合成生物学 ›› 2022, Vol. 3 ›› Issue (2): 415-427.DOI: 10.12211/2096-8280.2021-050
• 研究论文 • 上一篇
病毒-纳米金杂合导电网络结构在电化学分析的应用
- 1.中南民族大学生命科学学院,湖北 武汉 430074
2.中国科学院武汉病毒研究所,生物安全大科学研究中心,病毒学国家重点实验室,湖北 武汉 430071
3.中国科学院生物物理研究所,生物大分子科教融合卓越中心,生物大分子国家重点实验室,北京 100101
4.中国科学院大学,北京 100049
-
收稿日期:2021-04-26修回日期:2021-11-14出版日期:2022-04-30发布日期:2022-05-11 -
通讯作者:梁晓声 -
作者简介:梁晓声 (1984—),男,博士,讲师。研究方向为纳米生物技术、纳米材料检测应用等。 E-mail:liangxs@mail.scuec.edu.cn -
基金资助:国家重点研发计划(2019YFA0904800)
Hybrid systems of virus and nano-gold conducting networks for electrochemical analysis
LIANG Xiaosheng1( ), GUO Yongchao2, MEN Dong2,4, ZHANG Xian’en3,4
), GUO Yongchao2, MEN Dong2,4, ZHANG Xian’en3,4
- 1.College of Life Science,South-Central University for Nationalities,Wuhan 430074,Hubei,China
2.State Key Laboratory of Virology,Wuhan Institute of Virology,Center for Biosafety Mega-Science,Chinese Academy of Sciences,Wuhan 430071,Hubei,China
3.National Laboratory of Biomacromolecules,CAS Center for Excellence in Biomacromolecules,Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China
4.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2021-04-26Revised:2021-11-14Online:2022-04-30Published:2022-05-11 -
Contact:LIANG Xiaosheng
摘要:
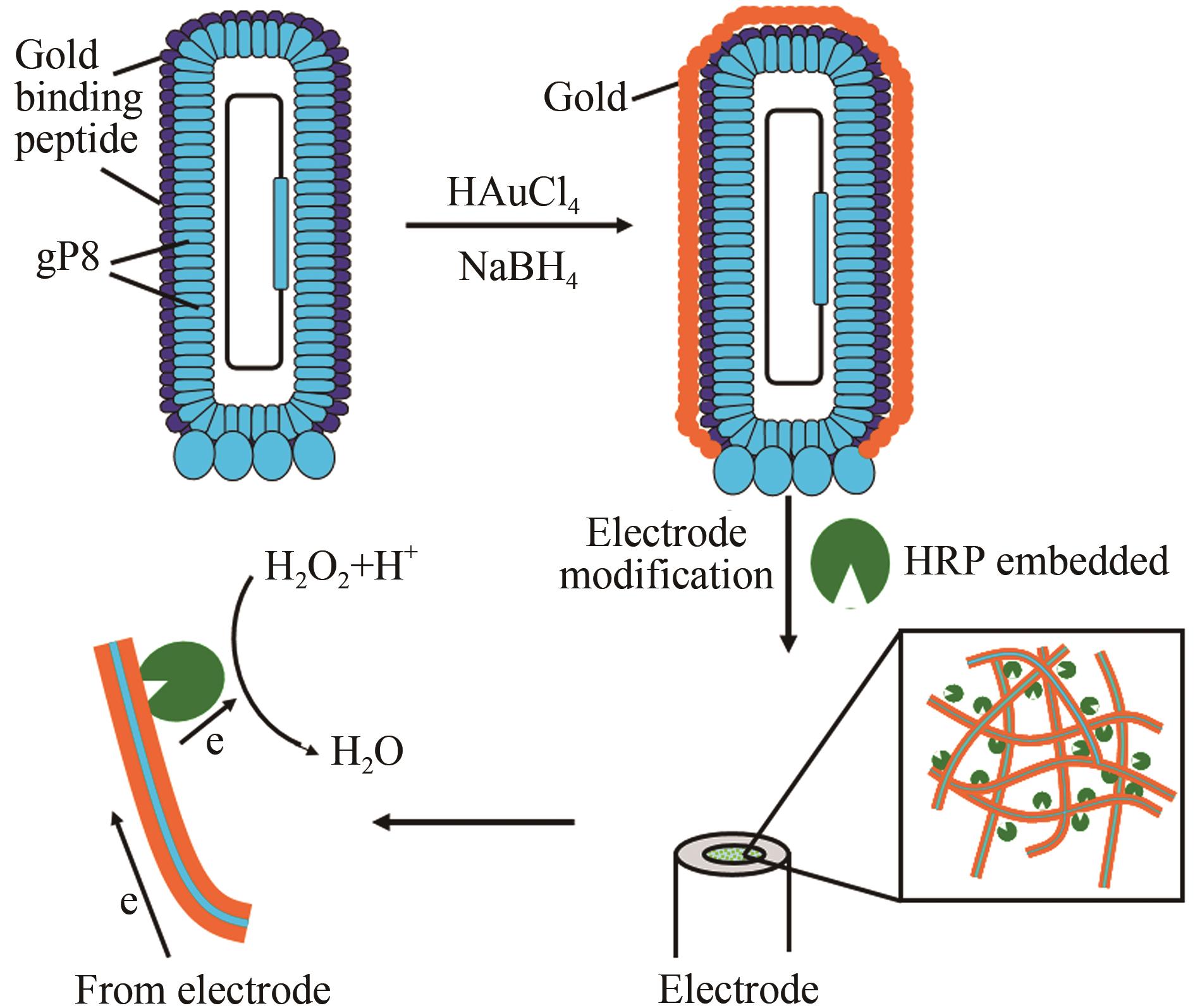
本文利用噬菌体展示技术将金结合肽展示在噬菌体M13主要衣壳蛋白(gP8)之上,构建了金结合肽展示的基因改造噬菌体M13(GM M13),并将这种基因改造噬菌体作为矿化成核模板在其表面沉积金,得到金-基因改造噬菌体复合物。利用壳聚糖将金-基因改造噬菌体复合物与辣根过氧化物酶(HRP)包埋修饰到玻碳电极上用于过氧化氢检测。修饰电极对过氧化氢具有高灵敏响应,线性范围2.5 μmol/L~60 mmol/L,检测限为0.32 μmol/L(S/N=3)。HRP/纳米金-噬菌体复合物/壳聚糖修饰玻碳电极对底物信号响应符合Michaelis-Menten动力学方程,Kmapp值经计算为0.3 mmol/L,说明该电极对底物具有高亲和性及高灵敏度。交流阻抗测试表明,HRP/纳米金-噬菌体复合物/壳聚糖修饰电极Ret值显著小于HRP/金纳米颗粒/壳聚糖修饰电极和HRP/壳聚糖修饰电极,说明该电极更有利于电子传递。不同修饰电极对过氧化氢响应信号比较结果表明,金-基因改造噬菌体复合物构建的酶电极与纳米金修饰的同类酶电极相比具有更高的灵敏度,相同底物浓度下可获得数倍的电流信号提升。过氧化氢酶电极的示例证明,金-基因改造噬菌体复合物作为一种酶电极修饰材料可显著提高电极导电面积,增大酶有效固定位点,从而获得显著的信号增益。
中图分类号:
引用本文
梁晓声, 郭永超, 门冬, 张先恩. 病毒-纳米金杂合导电网络结构在电化学分析的应用[J]. 合成生物学, 2022, 3(2): 415-427.
