合成生物学 ›› 2023, Vol. 4 ›› Issue (4): 651-675.DOI: 10.12211/2096-8280.2022-056
光酶催化合成进展
明阳, 陈彬, 黄小强
- 南京大学化学化工学院,南京大学化学与生物医药创新研究院,配位化学国家重点实验室,江苏 南京 210023
-
收稿日期:2022-10-10修回日期:2022-12-06出版日期:2023-08-31发布日期:2023-09-14 -
通讯作者:黄小强 -
作者简介:明阳 (1999—),女,博士研究生。研究方向为光酶催化不对称生物合成。E-mail:yang222ming@163.com黄小强 (1991—),男,特聘研究员,博士生导师。研究方向为交叉融合生物合成与化学合成。E-mail:huangx513@nju.edu.cn -
基金资助:国家自然科学基金面上项目(22277053);科技部重点研发计划(2022YFA0913000);江苏省青年基金(BK20220760)
Recent advances in photoenzymatic synthesis
MING Yang, CHEN Bin, HUANG Xiaoqiang
- State Key Laboratory of Coordination Chemistry,Chemistry and Biomedicine Innovation Center,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210023,Jiangsu,China
-
Received:2022-10-10Revised:2022-12-06Online:2023-08-31Published:2023-09-14 -
Contact:HUANG Xiaoqiang
摘要:
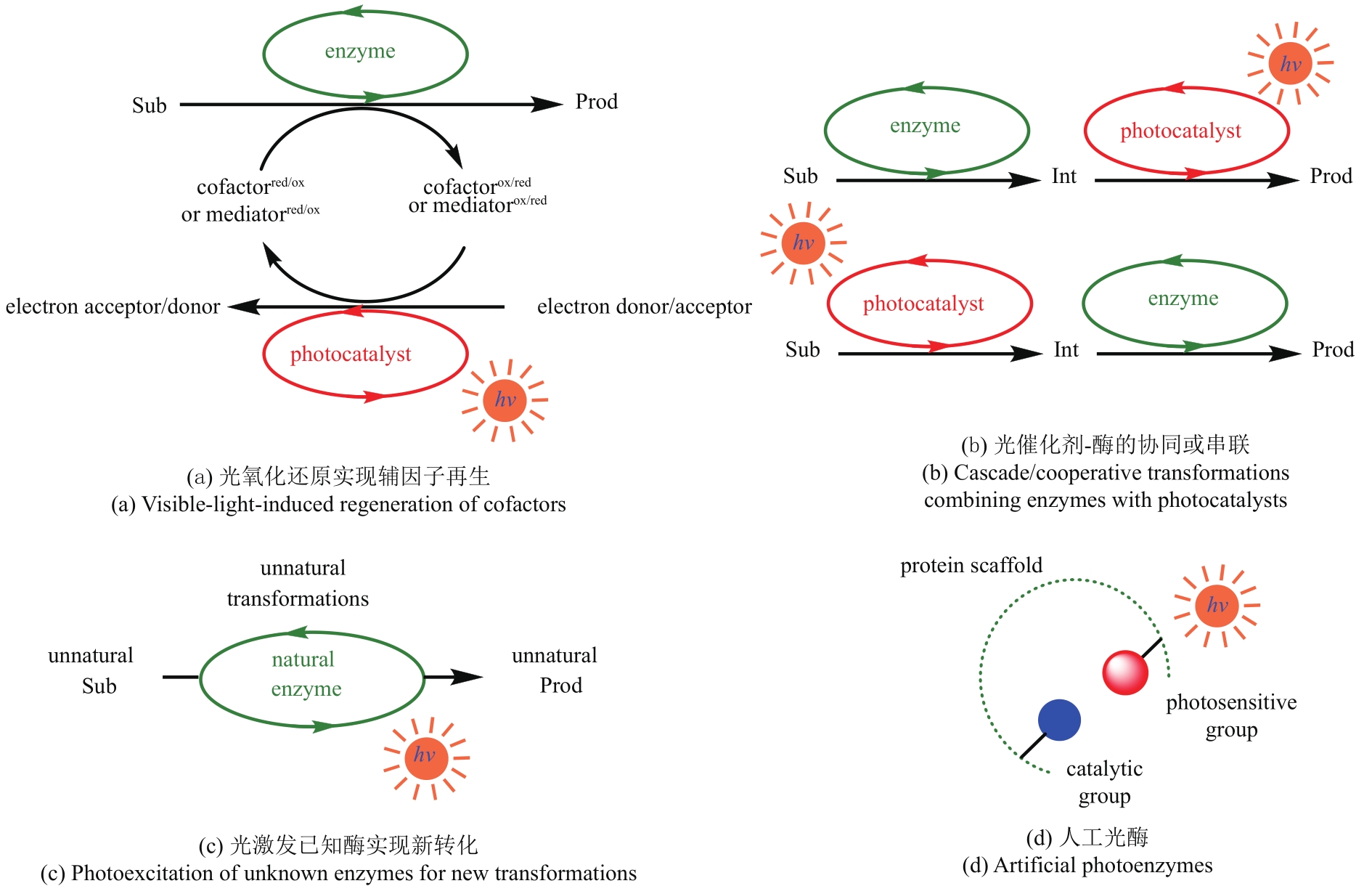
酶催化具有绿色温和、高效高选择性的优势,在工业生产和技术研发等领域发挥着重要作用。然而,酶能催化的反应类型相对有限,难以满足未来绿色生物合成的需要。光催化已成为在温和反应条件下生成活性反应中间体的有效策略,但是光化学反应的选择性调控一直是个挑战性难题。结合光催化与酶催化的光酶催化合成,能够突破天然酶催化功能的局限,并为光化学领域的选择性调控难题提供新的解决方案,成为合成科学领域的研究热点之一。本文综述了光酶催化合成的最新研究进展,根据光酶的结合模式分成四部分讨论:光氧化还原实现辅因子再生、光催化剂-酶的协同或串联反应、光激发已知酶实现新转化、人工光酶。本文归纳了近年来光酶催化合成的代表性工作,重点分析光酶催化反应的化学机制和实现新生物转化的策略。与此同时,通过分析该领域当下面临的瓶颈,本文展望了光酶催化未来的发展方向,希望能够为光酶催化新转化的开发和更多高附加值化学品的绿色不对称合成提供参考。
中图分类号:
引用本文
明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675.
MING Yang, CHEN Bin, HUANG Xiaoqiang. Recent advances in photoenzymatic synthesis[J]. Synthetic Biology Journal, 2023, 4(4): 651-675.
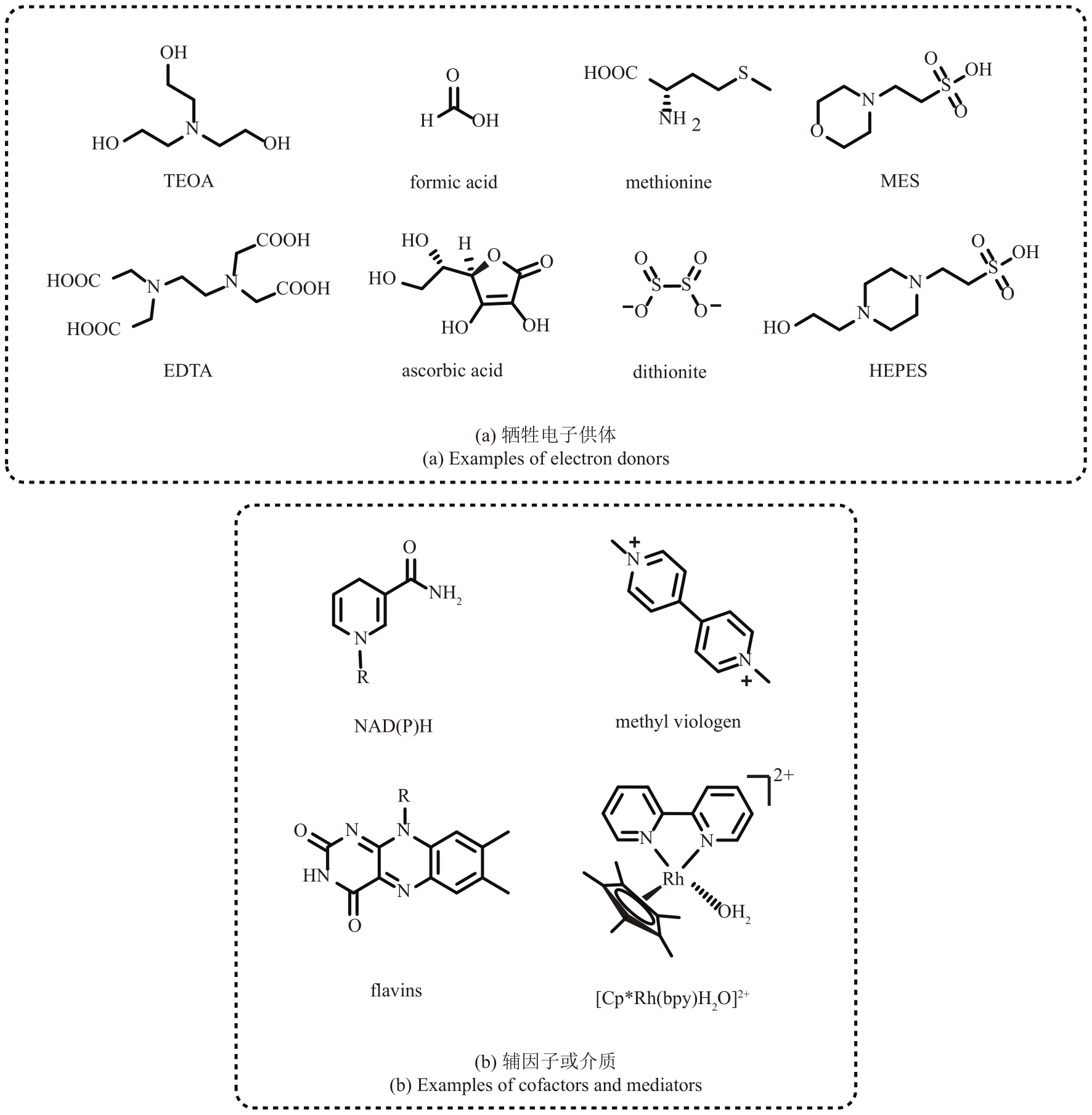
图2 光氧化还原实现辅因子再生系统中常见的牺牲电子供体、辅因子或介质
Fig. 2 Common sacrificial electron donors and cofactors/mediators in photoredox-enabled cofactor regeneration system
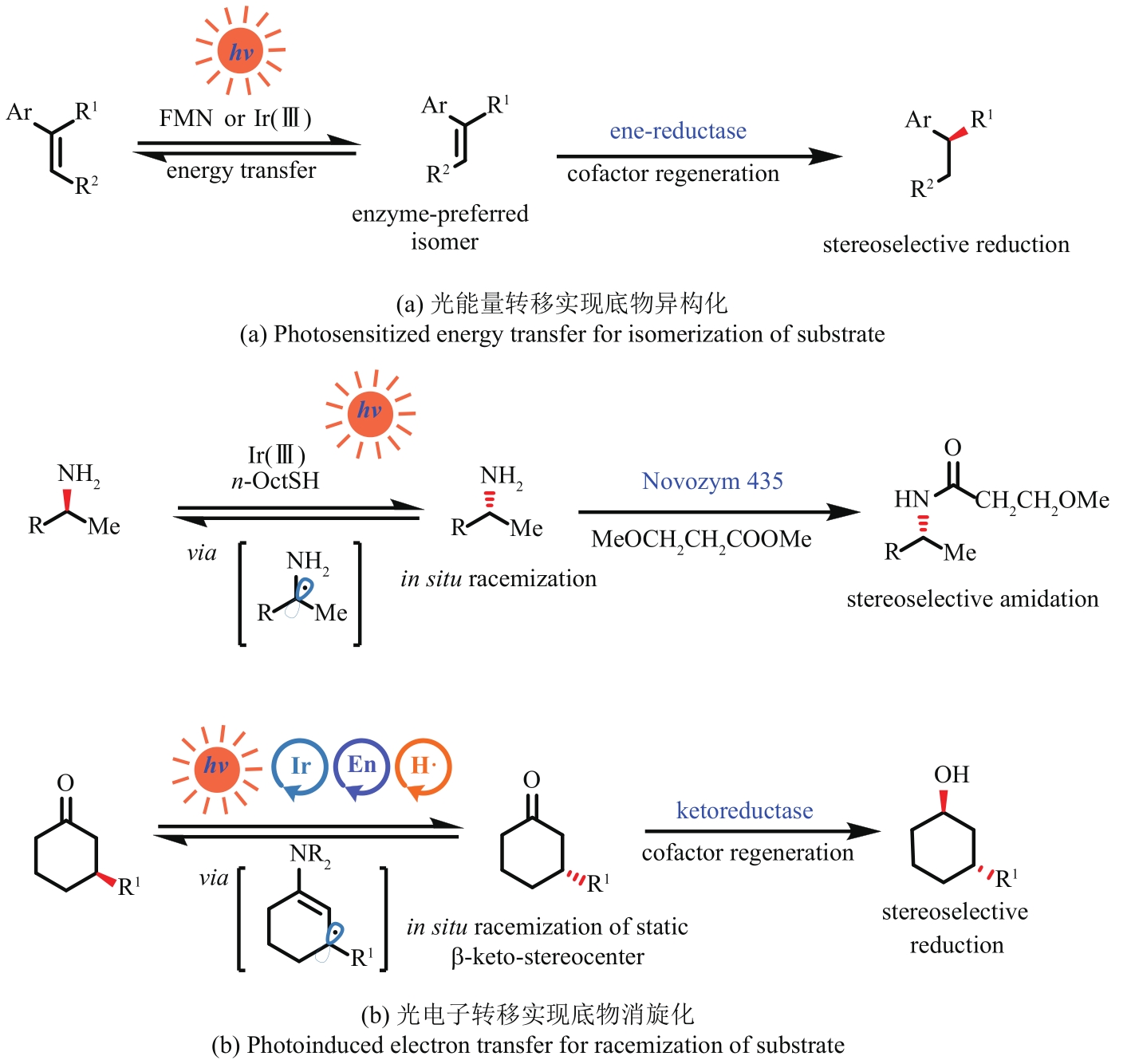
图4 光引发的能量/电子转移生成酶优选底物En—烯胺催化;H•—氢原子转移催化
Fig. 4 Convert substrate into enzyme-preferred ones by photoinduced energy/electron transferEn—enamine catalysis; H•—hydrogen atom transfer catalysis
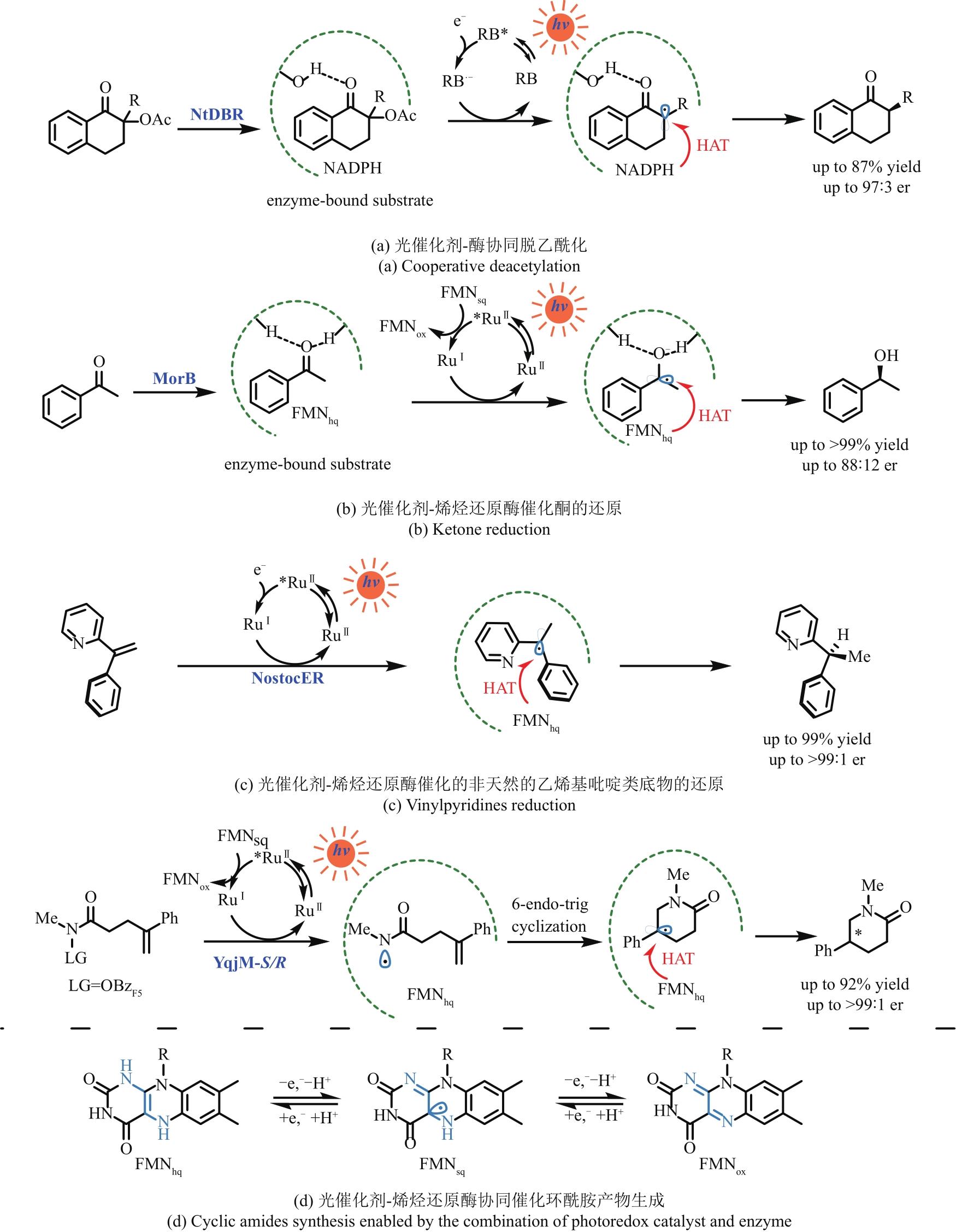
图5 光催化剂选择性还原酶活化的底物以完成非天然转化NtDBR—来自烟草的烟酰胺依赖性双键还原酶DBR;RB—孟加拉玫瑰红;MorB—来自普氏疟原虫的吗啡酮还原酶;RuⅡ—Ru(bpy)32+;NostocER—来自点型念珠蓝细菌的烯烃还原酶; YqjM-S/R—来自枯草芽孢杆菌的(S/R选择性)烯烃还原酶;OBzF5—五氟苯甲酰氧基
Fig. 5 Selected reductions of the enzyme activated substrates by photocatalysts to achieve unnatural transformationsRB—Rose Bengal; MorB—morphinone reductase from P. putida; RuⅡ—Ru(bpy)32+; NtDBR—Double bond reductase from Nicotiana tabacum; NostocER—Ene-Reductase from N. punctiforme; YqjM-S/R—Ene-Reductase from Bacillus subtili; OBzF5—perfluorobenzoyloxy
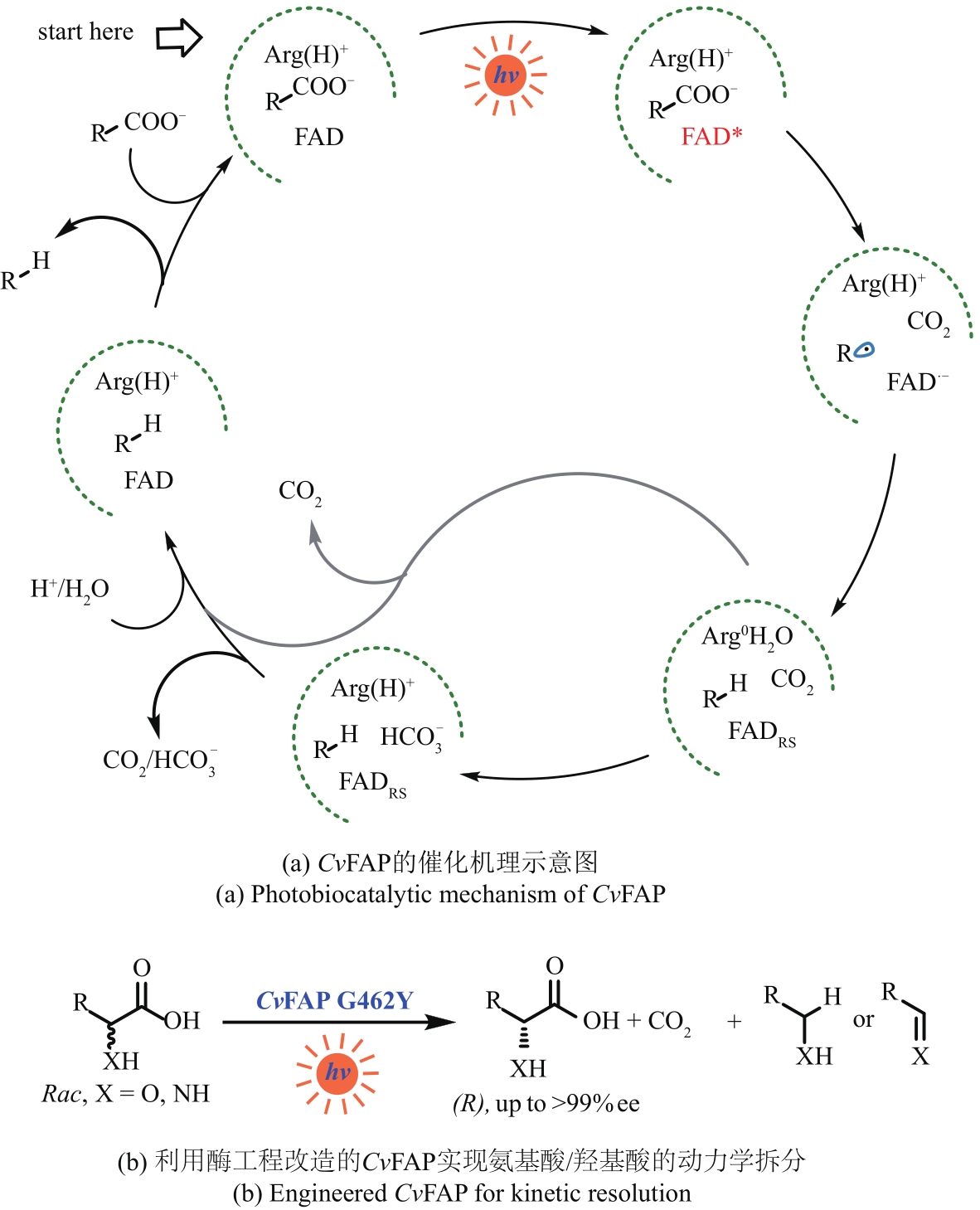
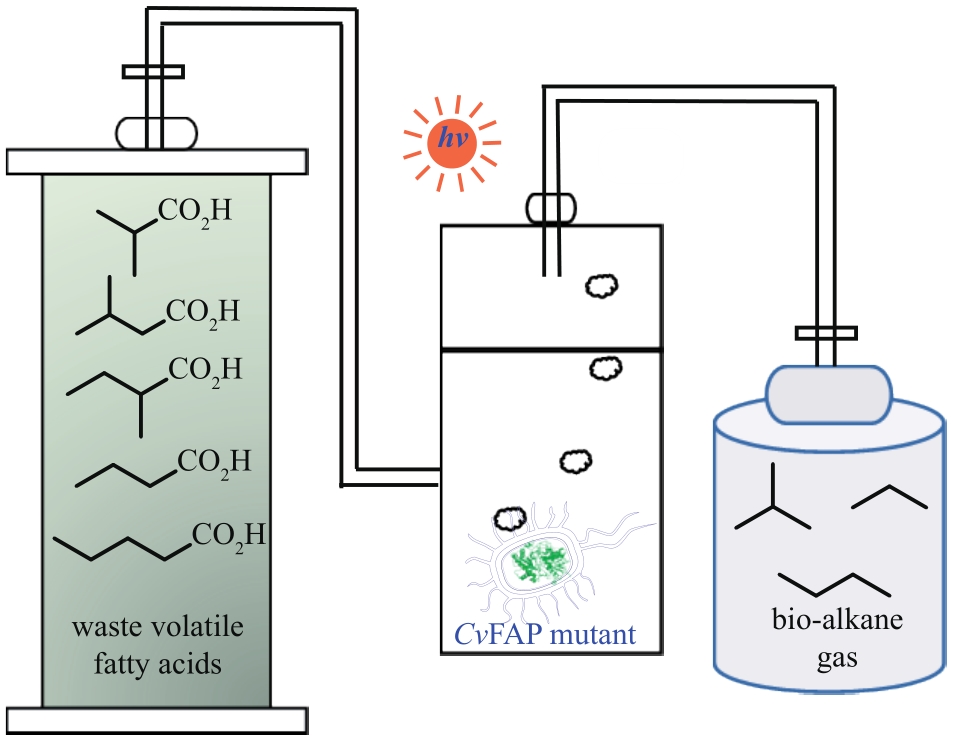
图7 CvFAP的催化机理及其应用实例Arg—精氨酸;FAD—核黄素腺嘌呤二核苷酸;FADRS—红移态的核黄素腺嘌呤二核苷酸;Rac—外消旋
Fig. 7 Photobiocatalytic mechanism of CvFAP and the application exampleArg—Arginine; FAD—flavin adenine dinucleotide; FADRS—red-shifted oxidized flavin; Rac—racemic
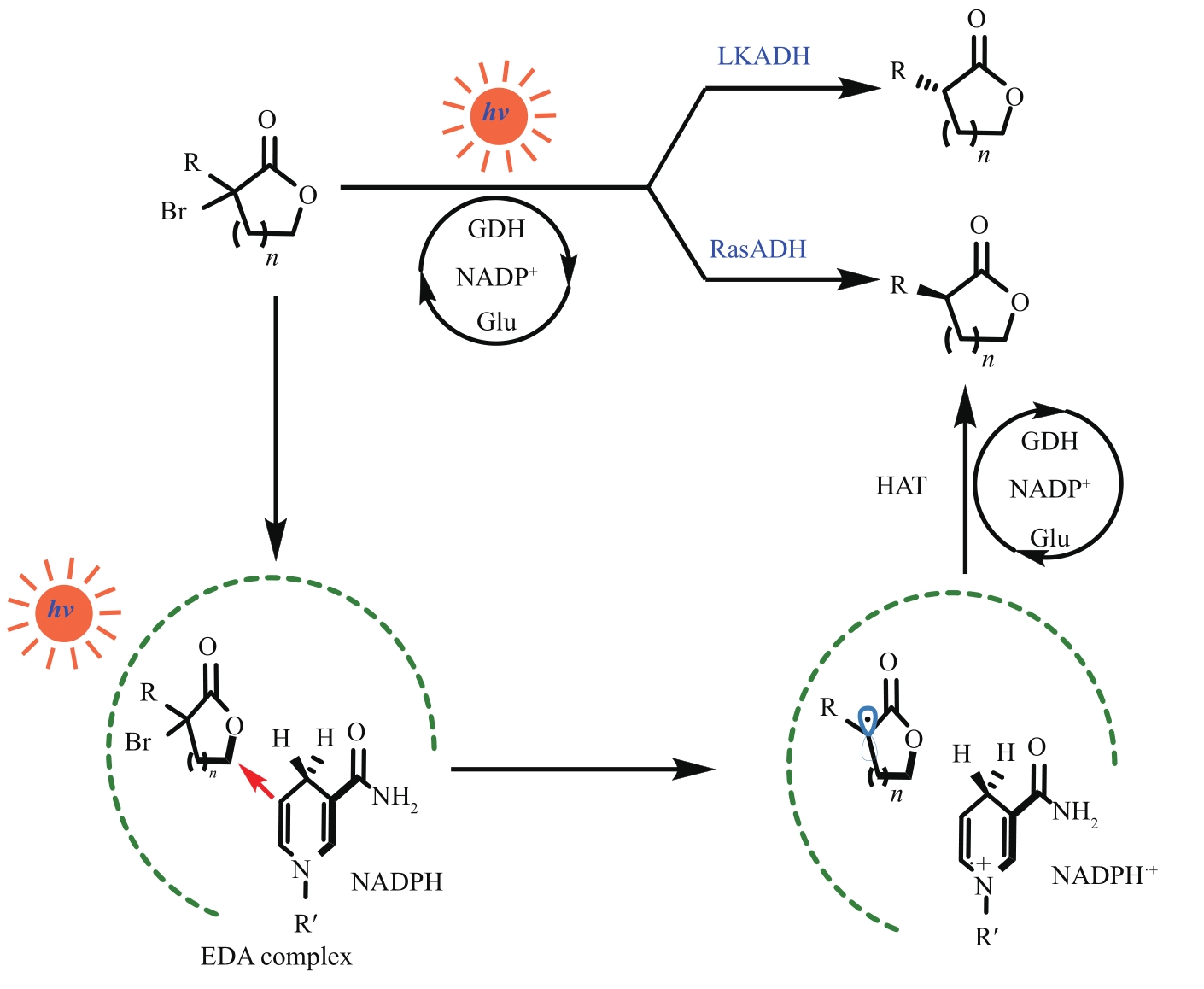
图9 光诱导醇脱氢酶实现卤代内酯的脱卤化LKADH—来自克菲里乳杆菌的短链脱氢酶;RasDH—来自雷氏菌属的短链脱氢酶
Fig. 9 Dehalogenation of halogenated lactones by light-induced alcohol dehydrogenaseLKADH—short-chain dehydrogenase from Lactobacillus kefiri; RasDH—short-chain dehydrogenase from Ralstonia species
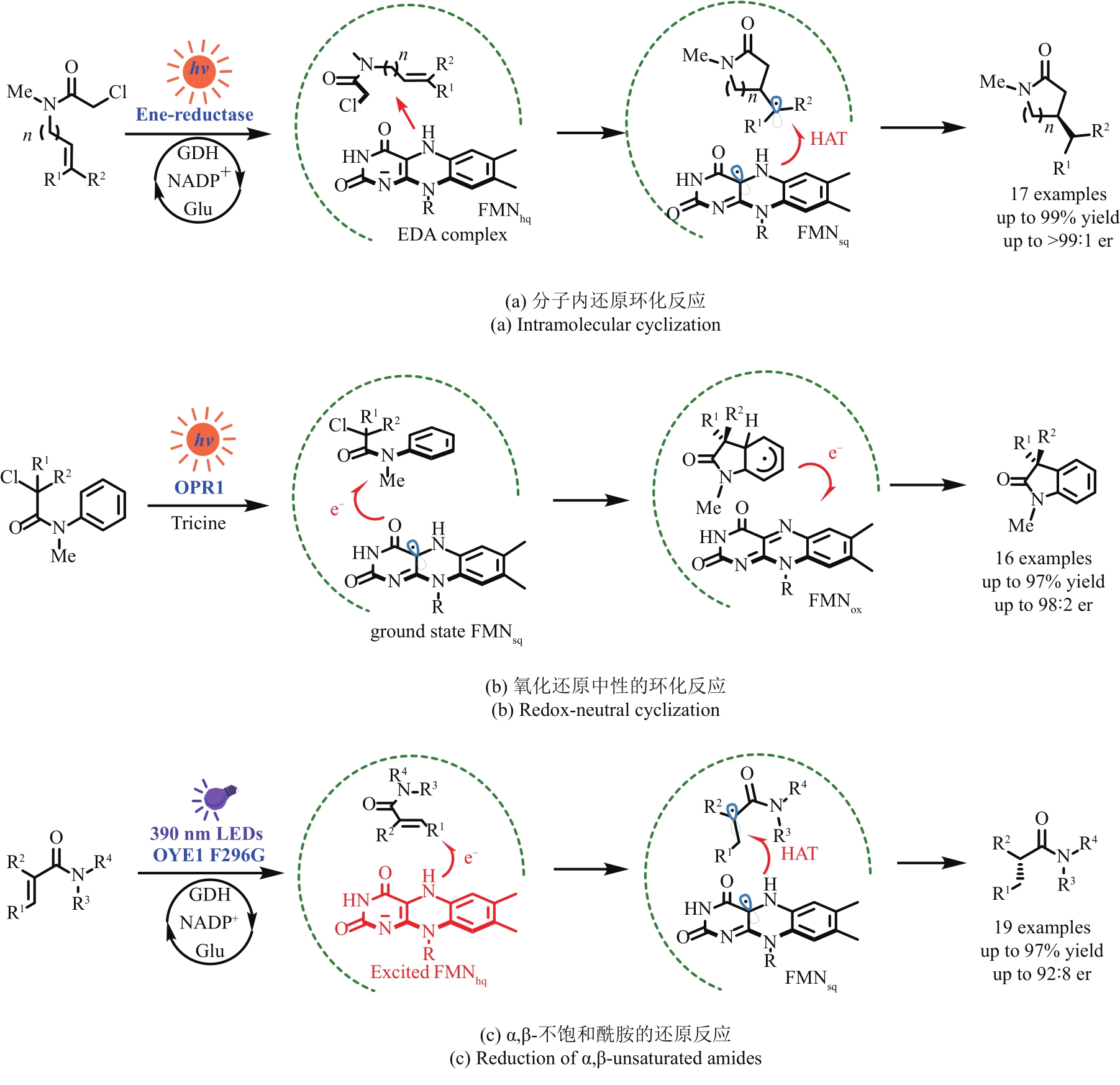
图11 光诱导烯烃还原酶实现的非天然转化OPR1—12-氧代二甲酸还原酶;OYE1 F298G—老黄酶1的F298G突变体
Fig. 11 Light-induced ene-reductase catalyzed unnatural transformationsOPR1—12-oxophytodienoate reductase; OYE1 F298G—mutants F298G of OYE1
