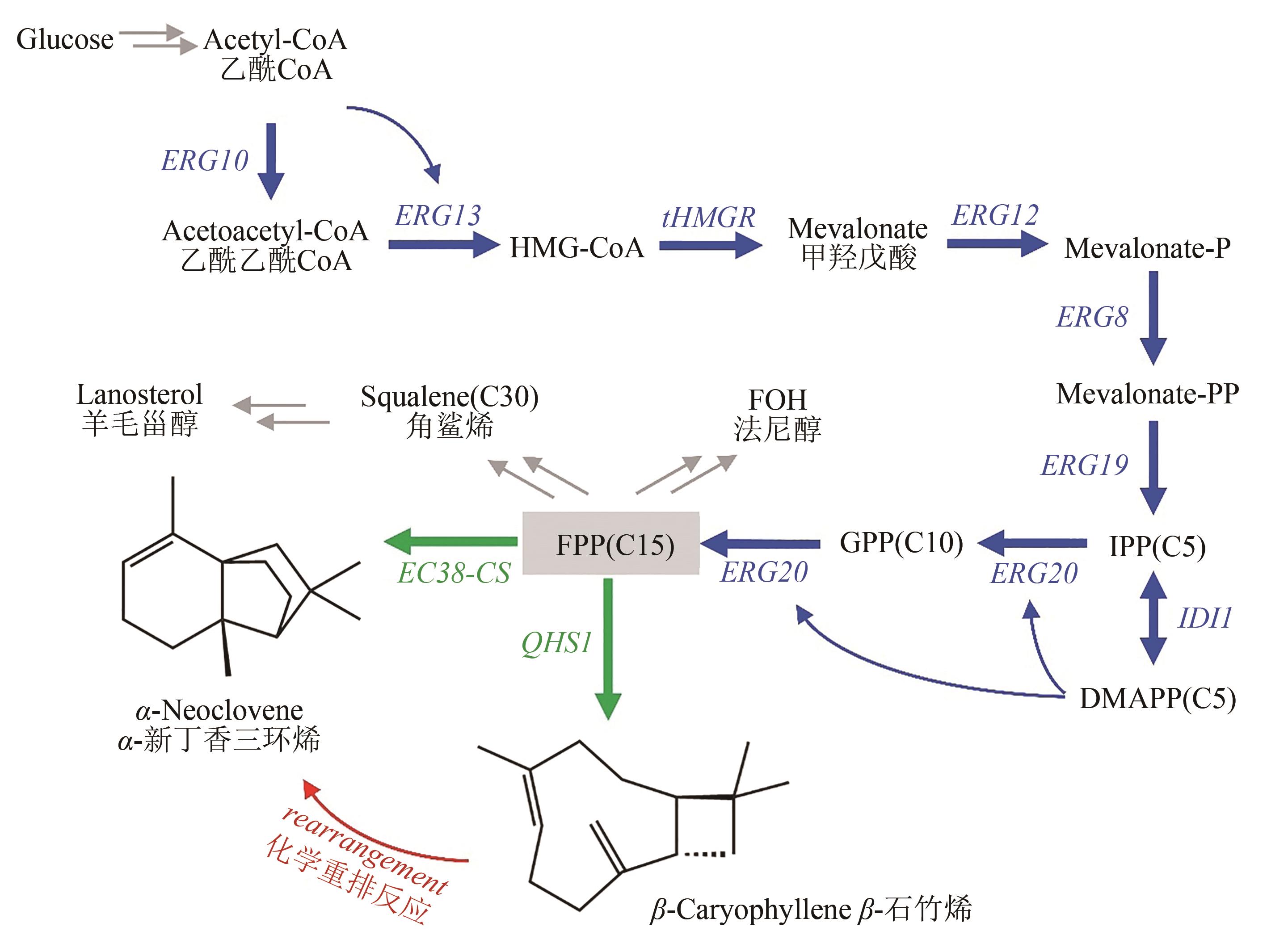
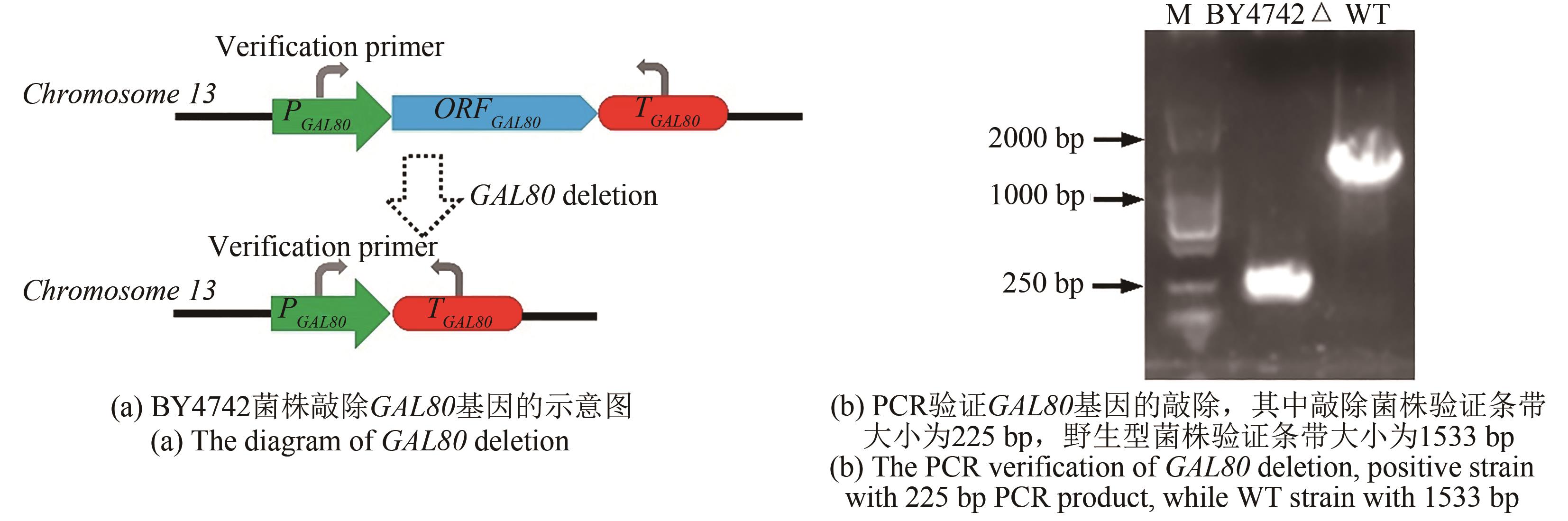
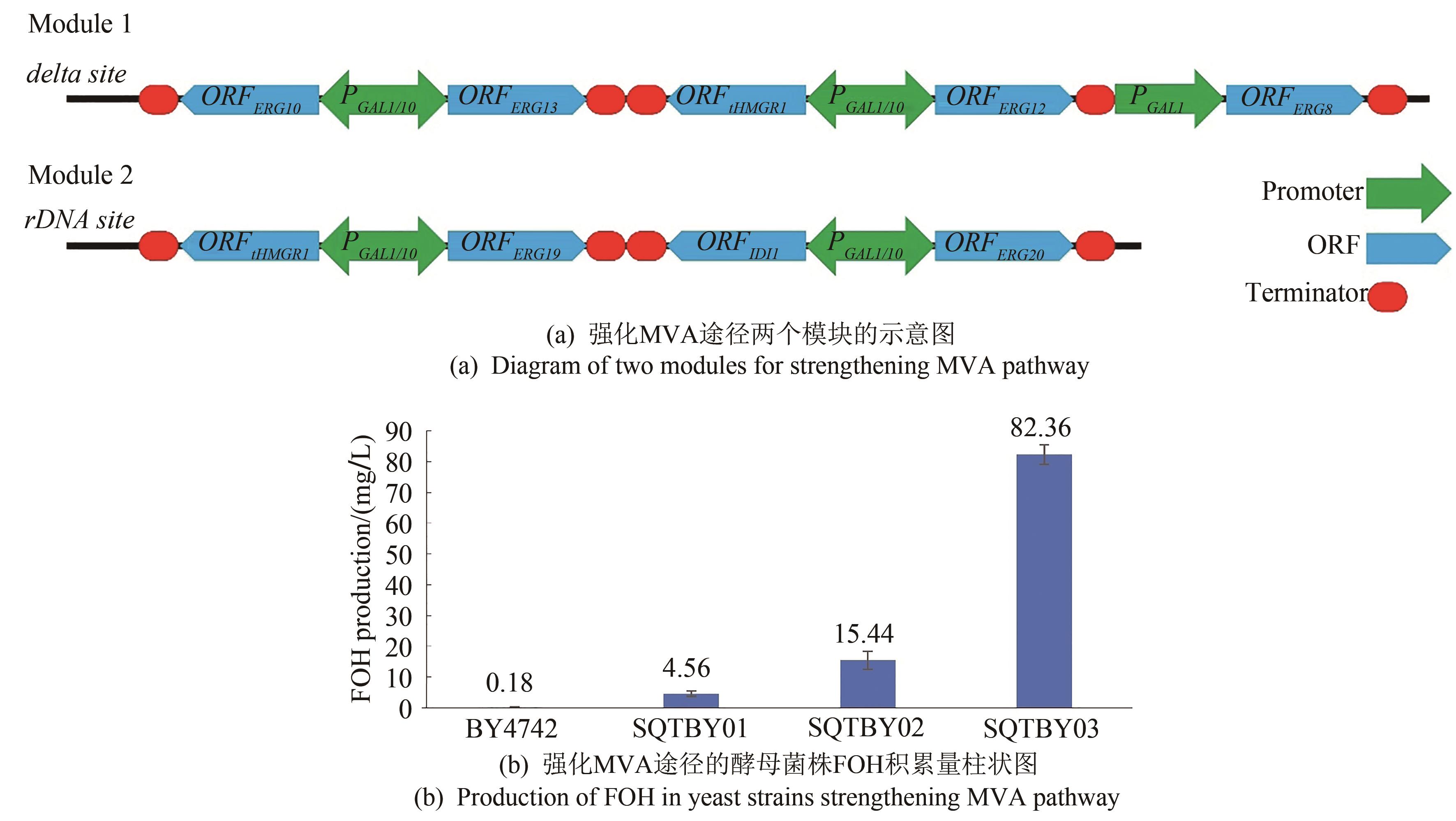
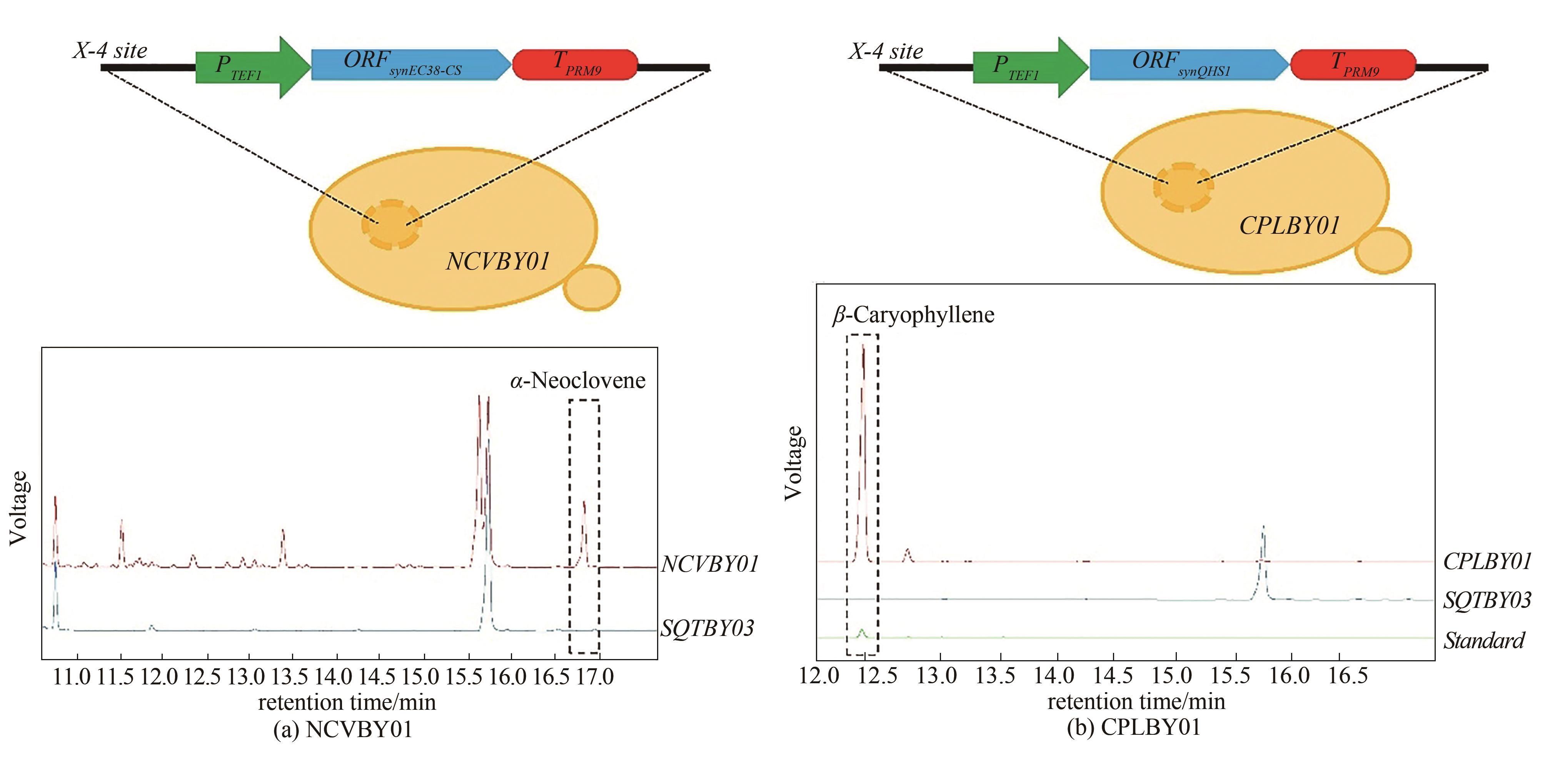
Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (5): 792-803.DOI: 10.12211/2096-8280.2021-014
Previous Articles Next Articles
Production of sesquiterpenoids α-neoclovene and β-caryophyllene by engineered Saccharomyces cerevisiae
