 | TRINH C T, WLASCHIN A, SRIENC F. Elementary mode analysis: a useful metabolic pathway analysis tool for characterizing cellular metabolism[J]. Applied Microbiology and Biotechnology, 2009, 81(5): 813-826. |
| 43 | ZAMBONI N, KÜMMEL A, HEINEMANN M. anNET: a tool for network-embedded thermodynamic analysis of quantitative metabolome data[J]. BMC Bioinformatics, 2008, 9(1): 199. |
| 44 | HENRY C S, BROADBELT L J, HATZIMANIKATIS V. Thermodynamics-based metabolic flux analysis[J]. Biophysical Journal, 2007, 92(5), 1792-1805. |
| 45 | BECKER S A, FEIST A M, MO M L, et al. Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox[J]. Nature Protocols, 2007, 2(3): 727-738. |
| 46 | SEGRE D, VITKUP D, CHURCH G M. Analysis of optimality in natural and perturbed metabolic networks[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(23): 15112-15117. |
| 47 | SHLOMI T, BERKMAN O, RUPPIN E. Regulatory on/off minimization of metabolic flux changes after genetic perturbations[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(21): 7695-7700. |
| 48 | BURGARD A P, PHARKYA P, MARANAS C D. Optknock: A bilevel programming framework for identifying gene knockout strategies for microbial strain optimization[J]. Biotechnology and Bioengineering, 2003, 84(6): 647-657. |
| 49 | HATZIMANIKATIS V, LI C IONITA J A, et al. Exploring the diversity of complex metabolic networks[J]. Bioinformatics, 2004, 21(8): 1603-1609. |
| 50 | EDWARDS J S, PALSSON B O. The Escherichia coli MG1655 in silico metabolic genotype: Its definition, characteristics, and capabilities[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(10): 5528-5533. |
| 51 | COVERT M W, SCHILLING C H, FAMILI I, et al. Metabolic modeling of microbial strains in silico[J]. Trends in Biochemical Sciences, 2001, 26(3): 179-186. |
| 52 | ÖSTERLUND T, NOOKAEW I, NIELSEN J. Fifteen years of large scale metabolic modeling of yeast: developments and impacts[J]. Biotechnology Advances, 2012, 30(5): 979-988. |
| 53 | FÖRSTER J, FAMILI I, FU P, et al. Genome-Scale Reconstruction of the Saccharomyces cerevisiae metabolic network[J]. Genome Research, 2003, 13(2): 244-253. |
| 54 | VARMA A, BOESCH B W, PALSSON B O. Biochemical production capabilities of Escherichia coli [J]. Biotechnology and Bioengineering, 1993, 42(1): 59-73. |
| 55 | VALLINO J J, STEPHANOPOULOS G. Carbon flux distributions at the glucose 6-phosphate branch point in Corynebacterium glutamicum during lysine overproduction[J]. Biotechnology Progress, 1994, 10(3): 327-334. |
| 56 | GULIK W M VAN, HEIJNEN J J. A metabolic network stoichiometry analysis of microbial growth and product formation[J]. Biotechnology and Bioengineering, 1995, 48(6): 681-698. |
| 57 | VANROLLEGHEM P A, DE JONG-GUBBELS P, GULIK W M VAN, et al. Validation of a metabolic network for Saccharomyces cerevisiae using mixed substrate studies[J]. Biotechnology Progress, 1996, 12(4): 434-448. |
| 58 | NISSEN T L, SCHULZE U, NIELSEN J, et al. Flux distributions in anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae [J]. Microbiology, 1997, 143(1): 203-218. |
| 59 | DUARTE N C. Reconstruction and validation of Saccharomyces cerevisiae iND750, a fully compartmentalized genome-scale metabolic model[J]. Genome Research, 2004, 14(7): 1298-1309. |
| 60 | DOBSON P D, SMALLBONE K, JAMESON D, et al. Further developments towards a genome-scale metabolic model of yeast[J]. BMC Systems Biology, 2010, 4(1): 145. |
| 61 | ORTH J D, CONRAD T M, NA J, et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011[J]. Molecular Systems Biology, 2011, 7(1): 535. |
| 62 | KJELDSEN K R, NIELSEN J. In silico genome-scale reconstruction and validation of the Corynebacterium glutamicum metabolic network[J]. Biotechnology and Bioengineering, 2009, 102(2): 583-597. |