LIANG Xiaosheng, GUO Yongchao, MEN Dong, ZHANG Xian’en. Hybrid systems of virus and nano-gold conducting networks for electrochemical analysis[J]. Synthetic Biology Journal, 2022, 3(2): 415-427.
| 引物名称 | 序列(5'→3') |
|---|---|
| 6264mS | CGCCAAGCTTGCATGCCGCAGGTCCTC |
| 6264dn | GATAGCCTTTGTAGATCTCTC |
| 1381up | GGCATTACGTATTTTACCC |
| 1381S | CTTTCGCTGCAGAGGGTGAGGATC |
| 1381A | CTTTTGCGGGATCCTCACCCTCTGC |
| 1381dn | GCTATTAATTAATTTTCCC |
| GbS | GTATCGGGTTCTTCTCCTGATTCT |
| GbA | GATCAGAATCAGGAGAAGAACCCGATACTGCA |
表1 构建基因改造噬菌体所用引物
Tab. 1 Primers for constructing of recombinant phage
| 引物名称 | 序列(5'→3') |
|---|---|
| 6264mS | CGCCAAGCTTGCATGCCGCAGGTCCTC |
| 6264dn | GATAGCCTTTGTAGATCTCTC |
| 1381up | GGCATTACGTATTTTACCC |
| 1381S | CTTTCGCTGCAGAGGGTGAGGATC |
| 1381A | CTTTTGCGGGATCCTCACCCTCTGC |
| 1381dn | GCTATTAATTAATTTTCCC |
| GbS | GTATCGGGTTCTTCTCCTGATTCT |
| GbA | GATCAGAATCAGGAGAAGAACCCGATACTGCA |
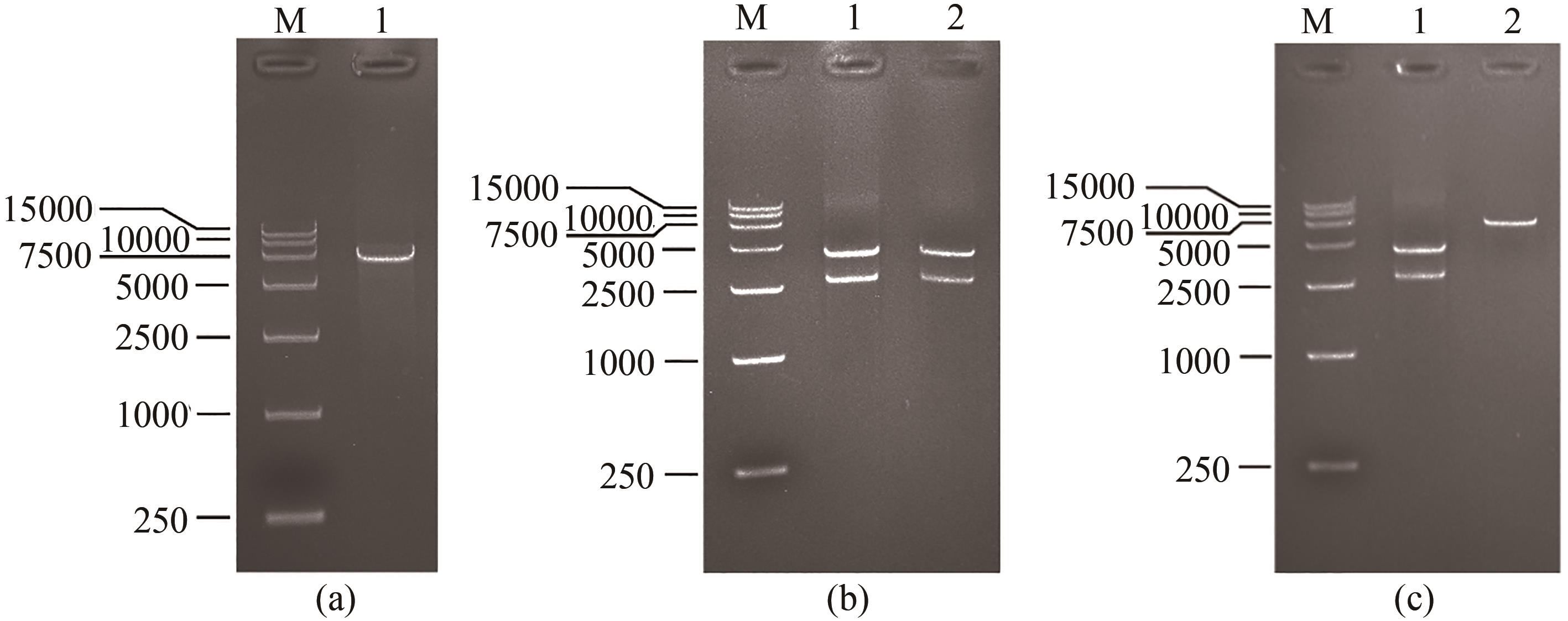
图2 展示金结合肽噬菌体的构建与鉴定M为takara DL15000 DNA ladder,(a)1泳道为使用PstⅠ和BglⅡ对6264处移码突变的病毒基因组进行双酶切;(b)1泳道为使用PstⅠ和PacⅠ,2泳道为使用BamHⅠ和PacⅠ分别对1372及1381处突变的病毒基因组进行双酶切;(c)1泳道为使用PstⅠ和PacⅠ对gp8基因插入了金结合肽序列的病毒基因组进行双酶切,2泳道为使用BamHⅠ和PacⅠ对gp8基因插入了金结合肽序列的病毒基因组进行双酶切