LI Xiaodong1,2, YANG Chengshuai3, WANG Pingping1, YAN Xing1, ZHOU Zhihua1
- 1.CAS-key Laboratory of Synthetic Biology,Center for Excellence in Molecular Plant Sciences,Chinese Academy of Sciences,Shanghai 200032,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
3.CAS Key Laboratory of Quantitative Engineering Biology,Shenzhen Institute of Synthetic Biology,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,Guangdong,China
-
Received:2021-01-31Revised:2021-03-10Online:2021-11-19Published:2021-10-31 -
Contact:ZHOU Zhihua
构建酿酒酵母细胞工厂从头合成倍半萜类化合物α-新丁香三环烯和β-石竹烯
李晓东1,2, 杨成帅3, 王平平1, 严兴1, 周志华1
- 1.中国科学院分子植物科学卓越创新中心,合成生物学重点实验室,上海 200032
2.中国科学院大学,北京 100049
3.中国科学院深圳先进技术研究院,深圳合成生物学创新研究院,中国科学院定量工程生物学重点实验室,广东 深圳 518055
-
通讯作者:周志华 -
作者简介:李晓东 (1992—),男,博士研究生。研究方向为天然产物合成生物学。E-mail:lixiaodong@cemps.ac.cn周志华 (1966—),女,研究员,博士,博士生导师。研究方向为天然化合物合成生物学、丝状真菌分子生物学及基因组编辑技术等。E-mail:zhouzhihua@sippe.ac.cn -
基金资助:国家重点研发计划(2019YFA0904300);国家自然科学基金(31921006)
CLC Number:
Cite this article
LI Xiaodong, YANG Chengshuai, WANG Pingping, YAN Xing, ZHOU Zhihua. Production of sesquiterpenoids α-neoclovene and β-caryophyllene by engineered Saccharomyces cerevisiae[J]. Synthetic Biology Journal, 2021, 2(5): 792-803.