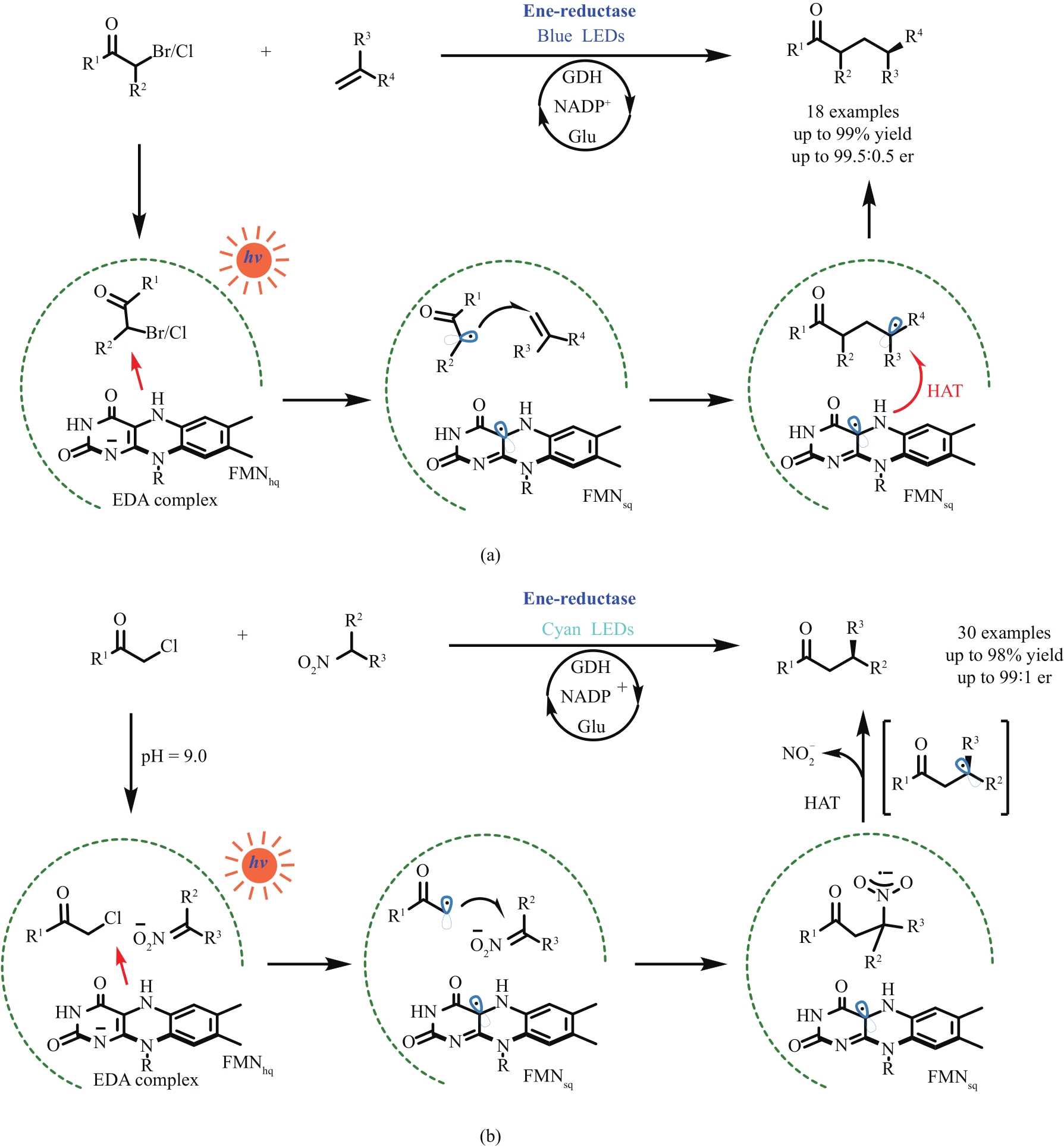
图12 光激发烯烃还原酶实现的分子间烯烃氢烷基化反应(a)和Csp3—Csp3亲电交叉偶联反应(b)
Fig. 12 Photoactivated ene-reductases enabled intermolecular reductive coupling couplings for alkene hydroalkylations (a) and Csp3—Csp3 bond formations (b)
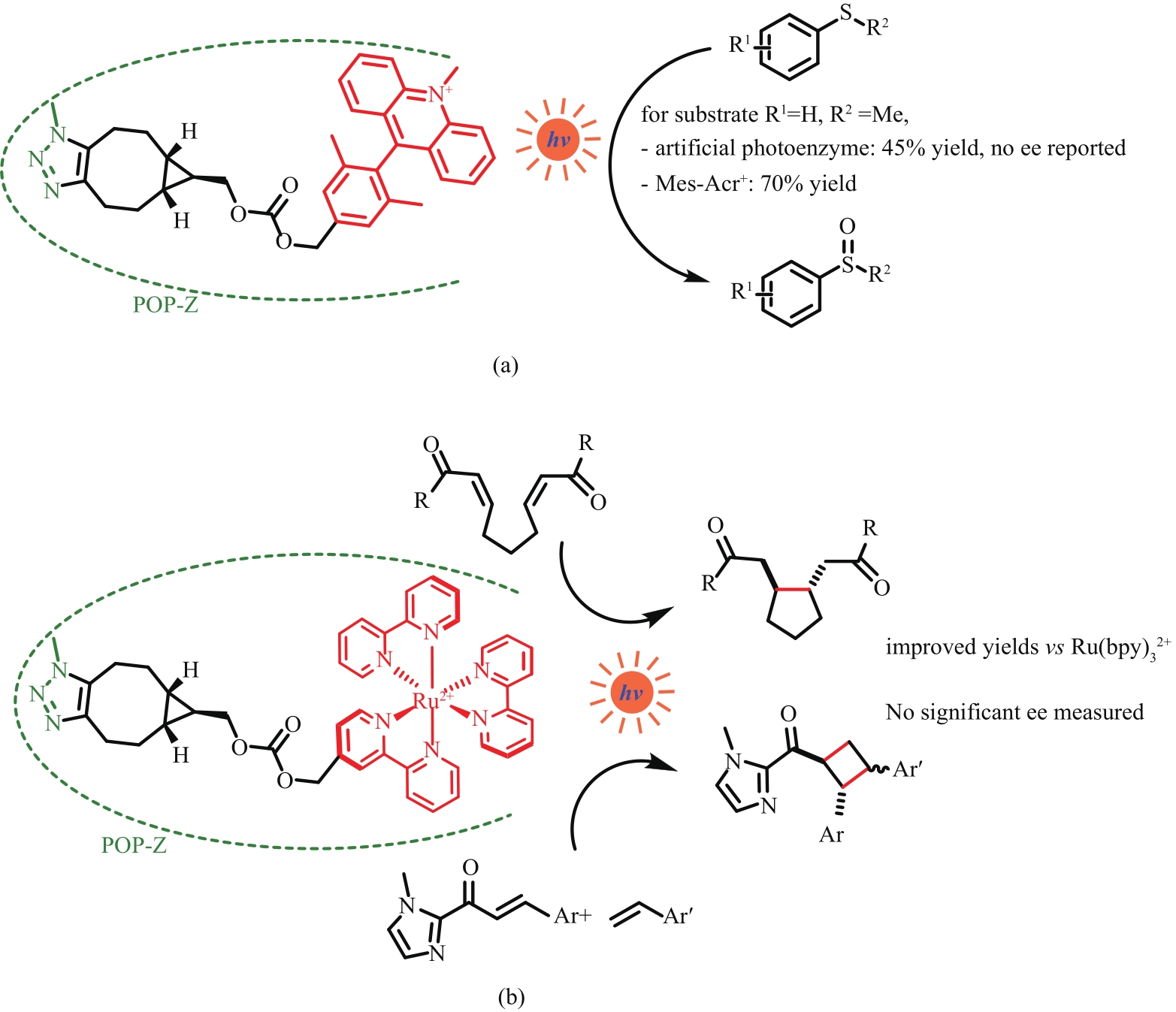
图13 通过点击化学将吖啶类光敏剂(a)、三联吡啶钌(b)引入蛋白POP-Z—引入非天然4-叠氮基-L-苯丙氨酸脯氨酰寡肽酶
Fig. 13 Introduction of acridine photosensitizer (a) and tris(2,2′-bipyridyl) rutheniumⅡ (b) into protein by clicking chemistryPOP-Z—p-azido-L-phenylalanine (Z) incorporated prolyl oligopeptidase (POP)
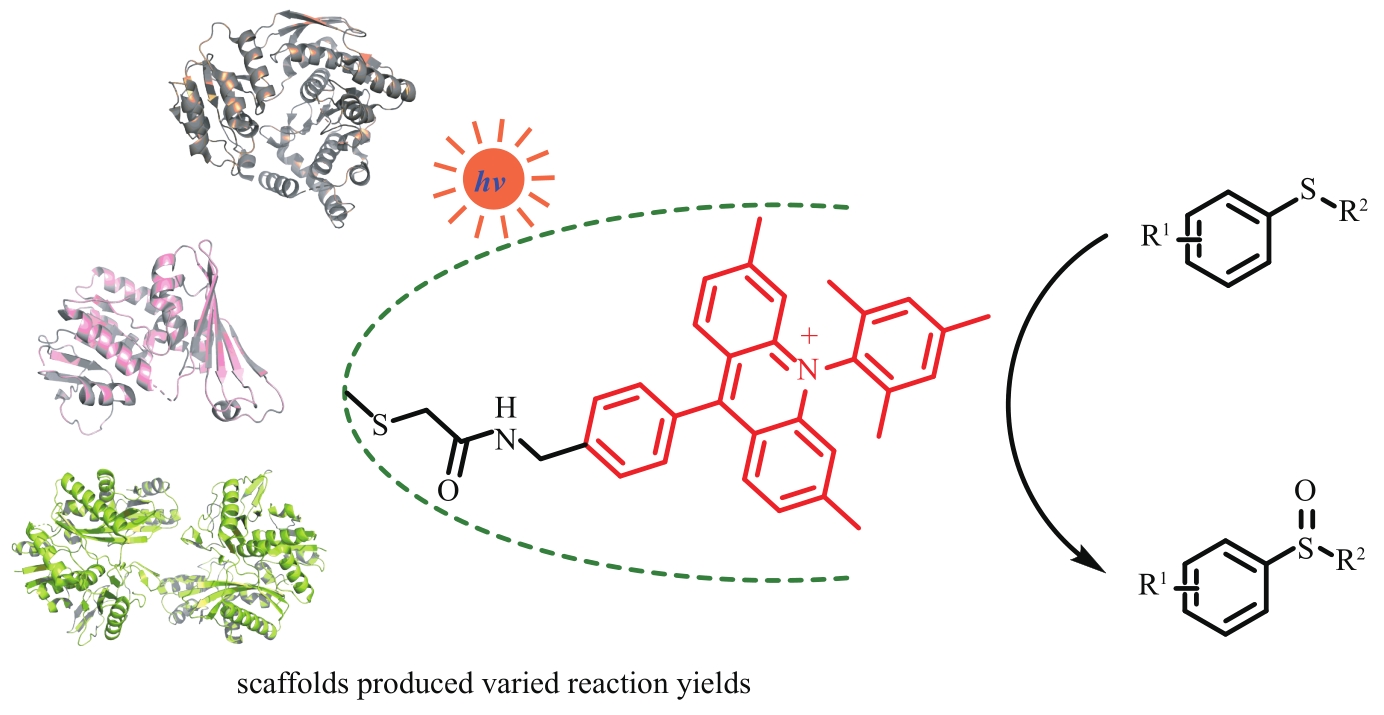
图14 通过半胱氨酸残基与碘代乙酰胺衍生物共价交联引入光敏剂构建不同人工光酶
Fig. 14 Construction of different artificial photoenzymes by introducing photosensitizers through covalent cross-linking of cysteine residues with iodoacetamide derivatives
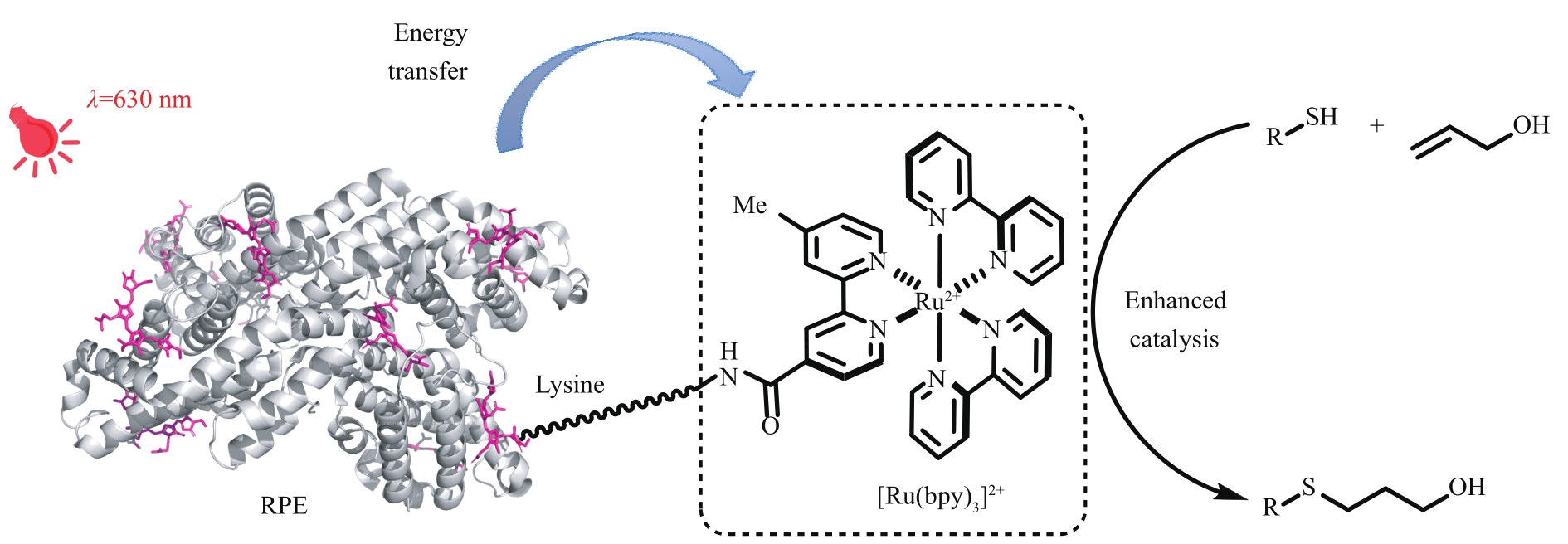
图15 通过光合吸光蛋白与光催化剂结合构建吸收低能光的光酶RPE—R-藻红蛋白,PDB 1EYX
Fig. 15 Construction of low energy absorption photoenzyme via the combination of photosynthetic light-harvesting protein and photocatalystRPE—R-phycoerythrin, PDB 1EYX
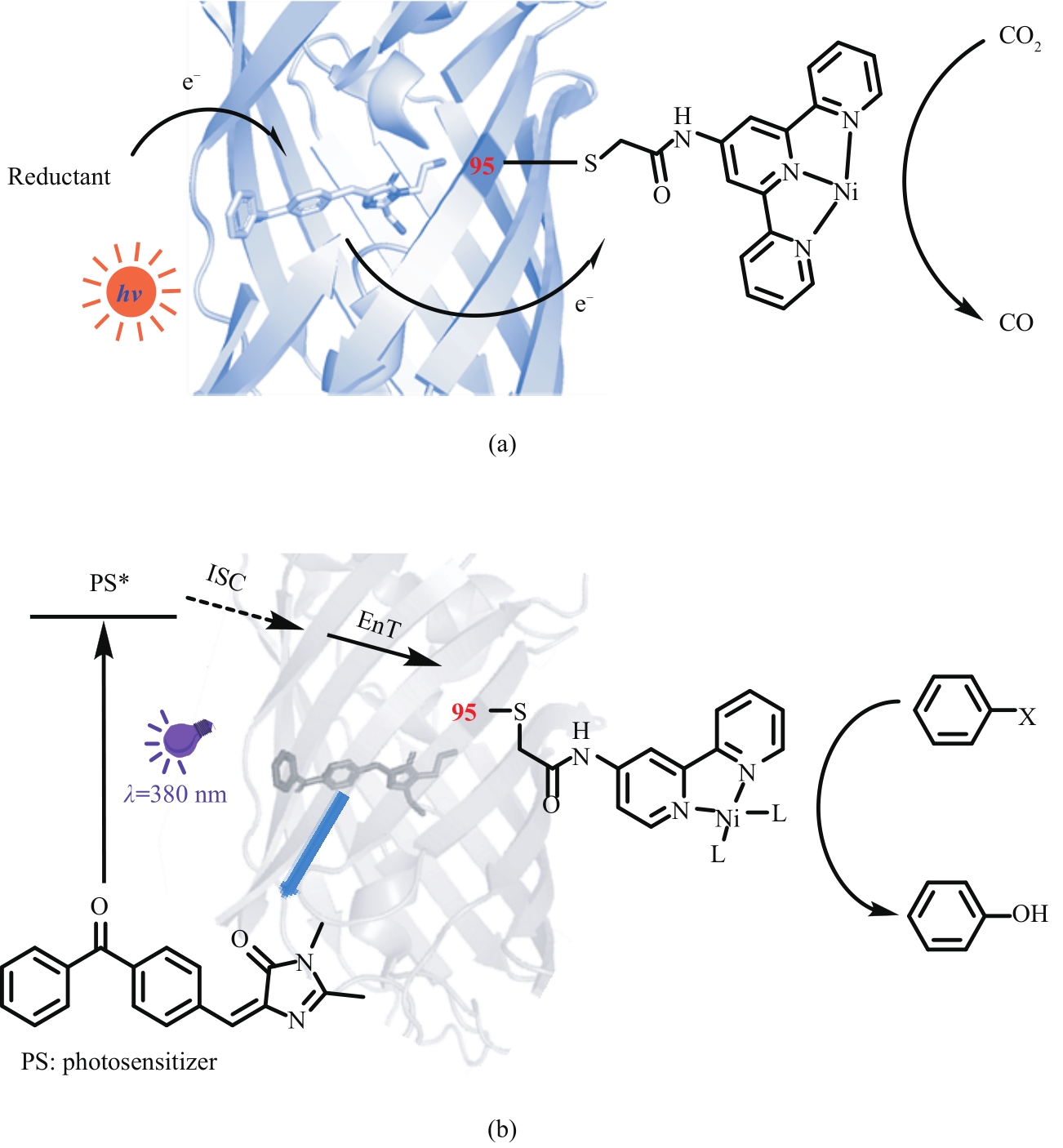
图16 具有二苯甲酮光敏基团的人工光酶催化二氧化碳的还原(a);卤代芳烃的脱卤羟化反应(b)
Fig. 16 Artificial photoenzymes with benzophenone photosensitive groups catalyze the reduction of carbon dioxide (a) and the dehalogenation and hydroxylation of aryl halides (b)
| 酶的种类 | 反应类型 | 参考文献 |
|---|---|---|
黄素依赖的 烯烃还原酶 | 利用光诱导能量转移促进烯烃异构化,酶优选底物发生对映选择性还原,实现立体汇聚式还原 | [ |
| 光催化剂-酶协同实现非天然底物的C=O、C=C双键的还原 | [ | |
| 光催化剂-酶协同实现不对称氢胺化反应 | [ | |
| 分子内自由基环化反应 | [ | |
| 氧化还原中性的不对称自由基环化反应 | [ | |
| 非天然底物α,β-不饱和酰胺的对映选择性还原 | [ | |
| α-卤代羰基化合物和烯烃的分子间氢烷基化反应 | [ | |
| 卤代烷烃和硝基烷烃的不对称Csp3—Csp3亲电交叉偶联反应 | [ | |
黄素依赖的 环己酮单加氧酶 | 光诱导酶催化的α-卤代-α-氟代酮的对映选择性还原脱卤反应 | [ |
烟酰胺依赖的 酮还原酶 | 结合光-小分子胺催化和酶催化,实现远程惰性C—H键的去消旋化 | [ |
| 卤代内酯的不对称自由基脱卤化反应 | [ | |
| 分子间自由基共轭加成反应 | [ | |
烟酰胺依赖的 双键还原酶 | 光催化剂-酶协同实现对映选择性脱乙酰氧基反应 | [ |
黄素依赖的 脂肪酸光脱羧酶 | 选择性催化S-构型底物的光脱羧反应,实现动力学拆分 | [ |
| 短链脂肪酸光脱羧、光脱羧氘化、反式脂肪酸的选择性光脱羧、生物燃料制造 | [ | |
| 人工光酶 | 催化硫茴香醚的氧化、二烯酮的分子内还原环化、[2+2]环加成反应以及硫醇与烯烃的偶联等 | [ |
| 二氧化碳还原、卤代芳烃脱卤羟化反应以及C—N键构建反应 | [ | |
| 紫外光激发插入的光敏非天然氨基酸,通过能量转移,实现对映选择性[2+2]环加成反应 | [ | |
| 过氧合酶 | 光催化剂-酶串联实现H2O2的原位生成及利用 | [ |
| 脂肪酶 | 通过光引发的电子转移促进底物消旋化,酶优选底物发生选择性酰胺化,实现动态动力学拆分 | [ |
| 光催化剂-酶串联实现2,2-二取代吲哚-3-酮的直接不对称合成 | [ |
表1 光酶种类及反应的总结
Table 1 Catalogue of photoenzymes and photoenzymatic reactions
| 酶的种类 | 反应类型 | 参考文献 |
|---|---|---|
黄素依赖的 烯烃还原酶 | 利用光诱导能量转移促进烯烃异构化,酶优选底物发生对映选择性还原,实现立体汇聚式还原 | [ |
| 光催化剂-酶协同实现非天然底物的C=O、C=C双键的还原 | [ | |
| 光催化剂-酶协同实现不对称氢胺化反应 | [ | |
| 分子内自由基环化反应 | [ | |
| 氧化还原中性的不对称自由基环化反应 | [ | |
| 非天然底物α,β-不饱和酰胺的对映选择性还原 | [ | |
| α-卤代羰基化合物和烯烃的分子间氢烷基化反应 | [ | |
| 卤代烷烃和硝基烷烃的不对称Csp3—Csp3亲电交叉偶联反应 | [ | |
黄素依赖的 环己酮单加氧酶 | 光诱导酶催化的α-卤代-α-氟代酮的对映选择性还原脱卤反应 | [ |
烟酰胺依赖的 酮还原酶 | 结合光-小分子胺催化和酶催化,实现远程惰性C—H键的去消旋化 | [ |
| 卤代内酯的不对称自由基脱卤化反应 | [ | |
| 分子间自由基共轭加成反应 | [ | |
烟酰胺依赖的 双键还原酶 | 光催化剂-酶协同实现对映选择性脱乙酰氧基反应 | [ |
黄素依赖的 脂肪酸光脱羧酶 | 选择性催化S-构型底物的光脱羧反应,实现动力学拆分 | [ |
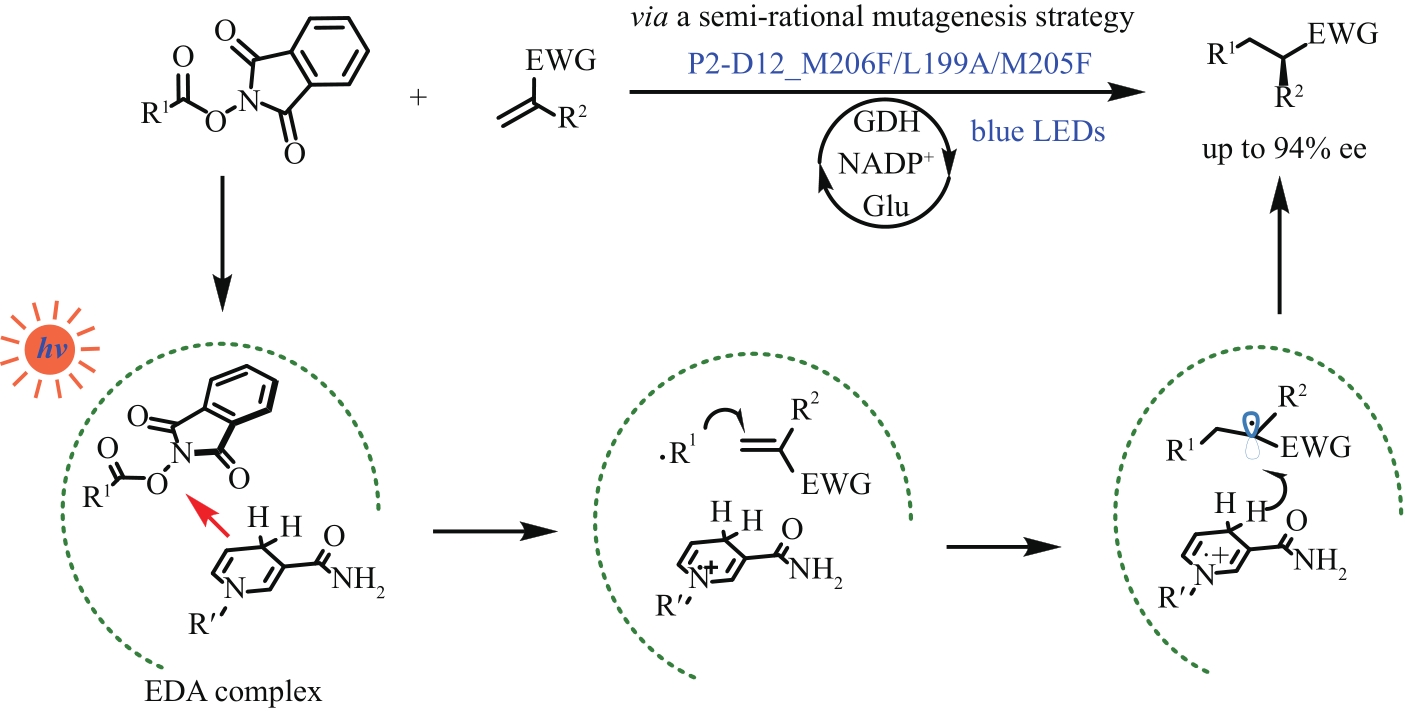
| 短链脂肪酸光脱羧、光脱羧氘化、反式脂肪酸的选择性光脱羧、生物燃料制造 | [ | |
| 人工光酶 | 催化硫茴香醚的氧化、二烯酮的分子内还原环化、[2+2]环加成反应以及硫醇与烯烃的偶联等 | [ |
| 二氧化碳还原、卤代芳烃脱卤羟化反应以及C—N键构建反应 | [ | |
| 紫外光激发插入的光敏非天然氨基酸,通过能量转移,实现对映选择性[2+2]环加成反应 | [ | |
| 过氧合酶 | 光催化剂-酶串联实现H2O2的原位生成及利用 | [ |
| 脂肪酶 | 通过光引发的电子转移促进底物消旋化,酶优选底物发生选择性酰胺化,实现动态动力学拆分 | [ |
| 光催化剂-酶串联实现2,2-二取代吲哚-3-酮的直接不对称合成 | [ |
