合成生物学 ›› 2025, Vol. 6 ›› Issue (3): 701-714.DOI: 10.12211/2096-8280.2025-040
• 特约评述 • 上一篇
生物制造标准体系建设的现状、问题与建议
黄怡1, 司同1, 陆安静2
- 1.中国科学院深圳先进技术研究院,定量合成生物学全国重点实验室,深圳合成生物学创新研究院,广东 深圳 518055
2.中国电子信息产业发展研究院,北京 100081
-
收稿日期:2025-04-29修回日期:2025-06-04出版日期:2025-06-30发布日期:2025-06-27 -
通讯作者:陆安静 -
作者简介:黄怡 (1996—),女,助理研究员。研究方向为生物制造科技情报分析与政策研究。E-mail:y.huang2@siat.ac.cn陆安静 (1987—),男,副研究员。研究方向为生物制造产业分析、市场与政策研究。E-mail:legendblue@foxmail.com
Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward
HUANG Yi1, SI Tong1, LU Anjing2
- 1.State Key Laboratory of Quantitative Synthetic Biology,Shenzhen Institute of Synthetic Biology,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,Guangdong,China
2.China Center for Information Industry Development,Beijing 100081,China
-
Received:2025-04-29Revised:2025-06-04Online:2025-06-30Published:2025-06-27 -
Contact:LU Anjing
摘要:
生物制造作为全球科技革命与产业变革的战略制高点,正通过合成生物学和人工智能等前沿技术的深度融合和协同创新,驱动物质生产方式实现颠覆性重塑。生物制造领域的标准化工作是加速科学发现进程、大幅增进生产效能、确保产业健康发展的重要基础。全球主要经济体均已将生物制造标准化列为国家竞争核心要素,竞相争夺产业发展主导权。本文梳理了国内外生物制造领域标准建设现状,从技术研发、产业生态、国际协同三个维度识别出标准制定进程滞后、跨领域标准协同受阻、国际标准互认壁垒三大问题。基于此提出构建动态化标准转化机制、建立跨领域标准协同平台、实施标准国际化计划的标准体系协同发展路径,为我国加快建设生物制造标准体系、推动生物制造从“技术驱动”转向“标准主导”的发展阶段提供理论支撑和决策参考。
中图分类号:
引用本文
黄怡, 司同, 陆安静. 生物制造标准体系建设的现状、问题与建议[J]. 合成生物学, 2025, 6(3): 701-714.
HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward[J]. Synthetic Biology Journal, 2025, 6(3): 701-714.
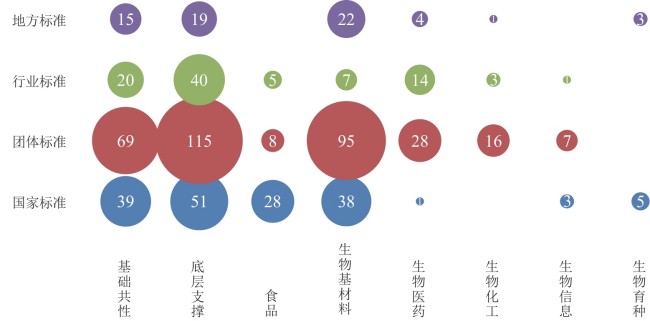
图2 生物制造相关标准分布图(数据来源:中国标准服务网https://www.cssn.net.cn/cssn/index)
Fig. 2 Overview of standards related to biomanufacturing in China(Date source: China Standard Service Network, https://www.cssn.net.cn/cssn/index)
| 1 | 国家标准化管理委员会.标准和标准化[EB/OL]. (2017-11-06)[2025-06-03]. . |
| Standardization Administration of the People’s Republic of China. Standard and standardization[EB/OL]. (2017-11-06)[2025-06-03]. . | |
| 2 | MENG F K, ELLIS T. The second decade of synthetic biology: 2010-2020[J]. Nature Communications, 2020, 11: 5174. |
| 3 | 赵国屏. 合成生物学: 开启生命科学“会聚” 研究新时代[J]. 中国科学院院刊, 2018, 33(11): 1135-1149. |
| ZHAO G P. Synthetic biology: unsealing the convergence era of life science research[J]. Bulletin of Chinese Academy of Sciences, 2018, 33(11): 1135-1149. | |
| 4 | 刘陈立. 拥抱生物经济发展新机遇[N]. 经济日报, 2024-07-05(10). |
| LIU C L. Embrace the new opportunities for the development of the bio-economy[N]. Economic Daily, 2024-07-05(10). | |
| 5 | MEIGE A, EAGAR R, SEHLSTEDT U, et al. The brave new world of synthetic biology: major impacts, significant challenges[R/OL]. 2024[2025-06-03]. . |
| 6 | LUO N, ZHAO G P, LIU C L. Quantitative synthetic biology[J]. Nature Reviews Bioengineering, 2024, 2(11): 911-913. |
| 7 | MUNSON M, MUNRO S, SALIT M. Synthetic biology standards consortium kick-off workshop report[R]. U.S. National Institute of Standards and Technology, 2015. |
| 8 | The White House. National biotechnology and biomanufacturing initiative[EB/OL]. (2022-09-12)[2025-06-03]. . |