李晓东, 杨成帅, 王平平, 严兴, 周志华. 构建酿酒酵母细胞工厂从头合成倍半萜类化合物α-新丁香三环烯和β-石竹烯[J]. 合成生物学, 2021, 2(5): 792-803.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-014
| Name | Genotype | Source |
|---|---|---|
| BY4742 | MATa, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0 | EUROSCARF |
| CEN.PK2-1 | MATa, wra3-52, trp1-289, leu2-3_112, hisΔ1 | EUROSCARF |
| SQTBY01 | BY4742(rDNA:: TEF1p-ERG8-PRM9t, TEF2p-tHMGR1-ENO2t, PGK1p-ERG10-CYC1t) | this study |
| SQTCEN01 | CEN.PK2-1(rDNA:: TEF1p-ERG8-PRM9t, TEF2p-tHMGR1-ENO2t, PGK1p-ERG10-CYC1t) | this study |
| BY4742Δ | BY4742(ΔGAL80) | this study |
| SQTBY02 | BY4742Δ(deltaDNA:: GAL1p-ERG10-TDH2t, GAL10p-ERG13-HXT7t, GAL1p-tHMGR1-tHMGR1t, GAL10p-ERG12-PYK1t, GAL1p-ERG8-PDC1t) | this study |
| SQTBY03 | SQTBY02(rDNA:: GAL1p-tHMGR1-CYC1t, GAL10p-ERG19-ADH1t, GAL1p-IDI1-ENO2t, GAL10p-ERG20-PGIt) | this study |
| NCVBY01 | SQTBY03(X-4:: TEF1p-synec38-cs-PRM9t) | this study |
| CPLBY01 | SQTBY03(X-4:: TEF1p-synQHS1-PRM9t) | this study |
Tab. 1 Strains used in this study
| Name | Genotype | Source |
|---|---|---|
| BY4742 | MATa, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0 | EUROSCARF |
| CEN.PK2-1 | MATa, wra3-52, trp1-289, leu2-3_112, hisΔ1 | EUROSCARF |
| SQTBY01 | BY4742(rDNA:: TEF1p-ERG8-PRM9t, TEF2p-tHMGR1-ENO2t, PGK1p-ERG10-CYC1t) | this study |
| SQTCEN01 | CEN.PK2-1(rDNA:: TEF1p-ERG8-PRM9t, TEF2p-tHMGR1-ENO2t, PGK1p-ERG10-CYC1t) | this study |
| BY4742Δ | BY4742(ΔGAL80) | this study |
| SQTBY02 | BY4742Δ(deltaDNA:: GAL1p-ERG10-TDH2t, GAL10p-ERG13-HXT7t, GAL1p-tHMGR1-tHMGR1t, GAL10p-ERG12-PYK1t, GAL1p-ERG8-PDC1t) | this study |
| SQTBY03 | SQTBY02(rDNA:: GAL1p-tHMGR1-CYC1t, GAL10p-ERG19-ADH1t, GAL1p-IDI1-ENO2t, GAL10p-ERG20-PGIt) | this study |
| NCVBY01 | SQTBY03(X-4:: TEF1p-synec38-cs-PRM9t) | this study |
| CPLBY01 | SQTBY03(X-4:: TEF1p-synQHS1-PRM9t) | this study |
| Gene | Description | Accession No. |
|---|---|---|
| GAL80 | Coding gene of transcription regulator | NM_001182409 |
| ERG10 | Coding gene of acetyl-CoA C-acetyltransferase | NM_001183842 |
| ERG13 | Coding gene of hydroxymethylglutaryl-CoA synthase | NM_001182489 |
| tHMGR1 | Coding gene of 3-hydroxy-3-methyl-glutaryl-CoA reductase | NM_001182434 |
| ERG12 | Coding gene of mevalonate kinase | NM_001182715 |
| ERG8 | Coding gene of phosphomevalonate kinase | NM_001182727 |
| ERG19 | Coding gene of diphosphomevalonate decarboxylase | NM_001183220 |
| IDI1 | Coding gene of isopentenyl-diphosphate delta-isomerase | NM_001183931 |
| ERG20 | Coding gene of (2E,6E)-farnesyl diphosphate synthase | NM_001181600 |
| ec38-cs | Coding gene of caryophyllene synthase Hypoxylon sp. EC38 | OTA59675 |
| QHS1 | Coding gene of β-caryophyllene synthase from Artemisia annua | AF472361 |