Fig. 2 Construction of gold-binding peptide displaying phage and its verificationM—Takara DL15000 DNA ladder. Lane (a) 1—Site 6264 mutated phage genome digested by PstⅠand BglⅡ. Lane (b) 1—Sites 1372 and 1381 mutated phage genome digested by PstⅠ and PacⅠ; 2—Sites 1372 and 1381 mutated phage genome digested by BamHⅠ and PacⅠ. Lane (c) 1—Gold-binding peptide sequence inserted phage genome digested by PstⅠ and PacⅠ; 2—Gold-binding peptide sequence inserted phage genome digested by BamHⅠ and PacⅠ
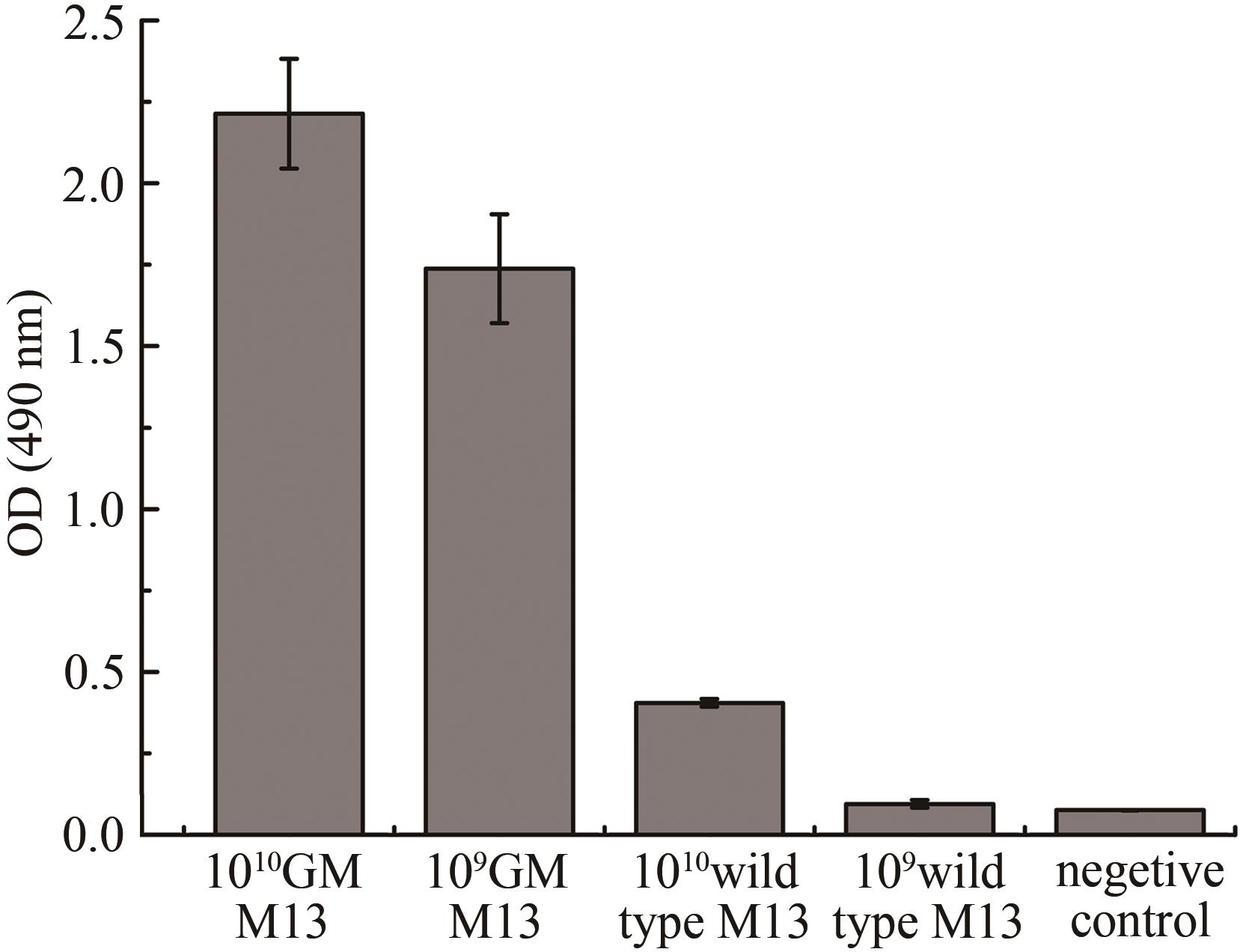
图3 银增强噬菌体ELISA实验结果(1010 GM M13 and 109 GM M13指滴度为1010 PFU/mL及109 PFU/mL基因改造的噬菌体M13;1010 Wild type M13 and 109 Wild type M13指滴度为1010 PFU/mL及109 PFU/mL野生型M13;阴性对照为未加噬菌体直接脱脂奶粉封阻后结合纳米金再进行银增强)
Fig. 3 Evaluation of gold-binding ability for GM M13 with silver enhancement(1010 GM M13 and 109 GM M13 refer to the genetically modified M13 with the titer of 1010 PFU and 109 PFU, respectively, and the silver enhancement GM M13 without gold nanoparticles is used as the control.)
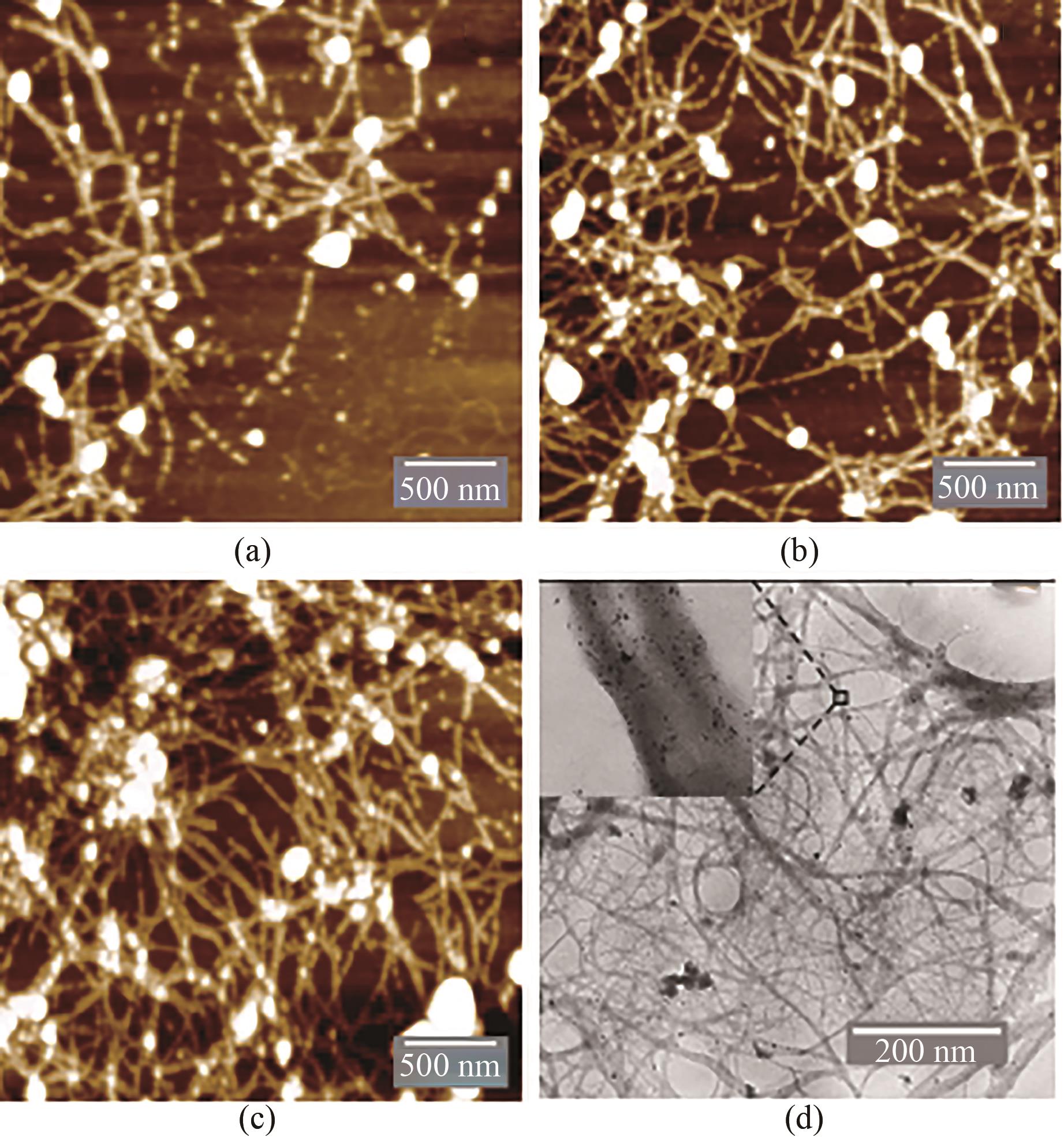