| 1 | QU G, LI A T, ACEVEDO-ROCHA C G, et al. The crucial role of methodology development in directed evolution of selective enzymes[J]. Angewandte Chemie International Edition, 2020, 59(32): 13204-13231. |
| 2 | DEVINE P N, HOWARD R M, KUMAR R, et al. Extending the application of biocatalysis to meet the challenges of drug development[J]. Nature Reviews Chemistry, 2018, 2(12): 409-421. |
| 3 | SCHMID A, DORDICK J S, HAUER B, et al. Industrial biocatalysis today and tomorrow[J]. Nature, 2001, 409(6817): 258-268. |
| 4 | YI D, BAYER T, BADENHORST C P S, et al. Recent trends in biocatalysis[J]. Chemical Society Reviews, 2021, 50(14): 8003-8049. |
| 5 | PYSER J B, CHAKRABARTY S, ROMERO E O, et al. State-of-the-art biocatalysis[J]. ACS Central Science, 2021, 7(7): 1105-1116. |
| 6 | SAJEEV SURAJ C, SUMANT O. Enzymes market: opportunities and forecast[R/OL]. 2022, 2021-2031. (2022-07-17)[2022-11-01]. . |
| 7 | HANEFELD U, HOLLMANN F, PAUL C E. Biocatalysis making waves in organic chemistry[J]. Chemical Society Reviews, 2022, 51(2): 594-627. |
| 8 | PENG Y Z, CHEN Z C, XU J, et al. Recent advances in photobiocatalysis for selective organic synthesis[J]. Organic Process Research & Development, 2022, 26(7): 1900-1913. |
| 9 | CHEN K, ARNOLD F H. Engineering new catalytic activities in enzymes[J]. Nature Catalysis, 2020, 3(3): 203-213. |
| 10 | YOON T P, ISCHAY M A, DU J A. Visible light photocatalysis as a greener approach to photochemical synthesis[J]. Nature Chemistry, 2010, 2(7): 527-532. |
| 11 | CIAMICIAN G. The photochemistry of the future[J]. Science, 1912, 36(926): 385-394. |
| 12 | XU C P, RAVI ANUSUYADEVI P, AYMONIER C, et al. Nanostructured materials for photocatalysis[J]. Chemical Society Reviews, 2019, 48(14): 3868-3902. |
| 13 | HUANG X Q, MEGGERS E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes[J]. Accounts of Chemical Research, 2019, 52(3): 833-847. |
| 14 | BUZZETTI L, CRISENZA G E M, MELCHIORRE P. Mechanistic studies in photocatalysis[J]. Angewandte Chemie International Edition, 2019, 58(12): 3730-3747. |
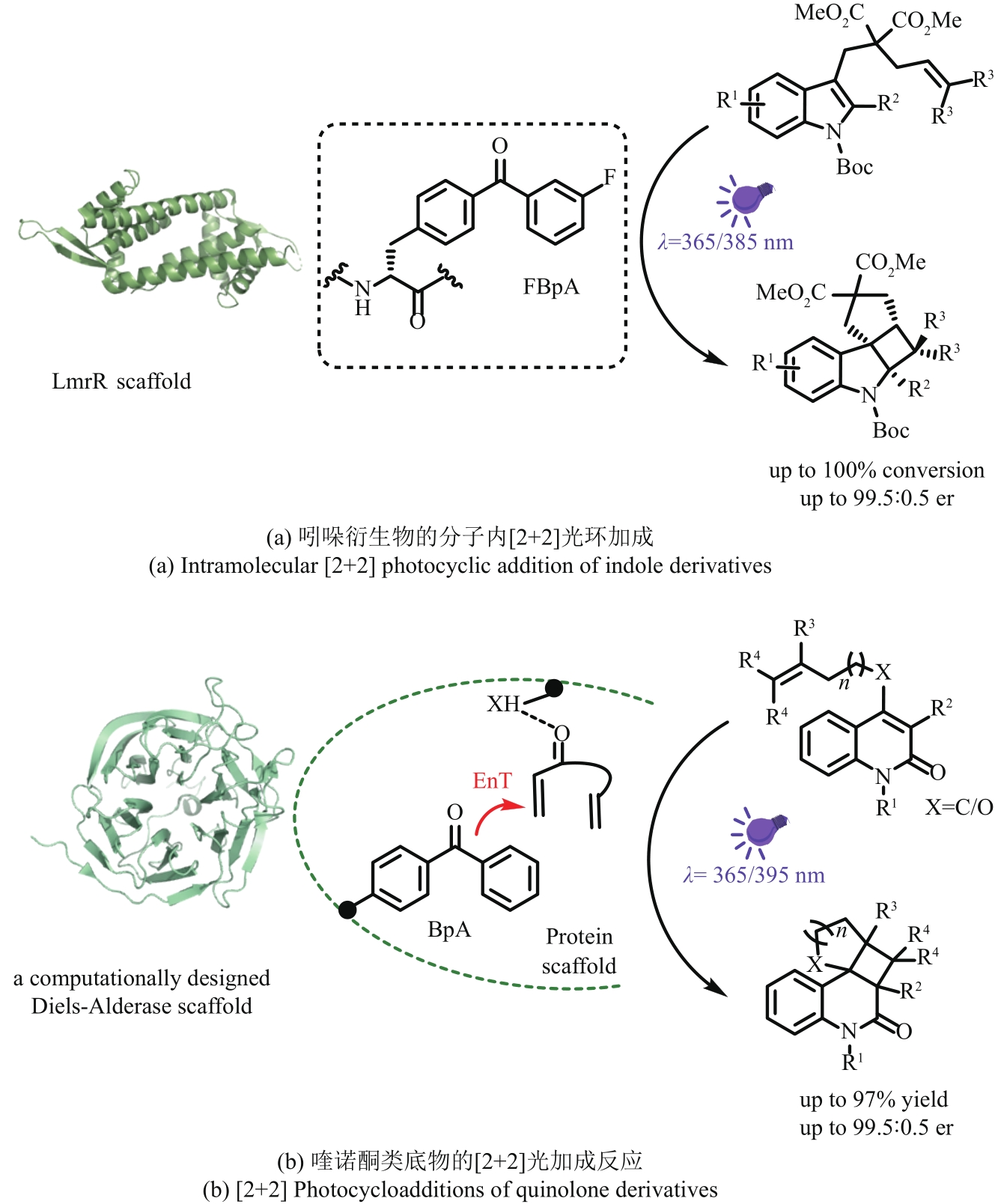
| 15 | ZHANG J N, HU W P, CAO S, et al. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting[J]. Nano Research, 2020, 13(9): 2313-2322. |
| 16 | FU J W, JIANG K X, QIU X Q, et al. Product selectivity of photocatalytic CO2 reduction reactions[J]. Materials Today, 2020, 32: 222-243. |
| 17 | CHEN D J, CHENG Y L, ZHOU N, et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: a review[J]. Journal of Cleaner Production, 2020, 268: 121725. |
| 18 | SILVESTRI S, FAJARDO A R, IGLESIAS B A. Supported porphyrins for the photocatalytic degradation of organic contaminants in water: a review[J]. Environmental Chemistry Letters, 2022, 20(1): 731-771. |
| 19 | XUAN J, HE X K, XIAO W J. Visible light-promoted ring-opening functionalization of three-membered carbo- and heterocycles[J]. Chemical Society Reviews, 2020, 49(9): 2546-2556. |
| 20 | YIN Y L, ZHAO X W, QIAO B K, et al. Cooperative photoredox and chiral hydrogen-bonding catalysis[J]. Organic Chemistry Frontiers, 2020, 7(10): 1283-1296. |
| 21 | CHAN A Y, PERRY I B, BISSONNETTE N B, et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis[J]. Chemical Reviews, 2022, 122(2): 1485-1542. |
| 22 | BRIMIOULLE R, LENHART D, MATURI M M, et al. Enantioselective catalysis of photochemical reactions[J]. Angewandte Chemie International Edition, 2015, 54(13): 3872-3890. |