Tab. 2 Genes used in this study
| Gene | Description | Accession No. |
|---|---|---|
| GAL80 | Coding gene of transcription regulator | NM_001182409 |
| ERG10 | Coding gene of acetyl-CoA C-acetyltransferase | NM_001183842 |
| ERG13 | Coding gene of hydroxymethylglutaryl-CoA synthase | NM_001182489 |
| tHMGR1 | Coding gene of 3-hydroxy-3-methyl-glutaryl-CoA reductase | NM_001182434 |
| ERG12 | Coding gene of mevalonate kinase | NM_001182715 |
| ERG8 | Coding gene of phosphomevalonate kinase | NM_001182727 |
| ERG19 | Coding gene of diphosphomevalonate decarboxylase | NM_001183220 |
| IDI1 | Coding gene of isopentenyl-diphosphate delta-isomerase | NM_001183931 |
| ERG20 | Coding gene of (2E,6E)-farnesyl diphosphate synthase | NM_001181600 |
| ec38-cs | Coding gene of caryophyllene synthase Hypoxylon sp. EC38 | OTA59675 |
| QHS1 | Coding gene of β-caryophyllene synthase from Artemisia annua | AF472361 |
| Strains | FOH/(mg/L) | Squalene/(mg/L) | Lanosterol/(mg/L) |
|---|---|---|---|
| BY4742 | 0.177±0.011 | 0.5±0.07 | 3.1±0.57 |
| SQTBY01 | 4.556±0.245 | 51.1±4.55 | 46.0±9.24 |
| CEN.PK2-1 | 0.044±0.002 | 0.3±0.05 | 3.5±0.16 |
| SQTCEN01 | 0.504±0.093 | 32.0±6.23 | 26.8±1.66 |
Tab. 3 Production of FOH, squalene and lanosterol in different yeast strains
| Strains | FOH/(mg/L) | Squalene/(mg/L) | Lanosterol/(mg/L) |
|---|---|---|---|
| BY4742 | 0.177±0.011 | 0.5±0.07 | 3.1±0.57 |
| SQTBY01 | 4.556±0.245 | 51.1±4.55 | 46.0±9.24 |
| CEN.PK2-1 | 0.044±0.002 | 0.3±0.05 | 3.5±0.16 |
| SQTCEN01 | 0.504±0.093 | 32.0±6.23 | 26.8±1.66 |
| Retention time/min | Products | Mass spectra match factors/% | Area/% |
|---|---|---|---|
| 11.538 | (-)-cyperene | 92 | 21.62 |
| 12.359 | β-caryophyllene | 95 | 8.52 |
| 12.752 | humulene | 97 | 2.53 |
| 12.932 | AC1LBUZB | 96 | 5.02 |
| 13.067 | germacrene D | 96 | 3.54 |
| 13.395 | β-caryophyllene Fcc | 86 | 18.87 |
| 16.865 | α-neoclovene | 89 | 39.90 |
Tab. 4 GC/MS analysis for identification of by-products produced by NCVBY01
| Retention time/min | Products | Mass spectra match factors/% | Area/% |
|---|---|---|---|
| 11.538 | (-)-cyperene | 92 | 21.62 |
| 12.359 | β-caryophyllene | 95 | 8.52 |
| 12.752 | humulene | 97 | 2.53 |
| 12.932 | AC1LBUZB | 96 | 5.02 |
| 13.067 | germacrene D | 96 | 3.54 |
| 13.395 | β-caryophyllene Fcc | 86 | 18.87 |
| 16.865 | α-neoclovene | 89 | 39.90 |
| Strain | Products | Yield/(mg/L) |
|---|---|---|
| NCVBY01 | α-neoclovene | 487.1 |
| (-)-cyperene | 247.6 | |
| β-caryophyllene Fcc | 221.2 | |
| β-caryophyllene | 109.2 | |
| CPLBY01 | β-caryophyllene | 2949.1 |
| α-humulene | 109.8 |
Tab. 5 Yield of sesquiterpenes in NCVBY01 and CPLBY01 by fed-batch fermentation.