| 63 | OLIVEIRA A, NIELSEN J, FORSTER J. Modeling Lactococcus lactis using a genome-scale flux model[J]. BMC Microbiology, 2005, 5(1): 39. |
| 64 | PARK S, SCHILLING C, PALSSON B O : Compositions and methods for modeling Bacillus subtilis metabolism: US 20030224363[P]. 2003-12-04. |
| 65 | LERMAN J A, HYDUKE D R, LATIF H, et al. In silico method for modelling metabolism and gene product expression at genome scale[J]. Nature Communications, 2012, 3(1): 929. |
| 66 | LIU J K, O'BRIEN E J, LERMAN J A, et al. Reconstruction and modeling protein translocation and compartmentalization in Escherichia coli at the genome-scale[J]. BMC Systems Biology, 2014, 8:110. |
| 67 | ZHANG Y, THIELE I, WEEKES D, et al. Three-dimensional structural view of the central metabolic network of Thermotoga maritima [J]. Science, 2009, 325(5947): 1544-1549. |
| 68 | CHANG R L, ANDREWS K, KIM D, et al. Structural systems biology evaluation of metabolic thermotolerance in Escherichia coli [J]. Science, 2013, 340(6137): 1220-1223. |
| 69 | CHANG R L, XIE L, BOURNE P E, et al. Antibacterial mechanisms identified through structural systems pharmacology[J]. BMC Systems Biology, 2013, 7(1): 102. |
| 70 | CHANDRASEKARAN S, PRICE N D. Probabilistic integrative modeling of genome-scale metabolic and regulatory networks in Escherichia coli and Mycobacterium tuberculosis [J]. Proceedings of the National Academy Sciences of the United States of America, 2010, 107(41): 17845-17850. |
| 71 | HERRGARD M J. Integrated analysis of regulatory and metabolic networks reveals novel regulatory mechanisms in Saccharomyces cerevisiae [J]. Genome Research, 2006, 16(5): 627-635. |
| 72 | KARR J R, SANGHVI J C, MACKLIN D N, et al. A whole-cell computational model predicts phenotype from genotype[J]. Cell, 2012, 150(2): 389-401. |
| 73 | BECKER J, ZELDER O, HäFNER STEFAN, et al. From zero to hero-design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production[J]. Metabolic Engineering, 2011, 13(2): 159-168. |
| 74 | CHOI K R, JANG W D, YANG D, et al. Systems metabolic engineering strategies: integrating systems and synthetic biology with metabolic engineering[J]. Trends in Biotechnology, 2019, 37(8): 817-837. |
| 75 | JANSET M L, GULIK W M VAN. Towards large scale fermentative production of succinic acid[J]. Current Opinion in Biotechnology, 2014,30: 190-197. |
| 76 | BURK M J. Sustainable production of industrial chemicals from sugars[J]. International Sugar Journal, 2010, 112(1333): 30-35. |
| 77 | DATTA J, KASPRZYK P, BŁAŻEK K, et al. Synthesis, structure and properties of poly(ester-urethane)s obtained using bio-based and petrochemical 1,3-propanediol and 1,4-butanediol[J]. Journal of Thermal Analysis and Calorimetry, 2017, 130: 261-276. |
| 78 | NAKAMURA C E, WHITE G M. Metabolic engineering for the microbial production of 1,3-propanediol[J]. Current Opinion in Biotechnology, 2003, 14(5): 454-459. |
| 79 | Yield 10 bioscience agricultural company-Corporate Overview[OL]. . |
| 80 | BECKER J, LANGE A, FABARIUS J. Top value platform chemicals: bio-based production of organic acids[J]. Current Opinion in Biotechnology, 2015, 36: 168-175. |
| 81 | GUSTAVSSON M, LEE S Y. Prospects of microbial cell factories developed through systems metabolic engineering[J]. Microbial Biotechnology, 2016, 9(5): 610-617. |
| 82 | BUIJS N A, SIEWERS V, NIELSEN J. Advanced biofuel production by the yeast Saccharomyces cerevisiae [J]. Current Opinion in Chemical Biology, 2013, 17(3): 480-488. |
| 83 | GEORGE K W, ALONSO-GUTIERREZ J, KEASLING J D, et al. Isoprenoid drugs, biofuels, and chemicals-artemisinin, farnesene, and beyond[J]. Advances in Biochemical Engineering/Biotechnology, 2015, 355-389. |
| 84 | PADDON C J, KEASLING J D.Semi-synthetic artemisinin: a model for the use of synthetic biology inpharmaceutical development[J]. Nature Reviews Microbiology, 2014, 12(5): 355-367. |