| 23 | WANG C F, LU Z. Catalytic enantioselective organic transformations via visible light photocatalysis[J]. Organic Chemistry Frontiers, 2015, 2(2): 179-190. |
| 24 | MEGGERS E. Asymmetric catalysis activated by visible light[J]. Chemical Communications, 2015, 51(16): 3290-3301. |
| 25 | HARRISON W, HUANG X Q, ZHAO H M. Photobiocatalysis for abiological transformations[J]. Accounts of Chemical Research, 2022, 55(8): 1087-1096. |
| 26 | GUO X W, OKAMOTO Y, SCHREIER M R, et al. Enantioselective synthesis of amines by combining photoredox and enzymatic catalysis in a cyclic reaction network[J]. Chemical Science, 2018, 9(22): 5052-5056. |
| 27 | ZHANG W Y, BUREK B O, FERNÁNDEZ-FUEYO E, et al. Selective activation of C—H bonds in a cascade process combining photochemistry and biocatalysis[J]. Angewandte Chemie International Edition, 2017, 56(48): 15451-15455. |
| 28 | ÖZGEN F F, RUNDA M E, SCHMIDT S. Photo-biocatalytic cascades: combining chemical and enzymatic transformations fueled by light[J]. Chembiochem, 2021, 22(5): 790-806. |
| 29 | 蒋迎迎, 曲戈, 孙周通. 机器学习助力酶定向进化[J]. 生物学杂志, 2020, 37(4): 1-11. |
| JIANG Y Y, QU G, SUN Z T. Machine learning-assisted enzyme directed evolution[J]. Journal of Biology, 2020, 37(4): 1-11. | |
| 30 | WANG Y J, XUE P, CAO M F, et al. Directed evolution: methodologies and applications[J]. Chemical Reviews, 2021, 121(20): 12384-12444. |
| 31 | CHITNIS P R. PHOTOSYSTEM I: Function and physiology[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 2001, 52: 593-626. |
| 32 | GAO J L, WANG H, YUAN Q P, et al. Structure and function of the photosystem supercomplexes[J]. Frontiers in Plant Science, 2018, 9: 357. |
| 33 | KAVAKLI I H, BARIS I, TARDU M, et al. The photolyase/cryptochrome family of proteins as DNA repair enzymes and transcriptional repressors[J]. Photochemistry and Photobiology, 2017, 93(1): 93-103. |
| 34 | SANCAR A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors[J]. Chemical Reviews, 2003, 103(6): 2203-2237. |
| 35 | SORIGUÉ D, LÉGERET B, CUINÉ S, et al. An algal photoenzyme converts fatty acids to hydrocarbons[J]. Science, 2017, 357(6354): 903-907. |
| 36 | SORIGUÉ D, HADJIDEMETRIOU K, BLANGY S, et al. Mechanism and dynamics of fatty acid photodecarboxylase[J]. Science, 2021, 372(6538): eabd5687. |
| 37 | SCHMERMUND L, JURKAŠ V, ÖZGEN F F, et al. Photo-biocatalysis: biotransformations in the presence of light[J]. ACS Catalysis, 2019, 9(5): 4115-4144. |
| 38 | SEEL C J, GULDER T. Biocatalysis fueled by light: on the versatile combination of photocatalysis and enzymes[J]. Chembiochem, 2019, 20(15): 1871-1897. |
| 39 | LEE S H, CHOI D S, KUK S K, et al. Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons[J]. Angewandte Chemie International Edition, 2018, 57(27): 7958-7985. |
| 40 | 张武元, 袁波, 曲戈, 等. 光促酶催化反应设计及生物合成应用[J]. 生物学杂志, 2021, 38(5): 1-11. |
| ZHANG W Y, YUAN B, QU G, et al. Photobiocatalytic reaction design and its biosynthetic applications[J]. Journal of Biology, 2021, 38(5): 1-11. | |
| 41 | YUN C H, KIM J, HOLLMANN F, et al. Light-driven biocatalytic oxidation[J]. Chemical Science, 2022, 13(42): 12260-12279. |
| 42 | KIM J, LEE S H, TIEVES F, et al. Biocatalytic C=C bond reduction through carbon nanodot-sensitized regeneration of NADH analogues[J]. Angewandte Chemie International Edition, 2018, 57(42): 13825-13828. |