| Strain | Products | Yield/(mg/L) |
|---|---|---|
| NCVBY01 | α-neoclovene | 487.1 |
| (-)-cyperene | 247.6 | |
| β-caryophyllene Fcc | 221.2 | |
| β-caryophyllene | 109.2 | |
| CPLBY01 | β-caryophyllene | 2949.1 |
| α-humulene | 109.8 |
| 1 | RU W W, WANG D L, XU Y P, et al. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.)[J]. Drug Discoveries and Therapeutics, 2015, 9(1): 23-32. |
| 2 | GUO M K, SHAO S, WANG D D, et al. Recent progress in polysaccharides from Panax ginseng C. A. Meyer[J]. Food and Function, 2020, 12(2): 494-518. |
| 3 | JIANG R, SUN L W, WANG Y B, et al. Chemical composition, and cytotoxic, antioxidant and antibacterial activities of the essential oil from ginseng leaves[J]. Natural Product Communications, 2014, 9(6): 865-868. |
| 4 | 赵岩, 王红, 蔡恩博, 等. 人参挥发油化学成分及其主要活性成分聚乙炔醇类药理作用研究进展[J]. 中国药房, 2017, 28(13): 1856-1859. |
| ZHAO Y, WANG H, CAI E B, et al. Research progress on chemical constituents of essential oil from Panax ginseng and pharmacological effects of polyacetylenol[J]. China Pharmacy, 2017, 28(13): 1856-1859. | |
| 5 | 彭雪, 李超英. 人参挥发油研究[J]. 吉林中医药, 2017, 37(1): 71-74. |
| PENG X, LI C Y. Advances in the research on volatile oil from ginseng[J]. Jilin Journal of Traditional Chinese Medicine, 2017, 37(1): 71-74. | |
| 6 | IN H C. Volatile compounds of ginseng (Panax sp.): a review[J]. Journal of the Korean Society for Applied Biological Chemistry, 2015, 58(1): 67-75. |
| 7 | JUNG J I, KIM E J, KWON G T, et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice[J]. Carcinogenesis, 2015, 36(9): 1028-1039. |
| 8 | SOTTO A D, MANCINELLI R, GULLI M, et al. Chemopreventive potential of caryophyllane sesquiterpenes: an overview of preliminary evidence[J]. Cancers, 2020, 12(10): 3034. |
| 9 | LEGAULT J, PICHETTE A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel[J]. Journal of Pharmacy and Pharmacology, 2010, 59(12): 1643-1647. |
| 10 | EDWARDS T, MOSES C, DRYER F. Evaluation of combustion performance of alternative aviation fuels[C]//46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit. Nashville, TN. Reston, Virginia: AIAA, 2010. |
| 11 | HARVEY B G, MERRIMAN W W, KOONTZ T A. High-density renewable diesel and jet fuels prepared from multicyclic sesquiterpanes and a 1-hexene-derived synthetic paraffinic kerosene[J]. Energy & Fuels, 2015, 29(4): 2431-2436. |
| 12 | LIU H B, LU X Y, HU Y H, et al. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy[J]. Pharmacological Research, 2020, 161: 1052-1063. |
| 13 | GALANIE S, THODEY K, TRENCHARD I J, et al. Complete biosynthesis of opioids in yeast[J]. Science, 2015, 349(6252): 1095-1100. |
| 14 | PADDON C J, WESTFALL P J, PITERA D J, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| 15 | YAN X, FAN Y, WEI W, et al. Production of bioactive ginsenoside compound K in metabolically engineered yeast[J]. Cell Research, 2014, 24(6): 770-773. |
| 16 | PING Y, LI X D, YOU W J, et al. De novo production of the plant-derived tropine and pseudotropine in yeast[J]. ACS Synthetic Biology, 2019, 8(6): 1257-1262. |
| 17 | BAI Y F, YIN H, BI H P, et al. De novo biosynthesis of Gastrodin in Escherichia coli [J]. Metabolic Engineering, 2016, 35: 138-147. |
| 18 | WU W H, TRAN W, TAATJES C, et al. Rapid discovery and functional characterization of terpene synthases from four endophytic Xylariaceae[J]. PLoS One, 2016, 11(2): e0146983. |
| 19 | CAI Y, JIA J W, CROCK J, et al. A cDNA clone for beta-caryophyllene synthase from Artemisia annua[J]. Phytochemistry, 2002, 61(5): 523-529. |
| 20 | GIETZ R D, SCHIESTL R H. Large-scale high-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method[J]. Nature Protocols, 2007, 2(1): 38-41. |