| 85 | PADDON C J, WESTFALL P J, PITERA D J, et al. High level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 86 | FREED E F, WINKLER J D, WEISS S J, et al. Genome-wide tuning of protein expression levels to rapidly engineer microbial traits[J]. ACS Synthetic Biology, 2015, 4(11): 1244-1253. |
| 87 | BABA T, ARA T, HASEGAWA M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection[J]. Molecular Systems Biology, 2006. DOI: 10.1038/msb4100050 . |
| 88 | KOO B M, KRITIKOS G, FARELLI J D, et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis [J]. Cell systems, 2017, 4(3): 291-305. |
| 89 | WANG H H, ISAACS F J, CARR P A, et al. Programming cells by multiplex genome engineering and accelerated evolution[J]. Nature, 2009, 460(7257): 894-898. |
| 90 | WARNER J R, REEDER P J, KARIMPOUR-FARD A, et al. Rapid profiling of a microbial genome using mixtures of barcoded oligonucleotides[J]. Nature Biotechnology, 2010, 28(8): 856-862. |
| 91 | DOUDNA J A, CHARPENTIER E. The new frontier of genome engineering with CRISPR-Cas9[J]. Science, 2014, 346(6213): 1077. |
| 92 | QI L S, LARSON M H, GILBERT L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression[J]. Cell, 2013, 152(5): 1173-1183. |
| 93 | DONG C, FONTANA J, PATEL A, et al. Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria[J]. Nature Communications, 2018, 9(1): 2489. |
| 94 | MANS R, ROSSUM H M VAN, WIJSMAN M, et al. CRISPR/Cas9: a molecular Swiss army knife for simultaneous introduction of multiple genetic modifications in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2015,15(2). DOI: 1093/femsyr/fov004 . |
| 95 | HALPERIN S O, TOU C J, WONG E B, et al. CRISPR-guided DNA polymerases enable diversification of all nucleotides in a tunable window[J]. Nature, 2018, 560(7717): 248-252. |
| 96 | JIANG W, BIKARD D, COX D, et al. RNA-guided editing of bacterial genomes using CRISPR-Cas systems[J]. Nature Biotechnology, 2013, 31: 233-239. |
| 97 | OH J H, van PIJKEREN J P. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri [J]. Nucleic Acids Research 2014, 42: e131. |
| 98 | CHOUDHARY E, THAKUR P, PAREEK M, et al. Gene silencing by CRISPR interference in mycobacteria[J]. Nature Communications, 2015, 6(1): 6267. |
| 99 | YAO L, CENGIC I, ANFELT J, et al. Multiple gene repression in cyanobacteria using CRISPRi[J]. ACS Synthetic Biology 2016, 5: 207-212. |
| 100 | XU T, LI Y, SHI Z, et al. Efficient genome editing in Clostridium cellulolyticum via CRISPR-Cas9 nickase.[J]. Applied and Environmental Microbiology, 2015, 81: 4423-4431. |
| 101 | PETERS J M, COLAVIN A, SHI H, et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria[J]. Cell, 2016, 165: 1493-1506. |
| 102 | JIANG Y, QIAN F, YANG J, et al. CRISPR-Cpf1 assisted genome editing of Corynebacterium glutamicum [J]. Nature Communications, 2017, 8(1): 15179. |
| 103 | NAYAK D D, METCALF W W. Cas9-mediated genome editing in the methanogenic archaeon Methanosarcina acetivorans [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114: 2976-2981. |
| 104 | 王天民.基于 CRISPR 干扰技术的微生物高通量功能基因组学方法[D]. 北京: 清华大学, 2018. |
| WANG T M. CRISPR interference based high-throughput functional genomics platform method in bacteria[D]. Beijing: Tsinghua University, 2018. | |
| 105 | DICARLO J E, CONLEY A J, PENTTILA M, et al. Yeast oligo-mediated genome engineering (YOGE)[J]. ACS Synthetic Biology, 2013, 2(12): 741-749. |
| 106 | GARST A D, BASSALO M C, PINES G,et al. Genome-wide mapping of mutations at single-nucleotide resolution for protein, metabolic and genome engineering[J]. Nature Biotechnology, 2016, 35(1): 48-55. |
| 107 | ANZALONE A V, RANDOLPH P B, DAVIS J R, et al. Search-and-replace genome editing without double-strand breaks or donor DNA[J]. Nature, 2019, 576(7785): 149-157. |
| 108 | NISHIDA K, ARAZOE T, YACHIE N, et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems[J]. Science, 2016, 353(6305): aaf8729. |