| 9 | THOMAS D. The U.S. biomanufacturing economy: value added, supply chains, cost, sustainability, and efficiency[R/OL]. National Institute of Standards and Technology, 2023[2025-06-04]. . |
| 10 | The White House. U.S. government national standards strategy for critical and emerging technologies (usg nsscet): implementation roadmap[EB/OL]. (2024-07-26)[2025-06-03]. . |
| 11 | FREEMONT P S, NI C, AURAND E, et al. Engineering biology metrics and technical standards for the global bioeconomy[R/OL]. London, UK, 2024[2025-06-03]. . |
| 12 | FREEMONT P, ADEOGUN M. Standards and metrics for engineering biology in the UK: driving growth, investment and engineering biology powered solutions for UK companies[R/OL]. London, UK, 2024[2025-06-03]. . |
| 13 | ORDOZGOITI E, PORCAR M, BALDWIN G, et al. Standardization in synthetic biology: a white book[R]. European Union: fostering synthetic biology standardization through international collaboration, 2015. |
| 14 | Commission European. Building the future with nature: boosting biotechnology and biomanufacturing in the EU[EB/OL]. (2024-03-27)[2025-06-03]. . |
| 15 | Standard terminology for industrial biotechnology and synthetic biology: [S/OL]. [2025-06-03]. . |
| 16 | Biotechnology — Cell counting — Part 1: General guidance on cell counting methods: [S/OL]. [2025-06-03]. . |
| 17 | Biotechnology — Cell counting — Part 2: Experimental design and statistical analysis to quantify counting method performance: [S/OL]. [2025-06-03]. . |
| 18 | 陈国强, 吴赴清, 郑爽, 等. 我国生物制造底盘菌种现状、问题及对策[J]. 中国科学院院刊, 2025, 40(1): 2-13. |
| CHEN G Q, WU F Q, ZHENG S, et al. Current status and applications of microbial chassis strains for Chinese biomanufacturing industry[J]. Bulletin of Chinese Academy of Sciences, 2025, 40(1): 2-13. | |
| 19 | Microbiology of food and animal feeding stuffs horizontal method for the detection and enumeration of presumptiveEscherichia coli-most probable number technique-AMENDMENT 1: inclusion of performance testing of culture media and reagents: Amd 1:2023[S/OL]. [2025-06-03]. . |
| 20 | Biotechnology — Biobanking — General requirements for biobanking: [S/OL]. [2025-06-03]. . |
| 21 | Biotechnology — Biobanking — Requirements for animal biological material: [S/OL]. [2025-06-03]. . |
| 22 | Biotechnology — Biobanking — Requirements for the biobanking of plant biological material for research and development: [S/OL]. [2025-06-03]. . |
| 23 | Biotechnology — Biobanking — General requirements for the validation and verification of processing methods for biological material in biobanks: [S/OL]. [2025-06-03]. . |
| 24 | Biotechnology — Biobanking — Requirements for human mesenchymal stromal cells derived from bone marrow: [S/OL]. [2025-06-03]. . |
| 25 | Biotechnology — Biobanking — Requirements for human neural stem cells derived from pluripotent stem cells: [S/OL]. [2025-06-03]. . |
| 26 | Biotechnology — Requirements for data formatting and description in the life sciences: [S/OL]. [2025-06-03]. . |