图4 不同反应条件下纳米金-噬菌体复合物的AFM及TEM图(a)0.25 mmol/L HAuCl4、1012 PFU/mL基因改造噬菌体、20 mmol/L Gly-Cl-及2.5 mmol/L NaBH4沉积条件下,AFM扫描图;(b)0.375 mmol/L HAuCl4、1012 PFU/mL基因改造噬菌体、20 mmol/L Gly-Cl-及2.5 mmol/L NaBH4沉积条件下,AFM扫描图;(c)0.5 mmol/L HAuCl4、1012 PFU/mL基因改造噬菌体、20 mmol/L Gly-Cl-及2.5 mmol/L NaBH4沉积条件下,AFM扫描图;(d)纳米金-噬菌体复合物TEM显微成像图,复合物形成条件: 0.5 mmol/L HAuCl4、1012 PFU/mL基因改造噬菌体、20 mmol/L Gly-Cl-及2.5 mmol/L NaBH4
Fig. 4 AFM and TEM images for the Au-GM M13 complex formed under different reaction conditions(a) AFM images for 0.25 mmol/L HAuCl4, 1012 PFU/mL GM M13, 20 mmol/L Gly-Cl- and 2.5 mmol/L NaBH4; (b) AFM images for 0.375 mmol/L HAuCl4, 1012 PFU/mL GM M13, 20 mmol/L Gly-Cl- and 2.5 mmol/L NaBH4; (c) AFM images for 0.5 mmol/L HAuCl4, 1012 PFU/mL GM M13, 20 mmol/L Gly-Cl- and 2.5 mmol/L NaBH4; (d) TEM image for the Au-GM M13 complex. Complex forming conditions: 0.5 mmol/L HAuCl4, 1012 PFU/mL GM M13, 20 mmol/L Gly-Cl- and 2.5 mmol/L NaBH4
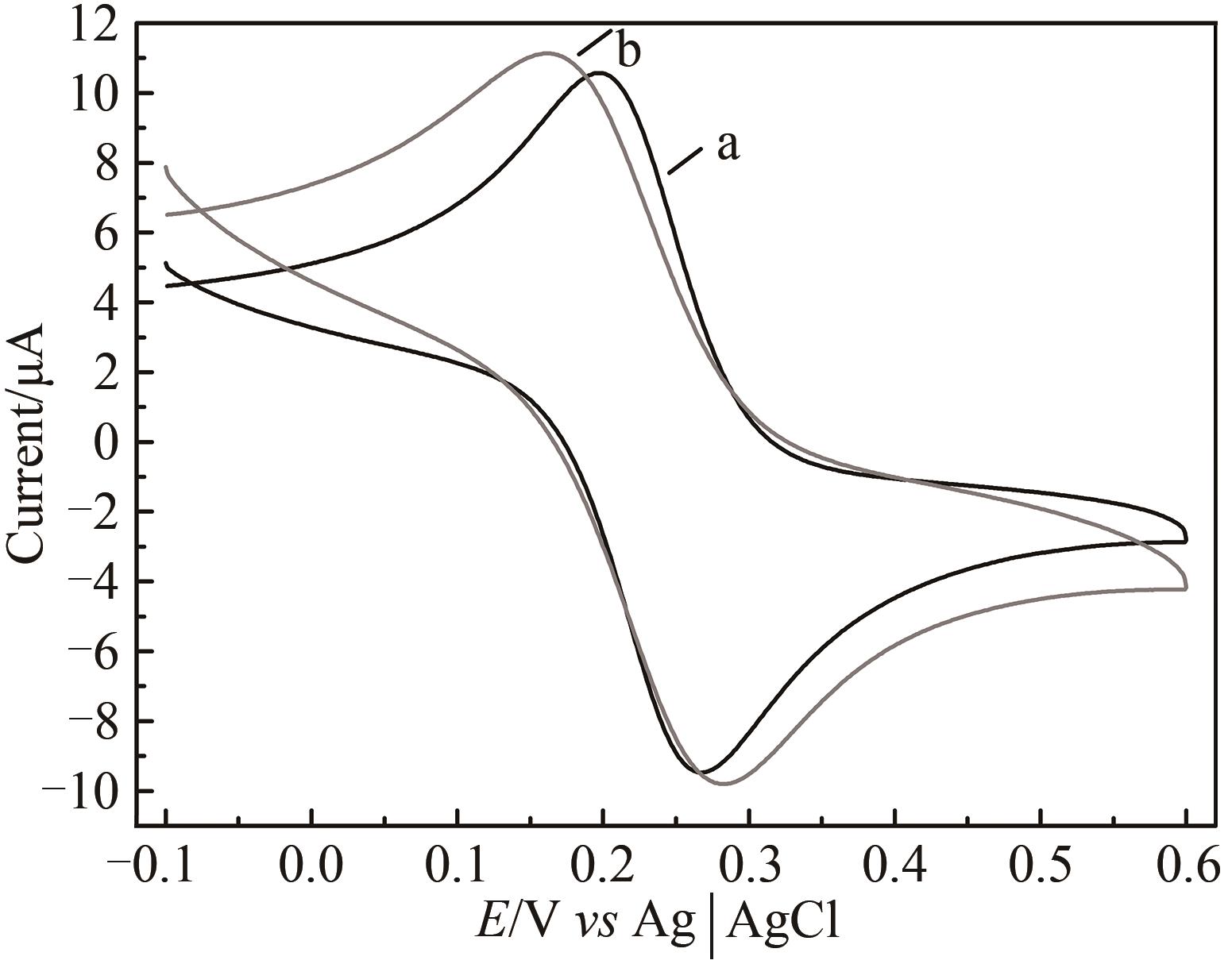
图5 HRP/纳米金-噬菌体复合物/壳聚糖修饰玻碳电极前后循环伏安图a—修饰前;b—修饰后[扫描速率50 mV/s;缓冲液含0.1 mol/L KCl及2 mmol/L [Fe(CN)6]3-/4-的PBS(10 mmol/L, pH 7.0)溶液]
Fig. 5 Voltammograms of the HRP/Au-GM M13 electrode without (a) and with chitosan modifications (b)[Scan rate: 50 mV/s; buffer: PBS (10 mmol/L, pH 7.0) solution containing 0.1 mol/L KCl and 2 mmol/L [Fe(CN)6]3-/4-.]