| 109 | MA Y, ZHANG J, YIN W, et al. Targeted AID-mediated mutagenesis (TAM) enables efficient genomic diversification in mammalian cells[J]. Nature Methods, 2016, 13(12): 1029-1035. |
| 110 | HESS G T, FRESARD L, HAN K, et al. Directed evolution using dCas9-targeted somatic hypermutation in mammalian cells[J]. Nature Methods, 2016, 13(12): 1036-1042. |
| 111 | LI Q, SEO J H, STRANGER B, et al. Integrative eQTL-Based analyses reveal the biology of breast cancer risk loci[J]. Cell, 2013, 152(3): 633-641. |
| 112 | WU J, ZHOU P, ZHANG X, et al. Efficient de novo synthesis of resveratrol by metabolically engineered Escherichia coli [J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(7): 1083-1095. |
| 113 | GILBERT L A, LARSON M H, MORSUT L, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes[J]. Cell, 2013, 154(2): 442-451. |
| 114 | HALPERIN S O, TOU C J, WANG E B, et al. CRISPR-guided DNA polymerases enable diversification of all nucleotides in a tunable window[J]. Nature, 2018, 560: 248-252. |
| 115 | WONG B G, MANCUSO C P, KIRIAKOV S, et al. Precise, automated control of conditions for high-throughput growth of yeast and bacteria with eVOLVER[J]. Nature Biotechnology, 2018, 36(7): 614-623. |
| 116 | JIAN X, GUO X, WANG J, et al. Microbial microdroplet culture system (MMC): an integrated platform for automated, high-throughput microbial cultivation and adaptive evolution[J]. Biotechnology and Bioengineering, 2019. DOI: 10.1101/2019.12. 19.883561 . |
| 117 | MICHENER J K, THODEY K, LIANG J C, et al. Applications of genetically-encoded biosensors for the construction and control of biosynthetic pathways[J]. Metabolic Engineering, 2012, 14(3): 212-222. |
| 118 | LIU D, EVANS T, ZHANG F Z, et al. Applications and advances of metabolite biosensors for metabolic engineering[J]. Metabolic Engineering, 2015, 31: 15-22. |
| 119 | FANG M, WANG T, ZHANG C, et al. Intermediate-sensor assisted push-pull strategy and its application in heterologous deoxyviolacein production in Escherichia coli [J]. Metabolic Engineering, 2016, 33: 41-51. |
| 120 | LIANG W, CUI L, CUI J, et al. Biosensor-assisted transcriptional regulator engineering for Methylobacterium extorquens AM1 to improve mevalonate synthesis by increasing the acetyl-CoA supply[J]. Metabolic Engineering, 2017, 39: 159-168. |
| 121 | BINDER S, SCHENDZIELORZ G, STABLER N, et al. A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level[J]. Genome Biology, 2012, 13: R40. |
| 122 | BENEYTON T, THOMAS S, GRIFFITHS A D, et al. Droplet-based microfluidic high-throughput screening of heterologous enzymes secreted by the yeast Yarrowia lipolytica [J]. Microbial Cell Factories, 2017, 16(1): 18. |
| 123 | SIEDLER S, KHATRI N K, ZSOHAR A, et al. Development of a bacterial biosensor for rapid screening of yeast p-coumaric acid production[J]. ACS Synthetic Biology, 2017, 6(10): 1860-1869. |
| 124 | BARET J C, MILLER O J, TALY V, et al. Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity[J]. Lab on a Chip, 2009, 9(13): 1850. |
| 125 | WAGNER J M, LIU L, YUAN S et. al. A comparative analysis of single cell and droplet-based FACS for improving production phenotypes : riboflavin overproduction in Yarrowia lipolytica [J]. Metabolic Engineering, 2018, 47: 346-356. |
| 126 | MA C, TAN Z L, LIN Y, et al. Gel microdroplet-based high-throughput screening for directed evolution of xylanase-producing Pichia pastoris [J]. Journal of Bioscience and Bioengineering, 2019, 128(6): 662-668. |
| 127 | WANG X, REN L, SU Y, et al. Raman-activated droplet sorting (RADS) for label-free high-throughput screening of microalgal single-cells[J]. Analytical Chemistry, 2017, 89(22): 12569-12577. |
| 128 | DIXIT A, PARNAS O, LI B, et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens[J]. Cell, 2016, 167: 1853-1866. |