| 27 | ROSS D, TONNER P D, VASILYEVA O B. Method for reproducible automated bacterial cell culture and measurement[J]. Synthetic Biology, 2022, 7(1): ysac013. |
| 28 | HADDOCK-ANGELLI T, BEAL J, FARNY N, et al. Standard iGEM cell measurement protocol[R/OL]. (2019-07-21)[2025-06-03] . |
| 29 | Biotechnology — Nucleic acid synthesis — Part 2: Requirements for the production and quality control of synthesized gene fragments, genes, and genomes: [S/OL]. [2025-06-03]. . |
| 30 | 中国生物工程学会. 合成生物学路线图2030:驱动下一代生物制造的引擎[M]. 北京: 科学出版社, 2024. |
| Chinese Society of Biotechnology. Synthetic biology roadmap 2030: the engine driving the next generation of biomanufacturing[M].Beijing: Science Press, 2024. | |
| 31 | ROMANTSEVA E, STRYCHALSKI E A. CELL-FREE (Comparable engineered living lysates for research education and entrepreneurship) workshop report[R/OL]. U.S. National Institute of Standards and Technology, 2020 [2025-06-03]. . |
| 32 | ROMANTSEVA E. Metrology for cell-free expression systems[R/OL].U.S. National Institute of Standards and Technology, 2025 [2025-06-03]. . |
| 33 | Foodstuffs — Methods of analysis for the detection of genetically modified organisms and derived products — General requirements and definitions — Amendment 1: Amd 1:2013[S/OL]. [2025-06-03]. . |
| 34 | Foodstuffs — Methods of analysis for the detection of genetically modified organisms and derived products — Quantitative nucleic acid based methods — Amendment 1: Amd 1:2013[S/OL]. [2025-06-03]. . |
| 35 | Bio-based products-Vocabulary: EN 16575:2014 [S/OL]. [2025-06-03]. . |
| 36 | Rubber and rubber products — Determination of biobased content — Part 3: Biobased mass content: [S/OL]. [2025-06-03]. . |
| 37 | Bio-based products. Bio-based content-Determination of the bio-based content using the material balance method: [S/OL]. [2025-06-03]. . |
| 38 | Plastics — Biobased content — Part 4: Determination of biobased mass content: [S/OL]. [2025-06-03]. . |
| 39 | Bio-based products-Life Cycle Assessment: EN 16760:2015 [S/OL]. [2025-06-03]. . |
| 40 | Bio-based products-Guidelines for Life Cycle Inventory (LCI) for the End-of-life phase: CEN/TR 16957:2016 [S/OL]. [2025-06-03]. . |
| 41 | Determination of the ultimate biodegradation of plastics materials in an aqueous system under anoxic (denitrifying) conditions - Method by measurement of pressure increase: EN 17417:2020 [S/OL]. [2025-06-03]. . |
| 42 | Plastics-Biodegradable mulch films for use in agriculture and horticulture-Requirements and test methods: EN 17033:2018 [S/OL]. [2025-06-03]. . |
| 43 | Plastics-Determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system-Method by measurement of biogas production: EN [S/OL]. [2025-06-03]. . |
| 44 | Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium — Method by measuring the oxygen demand in a closed respirometer: [S/OL]. [2025-06-03]. . |
| 45 | Plastics — Evaluation of the action of microorganisms: [S/OL]. [2025-06-03]. . |