| 43 | SCHROEDER L, FRESE M, MÜLLER C, et al. Photochemically driven biocatalysis of halogenases for the green production of chlorinated compounds[J]. ChemCatChem, 2018, 10(15): 3336-3341. |
| 44 | CHENG Y Q, SHI J F, WU Y Z, et al. Intensifying electron utilization by surface-anchored Rh complex for enhanced nicotinamide cofactor regeneration and photoenzymatic CO2 reduction[J]. Research, 2021, 2021: 8175709. |
| 45 | ZHANG S H, ZHANG Y S, CHEN Y, et al. Metal hydride-embedded titania coating to coordinate electron transfer and enzyme protection in photo-enzymatic catalysis[J]. ACS Catalysis, 2021, 11(1): 476-483. |
| 46 | JIA J S, HUO Q, YANG D, et al. Granum-inspired photoenzyme-coupled catalytic system via stacked polymeric carbon nitride[J]. ACS Catalysis, 2021, 11(15): 9210-9220. |
| 47 | SUN Y Y, SHI J F, WANG Z, et al. Thylakoid membrane-inspired capsules with fortified cofactor shuttling for enzyme-photocoupled catalysis[J]. Journal of the American Chemical Society, 2022, 144(9): 4168-4177. |
| 48 | STRIETH-KALTHOFF F, JAMES M J, TEDERS M, et al. Energy transfer catalysis mediated by visible light: principles, applications, directions[J]. Chemical Society Reviews, 2018, 47(19): 7190-7202. |
| 49 | ZHANG Y, LIU H Z, SHI Q S, et al. Enantiocomplementary synthesis of vicinal fluoro alcohols through photo-bio cascade reactions[J]. Green Chemistry, 2022, 24(20): 7889-7893. |
| 50 | SUN Y Y, LI W P, WANG Z, et al. General framework for enzyme-photo-coupled catalytic system toward carbon dioxide conversion[J]. Current Opinion in Biotechnology, 2022, 73: 67-73. |
| 51 | LITMAN Z C, WANG Y J, ZHAO H M, et al. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis[J]. Nature, 2018, 560(7718): 355-359. |
| 52 | WANG Y J, HUANG X Q, HUI J S, et al. Stereoconvergent reduction of activated alkenes by a nicotinamide free synergistic photobiocatalytic system[J]. ACS Catalysis, 2020, 10(16): 9431-9437. |
| 53 | VERHO O, BÄCKVALL J E. Chemoenzymatic dynamic kinetic resolution: a powerful tool for the preparation of enantiomerically pure alcohols and amines[J]. Journal of the American Chemical Society, 2015, 137(12): 3996-4009. |
| 54 | BHAT V, WELIN E R, GUO X L, et al. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations[J]. Chemical Reviews, 2017, 117(5): 4528-4561. |
| 55 | YANG Q, ZHAO F Q, ZHANG N, et al. Mild dynamic kinetic resolution of amines by coupled visible-light photoredox and enzyme catalysis[J]. Chemical Communications, 2018, 54(100): 14065-14068. |
| 56 | DEHOVITZ J S, LOH Y Y, KAUTZKY J A, et al. Static to inducibly dynamic stereocontrol: the convergent use of racemic β-substituted ketones[J]. Science, 2020, 369(6507): 1113-1118. |
| 57 | PIRNOT M T, RANKIC D A, MARTIN D B C, et al. Photoredox activation for the direct β-arylation of ketones and aldehydes[J]. Science, 2013, 339(6127): 1593-1596. |
| 58 | BIEGASIEWICZ K F, COOPER S J, EMMANUEL M A, et al. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases[J]. Nature Chemistry, 2018, 10(7): 770-775. |
| 59 | SANDOVAL B A, KURTOIC S I, CHUNG M M, et al. Photoenzymatic catalysis enables radical-mediated ketone reduction in ene-reductases[J]. Angewandte Chemie International Edition, 2019, 58(26): 8714-8718. |