| 129 | TROPINI C, EARLE K A, HUANG K C, et al. The gut microbiome: connecting spatial organization to function[J]. Cell Host Microbe, 2017, 21: 433-442. |
| 130 | SHI H, COLAVIN A, LEE T K, et al. Strain library imaging protocol for high-throughput, automated single-cell microscopy of large bacterial collections arrayed on multiwell plates[J]. Nature Protocols Erecipes for Researchers, 2017, 12: 429-438. |
| 131 | LAWSON M J, CAMSUND D, LARSSON J, et al. In situ genotyping of a pooled strain library after characterizing complex phenotypes[J]. Molecular Systems Biology, 2017, 13: 947. |
| 132 | ZONG Y, ZHANG H M, LYU C, et al. Insulated transcriptional elements enable precise design of genetic circuits[J]. Nature Communications, 2017, 8: 52. |
| 133 | BASSALO M C, GARST A D, CHOUDHURY A, et al. Deep scanning lysine metabolism in Escherichia coli [J]. Molecular Systems Biology, 2018, 14(11): e8371. |
| 134 | LIANG L, LIU R, GARST A D, et al. CRISPR EnAbled trackable genome engineering for isopropanol production in Escherichia coli [J]. Metabolic Engineering, 2017, 41: 1-10. |
| 135 | LIU R, LIANG L, GARST A D, et al. Directed combinatorial mutagenesis of Escherichia coli for complex phenotype engineering[J]. Metabolic Engineering, 2018, 47: 10-20. |
| 136 | LIANG L, LIU R, FOSTER K E, et al. Genome engineering of E. coli for improved styrene production[J]. Metabolic Engineering, 2020, 57: 74-84. |
| 137 | ANGERMUELLER C, PÄRNAMAA T, PARTS L, et al. Deep learning for computational biology[J]. Molecular Systems Biology, 2016, 12(7): 878. |
| 138 | COSTELLO Z, MARTIN H G. A machine learning approach to predict metabolic pathway dynamics from time-series multiomics data[J]. NPJ Systems Biology and Applications, 2018, 4(1): 1-14. |
| 139 | CUPERUS J T, GROVES B, KUCHINA A, et al. Deep learning of the regulatory grammar of yeast 5' untranslated regions from 500,000 random sequences[J]. Genome Research, 2017, 27(12): 2015-2024. |
| 140 | ZHOU Y, LI G, DONG J, et al. MiYA, an efficient machine-learning workflow in conjunction with the YeastFab assembly strategy for combinatorial optimization of heterologous metabolic pathways in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2018, 47: 294-302. |
| 141 | DENBY C M, LI R A, VU V T, et al. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer[J]. Nature Communications, 2018, 9(1): 965. |
| 142 | ZHANG J, PSTERSEN S D, RADIVOJEVIC T, et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism[J]. Nature Communications, 2020, 11(1): 4880. |
| 143 | GUO J, WANG T, GUAN C, et al. Improved sgRNA design in bacteria via genome-wide activity profiling[J]. Nucleic Acids Research, 2018, 46(14): 7052-7069. |
| [1] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [2] | 应汉杰, 柳东, 王振宇, 沈涛, 庄伟, 朱晨杰. 工业生物制造与“碳中和”目标探讨[J]. 合成生物学, 2025, 6(1): 1-7. |
| [3] | 张以恒, 陈雪梅, 石婷. 生物制造的市本率(PC值):定义与应用[J]. 合成生物学, 2025, 6(1): 8-17. |
| [4] | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6): 1231-1241. |
| [5] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [6] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [7] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [8] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [9] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [10] | 刘建明, 张炽坚, 张冰, 曾安平. 巴氏梭菌作为工业底盘细胞高效生产1,3-丙二醇——从代谢工程和菌种进化到过程工程和产品分离[J]. 合成生物学, 2024, 5(6): 1386-1403. |
| [11] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [12] | 禹伟, 高教琪, 周雍进. 一碳生物转化合成有机酸的研究进展[J]. 合成生物学, 2024, 5(5): 1169-1188. |
| [13] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| [14] | 谢皇, 郑义蕾, 苏依婷, 阮静怡, 李永泉. 放线菌聚酮类化合物生物合成体系重构研究进展[J]. 合成生物学, 2024, 5(3): 612-630. |
| [15] | 惠真, 唐啸宇. CRISPR/Cas9编辑系统在微生物天然产物研究中的应用[J]. 合成生物学, 2024, 5(3): 658-671. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