| 46 | Biotechnology — Bioprocessing — General requirements for the design of packaging to contain cells for therapeutic use: [S/OL]. [2025-06-03]. . |
| 47 | Biotechnology — Ancillary materials present during the production of cellular therapeutic products and gene therapy products: [S/OL]. [2025-06-03]. . |
| 48 | Biotechnology. Bioprocessing. General requirements and considerations for equipment systems used in the manufacturing of cells for therapeutic use: [S/OL]. [2025-06-03]. . |
| 49 | Biotechnology — Analytical methods — General requirements and considerations for the testing and characterization of cellular therapeutic products: [S/OL]. [2025-06-03]. . |
| 50 | Biotechnology — General requirements for transportation of cells for therapeutic use: [S/OL]. [2025-06-03]. . |
| 51 | 中共中央 国务院.国家标准化发展纲要[EB/OL]. (2021-10-10)[2025-06-03]. . |
| Central Committee of the Communist Party of China and State Council. National standardization development outline [EB/OL]. (2021-10-10)[2025-06-03]. . | |
| 52 | 工业和信息化部等四部门. 新产业标准化领航工程实施方案(2023—2035年)[EB/OL].(2023-08-03)[2025-06-03]. . |
| Ministry of Industry and Information Technology of the People’s Republic of China, Ministry of Science and Technology of the People’s Republic of China, National Energy Administration, et al. Implementation plan for new industry standardization pilot project (2023—2035) [EB/OL]. (2023-08-03)[2025-06-03]. . | |
| 53 | 工业和信息化部,教育部,科学技术部,等. 工业和信息化部等七部门关于推动未来产业创新发展的实施意见[EB/OL]. (2024-01-18)[2025-06-03]. . |
| Ministry of Industry and Information Technology of the People’s Republic of China, Ministry of Education of the People’s Republic of China, Ministry of Science and Technology of the People’s Republic of China, et al. The guideline to support development of future industries released by the Ministry of Industry and Information Technology and six other ministry and agencies[EB/OL]. (2024-01-18)[2025-06-03]. . | |
| 54 | 市场监管总局, 中央网信办, 国家发展改革委等18部门. 贯彻实施〈国家标准化发展纲要〉行动计划(2024—2025年)[EB/OL]. (2024-03-27)[2025-06-03]. . |
| State Administration for Market Regulation, Office of the Central Cyberspace Affairs Commission, National Development and Reform Commission, et al. Implement the action plan for the implementation of the national standardization development outline (2024-2025)[EB/OL]. (2024-03-27)[2025-06-03]. . | |
| 55 | 国家市场监督管理总局, 国家标准化管理委员会. 生物技术 生物样本保藏 动物生物样本保藏要求: [S]. 北京: 中国标准出版社, 2024. |
| State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. Biotechnology—Biobanking—Requirements for the biobanking of animal biological material: [S]. Beijing: Standards Press of China, 2024. | |
| 56 | 国家市场监督管理总局, 国家标准化管理委员会. 生物技术 生物样本保藏 用于研究和开发用途的植物生物样本保藏要求: [S]. 北京: 中国标准出版社, 2024. |
| State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. Biotechnology—Biobanking—Requirements for the biobanking of plant biological material for research and development: [S]. Beijing: Standards Press of China, 2024. | |
| 57 | 国家市场监督管理总局, 国家标准化管理委员会. 人感染病原微生物与样本保藏通用要求: [S]. 北京: 中国标准出版社, 2023. |