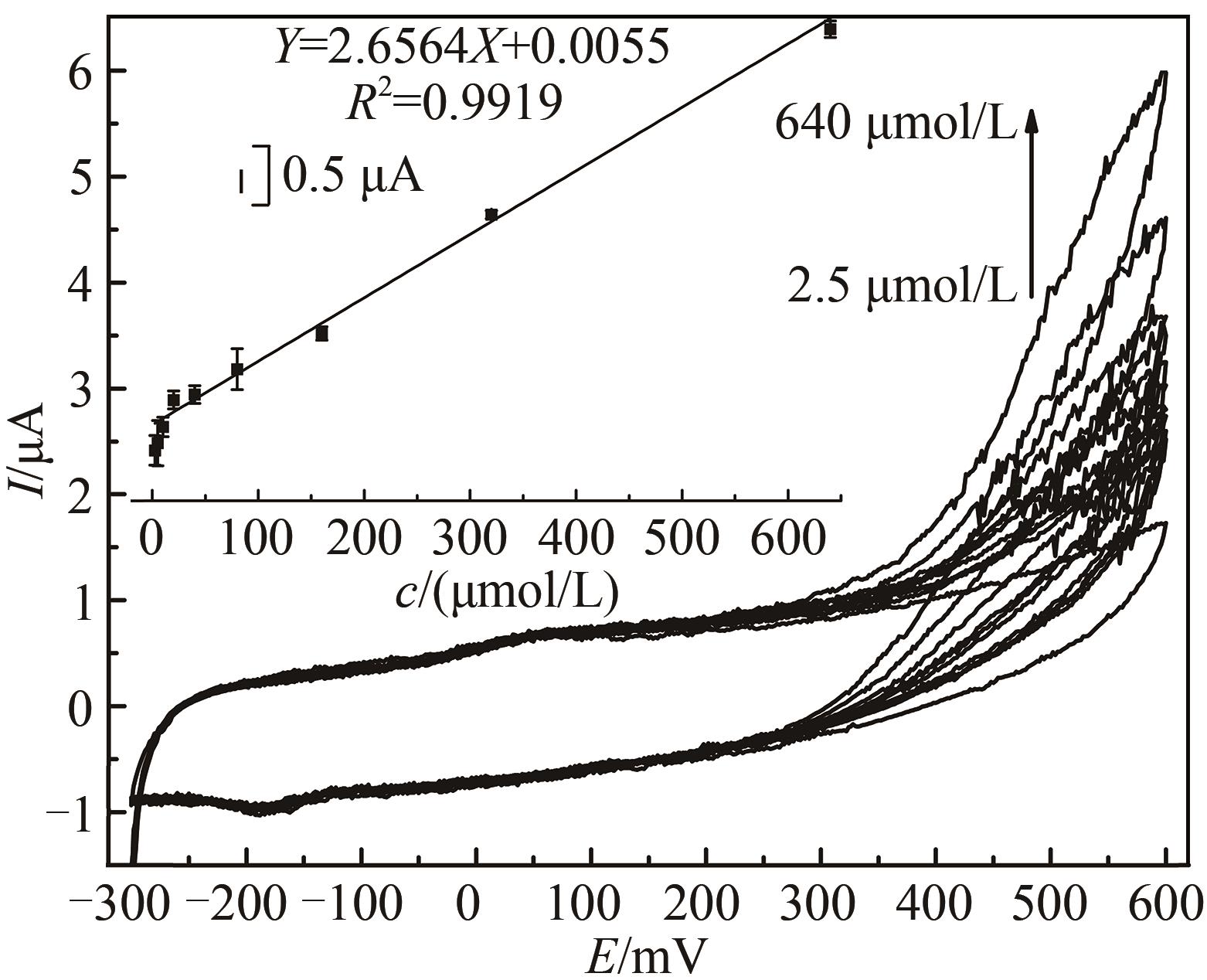
图6 不同过氧化氢浓度下HRP/纳米金-噬菌体复合物/壳聚糖修饰玻碳电极循环伏安图[Ar-饱和 PBS(0.1 mol/L, pH 7.0)中于20 mV/s速率扫描。H2O2浓度范围2.5~640 μmol/L。内插图为600 mV处峰电流(μA)对浓度(μmol/L)作图]
Fig. 6 Voltammograms of the HRP/Au-GM M13 electrode modified with chitosan at different concentrations of hydrogen peroxide[Scan rate: 20 mV/s for N2-saturated PBS (0.1 mol/L, pH 7.0). The curve peaks were obtained in response to H2O2 concentrations from 2.5 μmol/L to 640 μmol/L. The embedded image shows the calibration curve derived from the curves.]
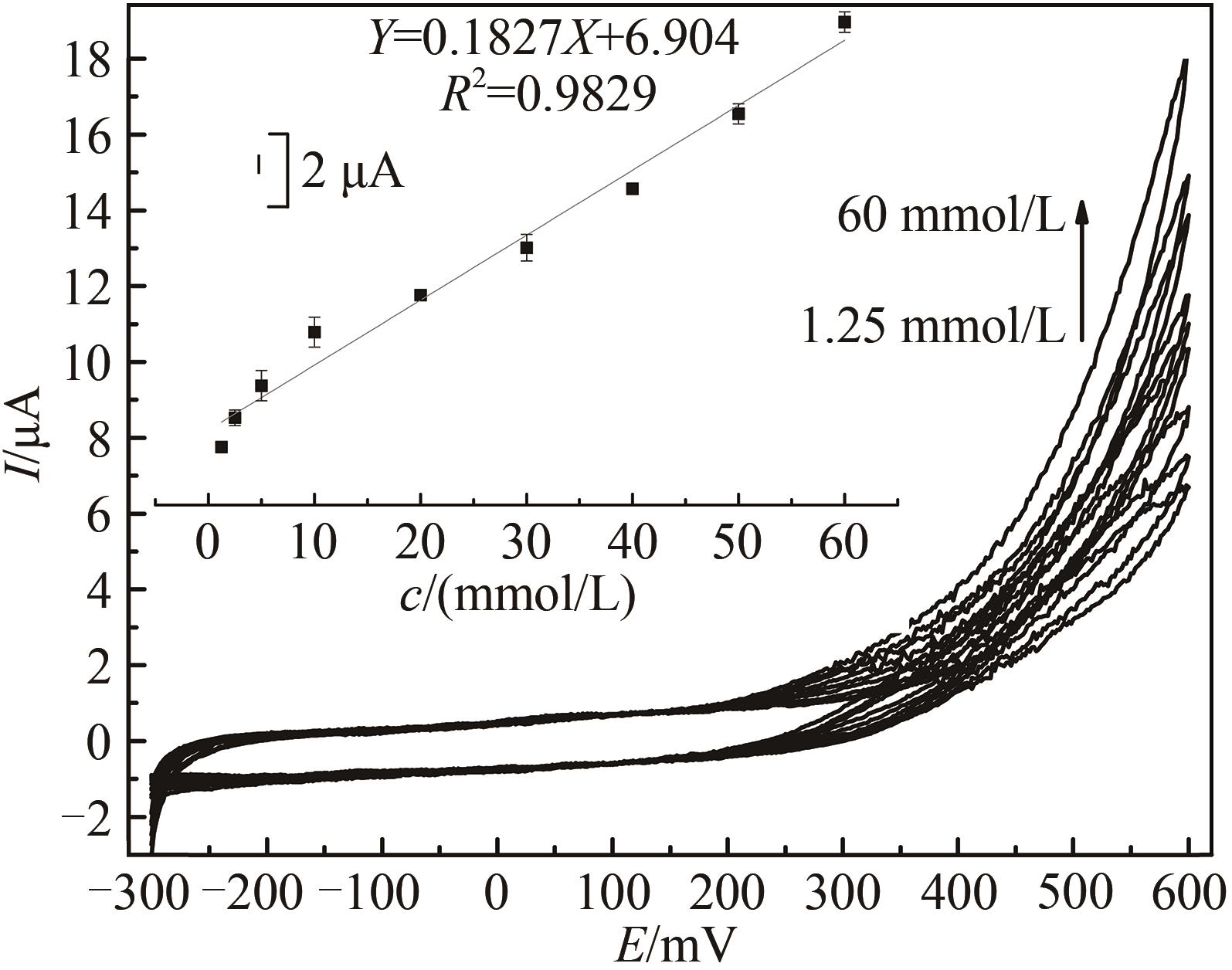
图7 不同过氧化氢浓度下HRP/纳米金-噬菌体复合物/壳聚糖修饰玻碳电极循环伏安图[Ar-饱和 PBS (0.1 mol/L, pH 7.0)中于20 mV/s速率扫描。H2O2浓度范围640 μmol/L~60 mmol/L。内插图为600 mV处峰电流(μA)对浓度(mmol/L)作图]
Fig. 7 Voltammograms of the HRP/Au-GM M13 electrode modified with chitosan at different concentrations of hydrogen peroxide from 640 μmol/L to 60 mmol/L[Scan rate: 20 mV/s for N2-saturated PBS (0.1 mol/L, pH 7.0). The embedded image shows the calibration curve derived from the curves.]
| 酶电极修饰方法 | 检测限 | Kmapp | 线性范围 | 来源 |
|---|---|---|---|---|
| Cerasomes with AuNPs@Poly(Ionic Liquid)s | 3.3 μmol/L | — | 10~70 μmol/L | [ |
| Silicahydroxyapatite hybrid film | 0.35 μmol/L | 21.8 μmol/L | 1.0~100 μmol/L | [ |
| Methanobactin functionalized gold nanoparticles | — | 0.787 mmol/L | 52.9 μmol/L~0.64 mmol/L | [ |
| Gold nanoparticles on indium/tin oxide electrode | 2 μmol/L | 0.4 mmol/L | 8.0 μmol/L~3.0 mmol/L | [ |
| Cerasome | 0.83 mmol/L | — | 2.5~325 mmol/L | [ |
| Carbon nanotubes | 0.1 μmol/L | — | 0.3~200 μmol/L | [ |
| 纳米金-噬菌体复合物 | 0.32 μmol/L | 0.3 mmol/L | 2.5 μmol/L~60 mmol/L | 本研究 |
表2 HRP/纳米金-噬菌体复合物/壳聚糖修饰电极与其他修饰过氧化氢酶电极的比较
Tab. 2 Comparison of the analytical performance of enzyme-phage-gold modified electrode with other electrochemical biosensors
| 酶电极修饰方法 | 检测限 | Kmapp | 线性范围 | 来源 |
|---|---|---|---|---|
| Cerasomes with AuNPs@Poly(Ionic Liquid)s | 3.3 μmol/L | — | 10~70 μmol/L | [ |
| Silicahydroxyapatite hybrid film | 0.35 μmol/L | 21.8 μmol/L | 1.0~100 μmol/L | [ |
| Methanobactin functionalized gold nanoparticles | — | 0.787 mmol/L | 52.9 μmol/L~0.64 mmol/L | [ |
| Gold nanoparticles on indium/tin oxide electrode | 2 μmol/L | 0.4 mmol/L | 8.0 μmol/L~3.0 mmol/L | [ |
| Cerasome | 0.83 mmol/L | — | 2.5~325 mmol/L | [ |
| Carbon nanotubes | 0.1 μmol/L | — | 0.3~200 μmol/L | [ |
| 纳米金-噬菌体复合物 | 0.32 μmol/L | 0.3 mmol/L | 2.5 μmol/L~60 mmol/L | 本研究 |