| 60 | NAKANO Y, BLACK M J, MEICHAN A J, et al. Photoenzymatic hydrogenation of heteroaromatic olefins using 'ene'-reductases with photoredox catalysts[J]. Angewandte Chemie International Edition, 2020, 59(26): 10484-10488. |
| 61 | YE Y X, CAO J Z, OBLINSKY D G, et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination[J]. Nature Chemistry, 2023, 15(2): 206-212. |
| 62 | LAUDER K, TOSCANI A, QI Y Y, et al. Photo-biocatalytic one-pot cascades for the enantioselective synthesis of 1, 3-mercaptoalkanol volatile sulfur compounds[J]. Angewandte Chemie International Edition, 2018, 57(20): 5803-5807. |
| 63 | YU Y, LIN R D, YAO Y, et al. Development of a metal- and oxidant-free enzyme-photocatalyst hybrid system for highly efficient C-3 acylation reactions of indoles with aldehydes[J]. ACS Catalysis, 2022, 12(20): 12543-12554. |
| 64 | DING X, DONG C L, GUAN Z, et al. Concurrent asymmetric reactions combining photocatalysis and enzyme catalysis: direct enantioselective synthesis of 2,2-disubstituted indol-3-ones from 2-arylindoles[J]. Angewandte Chemie International Edition, 2019, 58(1): 118-124. |
| 65 | BUREK B O, BORMANN S, HOLLMANN F, et al. Hydrogen peroxide driven biocatalysis[J]. Green Chemistry, 2019, 21(12): 3232-3249. |
| 66 | LÜTZ S, STECKHAN E, LIESE A. First asymmetric electroenzymatic oxidation catalyzed by a peroxidase[J]. Electrochemistry Communications, 2004, 6(6): 583-587. |
| 67 | ZHANG W Y, FERNÁNDEZ-FUEYO E, NI Y, et al. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalizations[J]. Nature Catalysis, 2018, 1(1): 55-62. |
| 68 | YUAN B, MAHOR D, FEI Q, et al. Water-soluble anthraquinone photocatalysts enable methanol-driven enzymatic halogenation and hydroxylation reactions[J]. ACS Catalysis, 2020, 10(15): 8277-8284. |
| 69 | ZHANG W Y, MA M, HUIJBERS M M E, et al. Hydrocarbon synthesis via photoenzymatic decarboxylation of carboxylic acids[J]. Journal of the American Chemical Society, 2019, 141(7): 3116-3120. |
| 70 | XU J, HU Y J, FAN J J, et al. Light-driven kinetic resolution of α-functionalized carboxylic acids enabled by an engineered fatty acid photodecarboxylase[J]. Angewandte Chemie International Edition, 2019, 58(25): 8474-8478. |
| 71 | LI D Y, WU Q, REETZ M T. Focused rational iterative site-specific mutagenesis (FRISM)[J]. Methods in Enzymology, 2020, 643: 225-242. |
| 72 | XU J, FAN J J, LOU Y J, et al. Light-driven decarboxylative deuteration enabled by a divergently engineered photodecarboxylase[J]. Nature Communications, 2021, 12: 3983. |
| 73 | LI D Y, HAN T, XUE J D, et al. Engineering fatty acid photodecarboxylase to enable highly selective decarboxylation of trans fatty acids[J]. Angewandte Chemie International Edtion, 2021, 60(38): 20695-20699. |
| 74 | GE R, ZHANG P P, DONG X T, et al. Photobiocatalytic decarboxylation for the synthesis of fatty epoxides from renewable fatty acids[J]. ChemSusChem, 2022, 15(20): e202201275. |
| 75 | AMER M, WOJCIK E Z, SUN C H, et al. Low carbon strategies for sustainable bio-alkane gas production and renewable energy[J]. Energy & Environmental Science, 2020, 13(6): 1818-1831. |
| 76 | BRUDER S, MOLDENHAUER E J, LEMKE R D, et al. Drop-in biofuel production using fatty acid photodecarboxylase from Chlorella variabilis in the oleaginous yeast Yarrowia lipolytica [J]. Biotechnology for Biofuels, 2019, 12: 202. |