| State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. General requirements for preservation of human pathogenic microorganisms and samples: [S]. Beijing: Standards Press of China, 2023. | |
| 58 | 人胃癌类器官构建、质量控制与保藏: T/1984 [S].北京: 中国标准出版社, 2024. |
| Human gastric cancer organoid construction, quality control and preservation: T/1984 [S]. Beijing: Standards Press of China, 2024. | |
| 59 | 人肝祖细胞类器官构建、质量控制与保藏操作指南: T/CRHA 017—2023 [S]. 北京: 中国标准出版社, 2023. |
| Operational guidelines for construction, quality control and preservation of human liver progenitor cell organoids: T/CRHA 017—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 60 | 人肝胆肿瘤细胞类器官构建、质量控制与保藏操作指南: T/CRHA 018—2023 [S]. 北京: 中国标准出版社, 2023. |
| Operational guidelines for construction, quality control and preservation of human hepatobiliary tumor cell organoids: T/CRHA 018—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 61 | 人胆系上皮组织类器官构建、质量控制与保藏操作指南: T/CRHA 019—2023 [S]. 北京: 中国标准出版社, 2023. |
| Operational guidelines for construction, quality control and preservation of human biliary epithelial tissue organoids: T/CRHA 019—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 62 | 原料药及中间体连续制造指导原则: T/PIAC 00001—2023 [S]. 北京: 中国标准出版社, 2023. |
| Guidelines for continuous manufacturing of drug substances and intermediates: T/PIAC 00001—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 63 | 张春鹏, 范宇婷, 董红霞. 美国新药技术成熟度评价研究[J]. 中国药物警戒, 2020, 17(6): 353-356. |
| ZHANG C P, FAN Y T, DONG H X. Research on the evaluation method of new drug technology readiness level in the United States[J]. Chinese Journal of Pharmacovigilance, 2020, 17(6): 353-356. |
| [1] | 吴柯, 罗家豪, 李斐然. 机器学习驱动的基因组规模代谢模型构建与优化[J]. 合成生物学, 2025, 6(3): 566-584. |
| [2] | 田晓军, 张日新. 合成基因回路面临的细胞“经济学窘境”[J]. 合成生物学, 2025, 6(3): 532-546. |
| [3] | 章益蜻, 刘高雯. 合成生物学视角下的基因功能探索与酵母工程菌株文库构建[J]. 合成生物学, 2025, 6(3): 685-700. |
| [4] | 宋成治, 林一瀚. AI+定向进化赋能蛋白改造及优化[J]. 合成生物学, 2025, 6(3): 617-635. |
| [5] | 张梦瑶, 蔡鹏, 周雍进. 合成生物学助力萜类香精香料可持续生产[J]. 合成生物学, 2025, 6(2): 334-356. |
| [6] | 张璐鸥, 徐丽, 胡晓旭, 杨滢. 合成生物学助力化妆品走进生物制造新时代[J]. 合成生物学, 2025, 6(2): 479-491. |
| [7] | 伊进行, 唐宇琳, 李春雨, 吴鹤云, 马倩, 谢希贤. 氨基酸衍生物在化妆品中的应用及其生物合成研究进展[J]. 合成生物学, 2025, 6(2): 254-289. |
| [8] | 韦灵珍, 王佳, 孙新晓, 袁其朋, 申晓林. 黄酮类化合物生物合成及其在化妆品中应用的研究[J]. 合成生物学, 2025, 6(2): 373-390. |
| [9] | 肖森, 胡立涛, 石智诚, 王发银, 余思婷, 堵国成, 陈坚, 康振. 可控分子量透明质酸的生物合成研究进展[J]. 合成生物学, 2025, 6(2): 445-460. |
| [10] | 王倩, 果士婷, 辛波, 钟成, 王钰. L-精氨酸的微生物合成研究进展[J]. 合成生物学, 2025, 6(2): 290-305. |
| [11] | 左一萌, 张姣姣, 连佳长. 酿酒酵母使能技术在化妆品原料合成中的应用[J]. 合成生物学, 2025, 6(2): 233-253. |
| [12] | 汤传根, 王璟, 张烁, 张昊宁, 康振. 功能肽合成和挖掘策略研究进展[J]. 合成生物学, 2025, 6(2): 461-478. |
| [13] | 郭婷婷, 韩湘凝, 黄熙婷, 张婷婷, 孔健. 乳酸菌的合成生物学工具及在合成益肤因子中的应用[J]. 合成生物学, 2025, 6(2): 320-333. |
| [14] | 张萍, 张维娇, 胥睿睿, 李江华, 陈坚, 康振. 防晒化合物类菌孢素氨基酸的生物合成[J]. 合成生物学, 2025, 6(2): 306-319. |
| [15] | 黄姝涵, 马赫, 罗云孜. 生物合成红景天苷的研究进展[J]. 合成生物学, 2025, 6(2): 391-407. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||