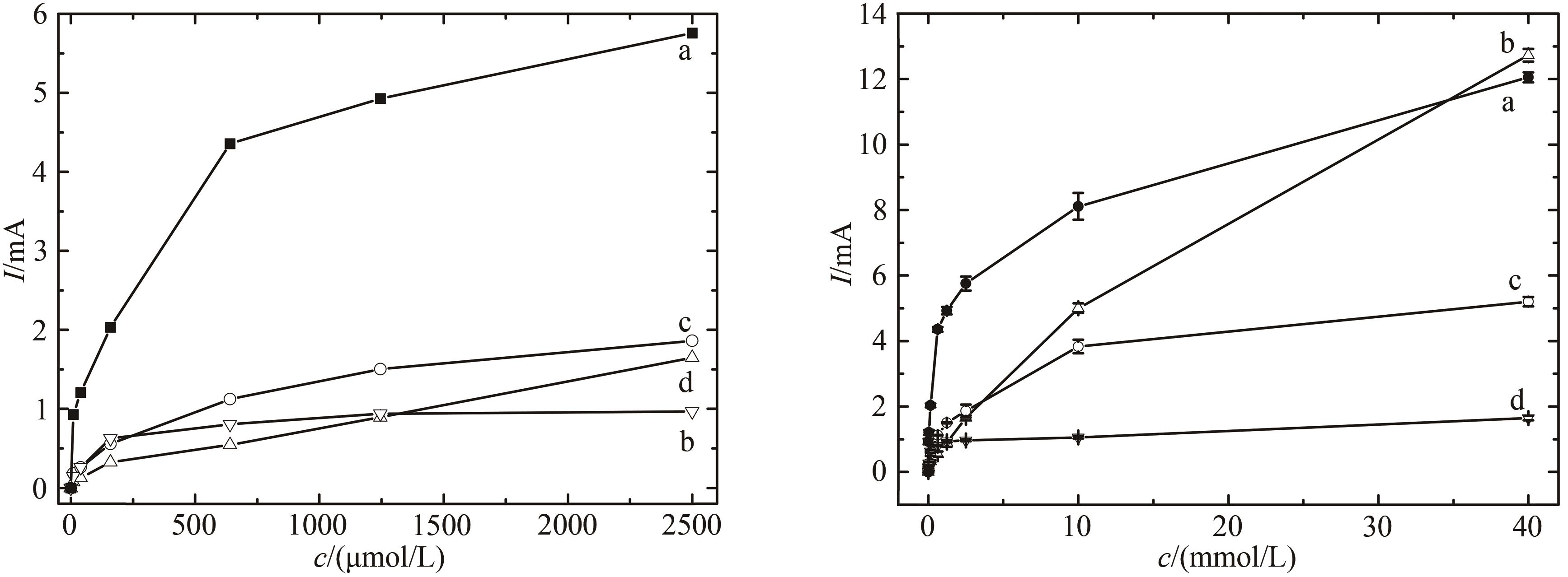
图8 随过氧化氢浓度变化不同修饰电极催化电流变化比较(a)HRP/纳米金-噬菌体复合物/壳聚糖修饰玻碳电极;(b)裸玻碳电极;(c)HRP/金纳米颗粒/壳聚糖修饰玻碳电极;(d)HRP壳聚糖包埋修饰玻碳电极
Fig. 8 Comparison of voltammograms for the electrode responses to increased H2O2 concentrations[The measurement was performed in the PBS without oxygen. HRP/Au-GM M13 electrode with (a) and without (b) chitosan modification, and HRP/Au nanoparticles electrode with (c) and without (d) chitosan modification]
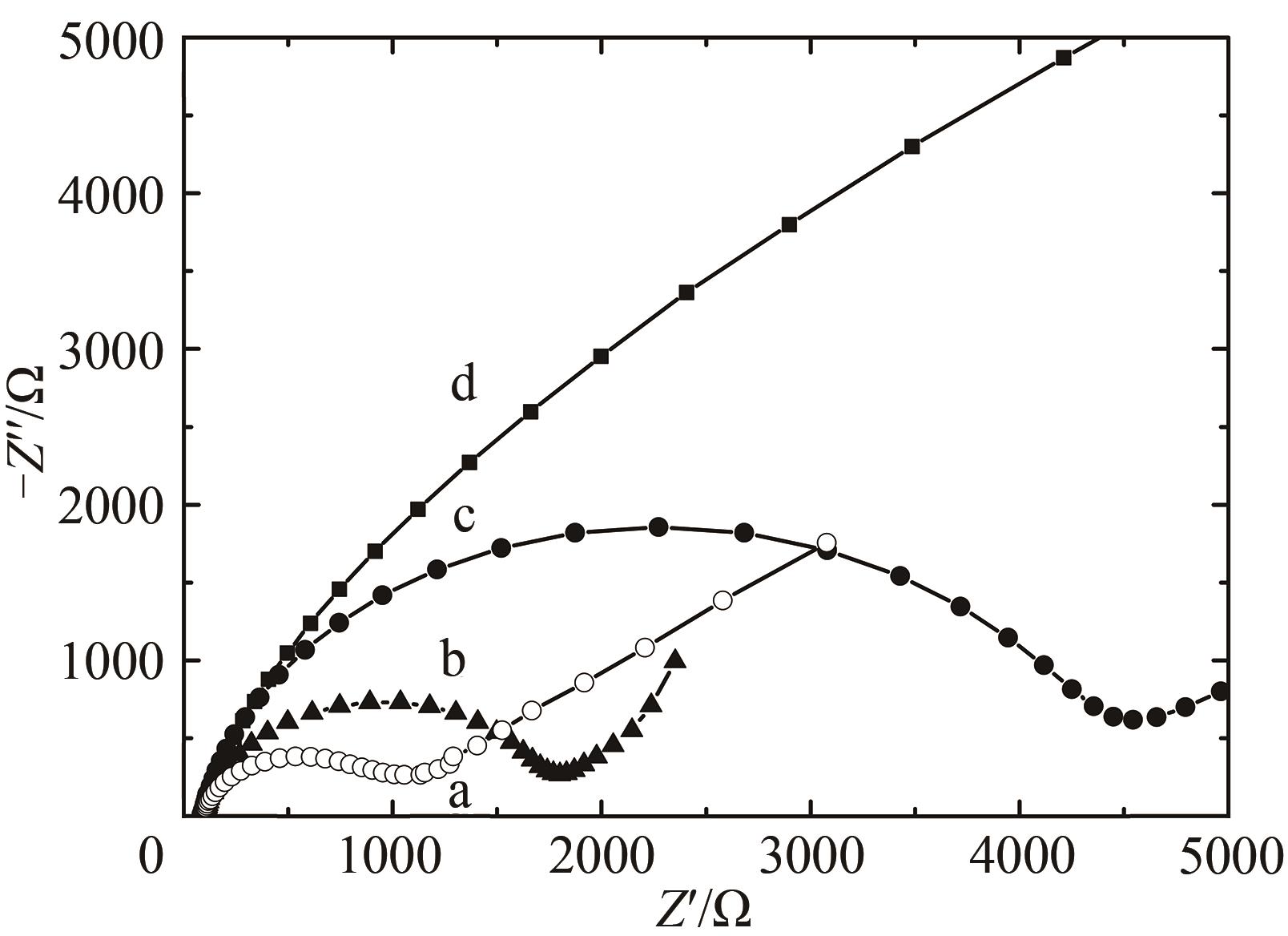
图9 交流阻抗Nyquist图(Z"vs Z')测试溶液:含0.1 mol/L KCl及2 mmol/L [Fe(CN)6]3-/4-的PBS(10 mmol/L,pH 7.0)溶液a—HRP/纳米金-噬菌体复合物/壳聚糖修饰玻碳电极;b—HRP/金纳米颗粒/壳聚糖修饰玻碳电极;c—HRP壳聚糖包埋修饰玻碳电极; d—裸玻碳电极
Fig. 9 Nyquist diagram (Z" vs Z') for Faradaic impedance spectra in the PBS (10 mmol/L, pH 7.0) containing 0.1 mol/L KCl and 2 mmol/L [Fe(CN)6]3-/4-a—HRP/Au-GM M13 electrode modified with chitosan; b—HRP/Au nanoparticles electrode modified with chitosan modified electrode; c—HRP electrode modified with chitosan; d—the glass-carbon electrode without the modification
| 1 | LUO H, SHI Z, LI N, et al. Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotube film on a glassy carbon electrode[J]. Analytical Chemistry, 2001, 73(5): 915-920. |
| 2 | HU M L, ABBASI-AZAD M, MORSALI A, et al. Electrochemical applications of ferrocene-based coordination polymers[J]. Chempluschem, 2021, 85(11): 2397-2418. |
| 3 | HUANG X C, ZHANG J R, ZHANG L L, et al. A sensitive H2O2 biosensor based on carbon nanotubes/tetrathiafulvalene and its application in detecting NADH[J]. Analytical Biochemistry, 2020, 589: 113493. |
| 4 | ITO K, OKUDA-SHIMAZAKI J, KOJIMA K, et al. Strategic design and improvement of the internal electron transfer of heme b domain-fused glucose dehydrogenase for use in direct electron transfer-type glucose sensors[J]. Biosensors and Bioelectronics, 2021, 176: 112911. |
| 5 | ZHAO M, GAO Y, SUN J Y, et al. Mediatorless glucose biosensor and direct electron transfer type glucose/air biofuel cell enabled with carbon nanodots[J]. Analytical Chemistry, 2015, 87(5): 2615-2622. |
| 6 | 袁盛建, 马迎飞. 噬菌体合成生物学研究进展和应用[J]. 合成生物学, 2020, 1(6): 635-655. |
| YUAN S J, MA Y F. Advances and applications of phage synthetic biology[J]. Synthetic Biology Journal, 2020, 1(6): 635-655. | |
| 7 | GUO Y C, ZHOU Y F, ZHANG X E, et al. Phage display mediated immuno-PCR[J]. Nucleic Acids Research, 2006, 34(8): e62. |