| 77 | XU W H, MOU K H, ZHOU H N, et al. Transformation of triolein to biogasoline by photo-chemo-biocatalysis[J]. Green Chemistry, 2022, 24(17): 6589-6598. |
| 78 | SELLÉS VIDAL L, KELLY C L, MORDAKA P M, et al. Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application[J]. Biochimica et Biophysica Acta-Proteins and Proteomics, 2018, 1866(2): 327-347. |
| 79 | TOOGOOD H S, SCRUTTON N S. Discovery, characterisation, engineering and applications of ene reductases for industrial biocatalysis[J]. ACS Catalysis, 2019, 8(4): 3532-3549. |
| 80 | MULLIKEN R S. Molecular compounds and their spectra. Ⅱ[J]. Journal of the American Chemical Society, 1952, 74(3): 811-824. |
| 81 | CRISENZA G E M, MAZZARELLA D, MELCHIORRE P. Synthetic methods driven by the photoactivity of electron donor-acceptor complexes[J]. Journal of the American Chemical Society, 2020, 142(12): 5461-5476. |
| 82 | EMMANUEL M A, GREENBERG N R, OBLINSKY D G, et al. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light[J]. Nature, 2016, 540(7633): 414-417. |
| 83 | PENG Y Z, WANG Z G, CHEN Y, et al. Photoinduced promiscuity of cyclohexanone monooxygenase for the enantioselective synthesis of α-fluoroketones[J]. Angewandte Chemie International Edtion, 2022, 61(50): e202211199. |
| 84 | HUANG X Q, FENG J Q, CUI J W, et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition[J]. Nature Catalysis, 2022, 5(7): 586-593. |
| 85 | XU J, CEN Y X, SINGH W, et al. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters[J]. Journal of the American Chemical Society, 2019, 141(19): 7934-7945. |
| 86 | BIEGASIEWICZ K F, COOPER S J, GAO X, et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization[J]. Science, 2019, 364(6446): 1166-1169. |
| 87 | LAGUERRE N, RIEHL P S, OBLINSKY D G, et al. Radical termination via β-scission enables photoenzymatic allylic alkylation using "ene"-reductases[J]. ACS Catalysis, 2022, 12(15): 9801-9805. |
| 88 | BLACK M J, BIEGASIEWICZ K F, MEICHAN A J, et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent 'ene'-reductases[J]. Nature Chemistry, 2020, 12(1): 71-75. |
| 89 | SANDOVAL B A, CLAYMAN P D, OBLINSKY D G, et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent 'ene'-reductases[J]. Journal of the American Chemical Society, 2021, 143(4): 1735-1739. |
| 90 | HUANG X Q, WANG B J, WANG Y J, et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation[J]. Nature, 2020, 584(7819): 69-74. |
| 91 | PAGE C G, COOPER S J, DEHOVITZ J S, et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins[J]. Journal of the American Chemical Society, 2021, 143(1): 97-102. |
| 92 | FU H G, LAM H, EMMANUEL M A, et al. Ground-state electron transfer as an initiation mechanism for biocatalytic C—C bond forming reactions[J]. Journal of the American Chemical Society, 2021, 143(25): 9622-9629. |
| 93 | FU H G, CAO J Z, QIAO T Z, et al. An asymmetric sp3-sp3 cross-electrophile coupling using 'ene'-reductases[J]. Nature, 2022, 610(7931): 302-307. |
| 94 | GU Y F, ELLIS-GUARDIOLA K, SRIVASTAVA P, et al. Preparation, characterization, and oxygenase activity of a photocatalytic artificial enzyme[J]. Chembiochem, 2015, 16(13): 1880-1883. |
| 95 | ZUBI Y S, LIU B Q, GU Y F, et al. Controlling the optical and catalytic properties of artificial metalloenzyme photocatalysts using chemogenetic engineering[J]. Chemical Science, 2022, 13(5): 1459-1468. |