| 8 | SMITH G P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface[J]. Science, 1985, 228(4705): 1315-1317. |
| 9 | KEHOE J W, KAY B K. Filamentous phage display in the new millennium[J]. Chemical Reviews, 2005, 105(11): 4056-4072. |
| 10 | SMITH G P, PETRENKO V A. Phage display[J]. Chemical Reviews, 1997, 97(2): 391-410. |
| 11 | OH D, QI J, LU Y C, et al. Biologically enhanced cathode design for improved capacity and cycle life for lithium-oxygen batteries[J]. Nature Communications, 2013, 4: 2756. |
| 12 | LEE Y J, YI H, KIM W J, et al. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes[J]. Science, 2009, 324(5930): 1051-1055. |
| 13 | LEE S W, MAO C B, FLYNN C E, et al. Ordering of quantum dots using genetically engineered viruses[J]. Science, 2002, 296(5569): 892-895. |
| 14 | 张文静, 李明, 周维, 等. 基于病毒组件的纳米材料的自组装合成、功能化及应用[J]. 合成生物学, 2020, 1(3): 298-318. |
| ZHANG W J, LI M, ZHOU W, et al. Self-assembly, biosynthesis, functionalization and applications of virus-based nanomaterials[J]. Synthetic Biology Journal, 2020, 1(3): 298-318. | |
| 15 | ZHANG J T, KANKALA R K, MA J Y, et al. Hollow tobacco mosaic virus coat protein assisted self-assembly of one-dimensional nanoarchitectures[J]. Biomacromolecules, 2021, 22(2): 540-545. |
| 16 | ZHANG W J, ZHANG X N, LI F. Virus-based nanoparticles of Simian virus 40 in the field of nanobiotechnology[J]. Biotechnology Journal, 2018, 13(6): 1700619. |
| 17 | ZHANG S, YU H M, YANG J, et al. Design of the nanoarray pattern Fe-Ni bi-metal nanoparticles@M13 virus for the enhanced reduction of p-chloronitrobenzene through the micro-electrolysis effect[J]. Environmental Science: Nano, 2017, 4(4): 876-885. |
| 18 | YOO P J, NAM K T, QI J, et al. Spontaneous assembly of viruses on multilayered polymer surfaces[J]. Nature Materials, 2006, 5(3): 234-240. |
| 19 | CHO W, FOWLER J D, FURST E M, Targeted binding of the M 13 bacteriophage to thiamethoxam organic crystals[J]. Langmuir, 2012, 28(14): 6013-6020. |
| 20 | HUANG Y, CHIANG C Y, LEE S K, et al. Programmable assembly of nanoarchitectures using genetically engineered viruses[J]. Nano Letters, 2005, 5(7): 1429-1434. |
| 21 | YAMAMOTO K, SHI G Y, ZHOU T S, et al. Study of carbon nanotubes-HRP modified electrode and its application for novel on-line biosensors[J]. The Analyst, 2003, 128(3): 249-254. |
| 22 | LIU D L, WU Q, ZOU S, et al. Surface modification of cerasomes with AuNPs@poly(ionic liquid)s for an enhanced stereo biomimetic membrane electrochemical platform[J]. Bioelectrochemistry, 2020, 132: 107411. |
| 23 | WANG B, ZHANG J J, PAN Z Y, et al. A novel hydrogen peroxide sensor based on the direct electron transfer of horseradish peroxidase immobilized on silica-hydroxyapatite hybrid film[J]. Biosensors and Bioelectronics, 2009, 24(5): 1141-1145. |
| 24 | XIN J Y, DOU B X, WANG Z X, et al. Direct electrochemistry of methanobactin functionalized gold nanoparticles on Au electrode[J]. Journal of Nanoscience and Nanotechnology, 2018, 18(7): 4805-4813. |
| 25 | WANG J W, WANG L P, DI J W, et al. Electrodeposition of gold nanoparticles on indium/tin oxide electrode for fabrication of a disposable hydrogen peroxide biosensor[J]. Talanta, 2009, 77(4): 1454-1459. |
| 26 | QIAO Y, TAHARA K, ZHANG Q, et al. Cerasomes: soft interface for redox enzyme electrochemical signal transmission[J]. Chemistry - A European Journal, 2016, 22(4): 1340-1348. |
| 27 | HAMES B D, HOOPER N M, HOUGHTON J D. Instant Notes in Biochemistry [M]. London: Taylor & Francis, 1997: 67. |
| 28 | ZHANG H L, LAI G S, HAN D Y, et al. An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres[J]. Analytical and Bioanalytical Chemistry, 2008, 390(3): 971-977. |
| 29 | MARCUS R A, SUTIN N. Electron transfers in chemistry and biology[J]. Biochimica et Biophysica Acta, 1985, 811(3): 265-322. |
| 30 | CHEN H, JIANG J H, HUANG Y, et al. An electrochemical impedance immunosensor with signal amplification based on Au-colloid labeled antibody complex[J]. Sensors and Actuators B: Chemical, 2006, 117(1): 211-218. |
| 31 | HLELI S, MARTELET C, ABDELGHANI A, et al. An immunosensor for haemoglobin based on impedimetric properties of a new mixed self-assembled monolayer[J]. Materials Science and Engineering: C, 2006, 26(2/3): 322-327. |
| [1] | 赵静宇, 张健, 祁庆生, 王倩. 基于细菌双组分系统的生物传感器的研究进展[J]. 合成生物学, 2024, 5(1): 38-52. |
| [2] | 杜瑶, 高宏丹, 刘家坤, 刘孝荣, 邢志浩, 张涛, 马东礼. CRISPR-Cas系统在病原核酸检测中的研究进展[J]. 合成生物学, 2024, 5(1): 202-216. |
| [3] | 杨璐, 吴楠, 白茸茸, 董维亮, 周杰, 姜岷. 基因回路型全细胞微生物传感器的设计、优化与应用[J]. 合成生物学, 2022, 3(6): 1061-1080. |
| [4] | 张萍, 魏文平, 周英, 叶邦策. 解脂耶氏酵母中光控表达系统的构建及其应用研究[J]. 合成生物学, 2021, 2(5): 778-791. |
| [5] | 张博, 马永硕, 尚轶, 黄三文. 植物合成生物学研究进展[J]. 合成生物学, 2020, 1(2): 121-140. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||