| 96 | SOSA V, MELKIE M, SULCA C, et al. Selective light-driven chemoenzymatic trifluoromethylation/hydroxylation of substituted arenes[J]. ACS Catalysis, 2018, 8(3): 2225-2229. |
| 97 | SCHWOCHERT T D, CRUZ C L, WATTERS J W, et al. Design and evaluation of artificial hybrid photoredox biocatalysts[J]. Chembiochem, 2020, 21(21): 3146-3150. |
| 98 | CESANA P T, LI B X, SHEPARD S G, et al. A biohybrid strategy for enabling photoredox catalysis with low-energy light[J]. Chem, 2022, 8(1): 174-185. |
| 99 | CESANA P T, PAGE C G, HARRIS D, et al. Photoenzymatic catalysis in a new light: Gluconobacter "ene"-reductase conjugates possessing high-energy reactivity with tunable low-energy excitation[J]. Journal of the American Chemical Society, 2022, 144(38): 17516-17521. |
| 100 | YU Y, LIU X H, WANG J Y. Expansion of redox chemistry in designer metalloenzymes[J]. Accounts of Chemical Research, 2019, 52(3): 557-565. |
| 101 | LIU X H, LIU P C, LI H J, et al. Excited-state intermediates in a designer protein encoding a phototrigger caught by an X-ray free-electron laser[J]. Nature Chemistry, 2022, 14(9): 1054-1060. |
| 102 | ZHENG D D, TAO M, YU L J, et al. Ultrafast photoinduced electron transfer in a photosensitizer protein[J]. CCS Chemistry, 2022, 4(4): 1217-1223. |
| 103 | LIU X H, KANG F Y, HU C, et al. A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2-reducing enzyme[J]. Nature Chemistry, 2018, 10(12): 1201-1206. |
| 104 | FU Y, HUANG J, WU Y Z, et al. Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase[J]. Journal of the American Chemical Society, 2021, 143(2): 617-622. |
| 105 | SUN N N, HUANG J J, QIAN J Y, et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes[J]. Nature, 2022, 611(7937): 715-720. |
| 106 | ROELFES G. LmrR: A privileged scaffold for artificial metalloenzymes[J]. Accounts of Chemical Research, 2019, 52(3): 545-556. |
| 107 | TRIMBLE J S, CRAWSHAW R, HARDY F J, et al. A designed photoenzyme for enantioselective [2+2]cycloadditions[J]. Nature, 2022, 611(7937): 709-714. |
| 108 | SIEGEL J B, ZANGHELLINI A, LOVICK H M, et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction[J]. Science, 2010, 329(5989): 309-313. |
| [1] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [2] | 付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049. |
| [3] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [4] | 叶伟, 李芮, 姜卫红, 顾阳. 二氧化碳微生物转化与体外酶催化体系研究进展[J]. 合成生物学, 2023, 4(6): 1223-1245. |
| [5] | 孙梦楚, 陆亮宇, 申晓林, 孙新晓, 王佳, 袁其朋. 基于荧光检测的高通量筛选技术和装备助力细胞工厂构建[J]. 合成生物学, 2023, 4(5): 947-965. |
| [6] | 康里奇, 谈攀, 洪亮. 人工智能时代下的酶工程[J]. 合成生物学, 2023, 4(3): 524-534. |
| [7] | 曾涛, 巫瑞波. 数据驱动的酶反应预测与设计[J]. 合成生物学, 2023, 4(3): 535-550. |
| [8] | 阮青云, 黄莘, 孟子钧, 全舒. 蛋白质稳定性计算设计与定向进化前沿工具[J]. 合成生物学, 2023, 4(1): 5-29. |
| [9] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [10] | 唐宇琦, 叶松涛, 刘嘉, 张鑫. 分子伴侣作用下的蛋白质稳定与进化[J]. 合成生物学, 2022, 3(3): 445-464. |
| [11] | 王盼盼, 于洪巍. 酶催化在维生素及其衍生物制备中的应用[J]. 合成生物学, 2022, 3(3): 500-515. |
| [12] | 汤恒, 韩鑫, 邹树平, 郑裕国. 多酶催化体系在医药化学品合成中的应用[J]. 合成生物学, 2021, 2(4): 559-576. |
| [13] | 吴淑可, 周颐, 王文, 张巍, 高鹏飞, 李智. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558. |
| [14] | 贺俊斌, 孟松, 潘海学, 唐功利. 多酶催化串联策略在复杂天然产物合成中的应用[J]. 合成生物学, 2020, 1(2): 226-246. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||