| 21 | WANG P P, WEI W, YE W, et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency[J]. Cell Discovery, 2019, 5: 5. |
| 22 | PARAMASIVAN K, RAJAGOPAL K, MUTTURI S. Studies on squalene biosynthesis and the standardization of its extraction methodology from Saccharomyces cerevisiae [J]. Applied Biochemistry and Biotechnology, 2019, 187(3): 691-707. |
| 23 | IGNEA C, TRIKKA F A, NIKOLAIDIS A K, et al. Efficient diterpene production in yeast by engineering Erg20p into a geranylgeranyl diphosphate synthase[J]. Metabolic Engineering, 2015, 27: 65-75. |
| 24 | APEL A R, D'ESPAUX L, WEHRS M, et al. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae [J]. Nucleic Acids Research, 2017, 45(1): 496-508. |
| 25 | PILAURI V, BEWLEY M, DIEP C, et al. Gal80 dimerization and the yeast GAL gene switch[J]. Genetics, 2005, 169(4): 1903-1914. |
| 26 | WESTFALL P, PITERA D J, LENIHAN J R, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(3): E111-8. |
| 27 | YANG J M, NIE Q J. Engineering Escherichia coli to convert acetic acid to β-caryophyllene [J]. Microbial Cell Factory, 2016, 15(5): 74-82. |
| 28 | WU W H, LIU F, DIVIS R W. Engineering Escherichia coli for the production of terpene mixture enriched in caryophyllene and caryophyllene alcohol as potential aviation fuel compounds[J]. Metabolic Engineering Communications, 2018, 5(6): 13-21. |
| 29 | PARKER W, RAPHAEL R A, ROBERTS J S. Neoclovene a novel rearrangement product of caryophyllene[J]. Tetrahedron Letters, 1965, 6(27): 2313-2315. |
| 30 | MCKILLOP T F W, MARTIN J, PARKER W, et al. The synthesis of neoclovene[J]. Journal of the Chemical Society, 1971(2): 162-162. |
| [1] | YING Hanjie, LIU Dong, WANG Zhenyu, SHEN Tao, ZHUANG Wei, ZHU Chenjie. Exploring industrial biomanufacturing and the goal of “carbon neutrality” [J]. Synthetic Biology Journal, 2025, 6(1): 1-7. |
| [2] | DIAO Zhidian, WANG Xixian, SUN Qing, XU Jian, MA Bo. Advances and applications of single-cell Raman spectroscopy testing and sorting equipment [J]. Synthetic Biology Journal, 2023, 4(5): 1020-1035. |
| [3] | WU Yujie, LIU Xinxin, LIU Jianhui, Yang Kaiguang, SUI Zhigang, ZHANG Lihua, ZHANG Yukui. Research progress of strain screening and quantitative analysis of key molecules based on high-throughput liquid chromatography and mass spectrometry [J]. Synthetic Biology Journal, 2023, 4(5): 1000-1019. |
| [4] | SUN Meili, WANG Kaifeng, LU Ran, JI Xiaojun. Rewiring and application of Yarrowia lipolytica chassis cell [J]. Synthetic Biology Journal, 2023, 4(4): 779-807. |
| [5] | GAO Xianyun, NIU Lingxue, JIAN Ni, GUAN Ningzi. Applications of microbial synthetic biology in the diagnosis and treatment of diseases [J]. Synthetic Biology Journal, 2023, 4(2): 263-282. |
| [6] | TU Ran, LI Shixin, LI Haoni, WANG Meng. Advances and applications of droplet-based microfluidics in evolution and screening of engineered microbial strains [J]. Synthetic Biology Journal, 2023, 4(1): 165-184. |
| [7] | WANG Xixian, SUN Qing, DIAO Zhidian, XU Jian, MA Bo. Advances with applications of Raman spectroscopy in single-cell phenotype sorting and analysis [J]. Synthetic Biology Journal, 2023, 4(1): 204-224. |
| [8] | PAN Yingjia, XIA Siyang, DONG Chang, CAI Jin, LIAN Jiazhang. Mutator-driven continuous genome evolution of Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2023, 4(1): 225-240. |
| [9] | LIU Qi, QIAN Zhilan, SONG Lili, YAO Chaoying, XU Mingqiang, REN Yanna, CAI Menghao. Rewiring and application of Pichia pastoris chassis cell [J]. Synthetic Biology Journal, 2022, 3(6): 1150-1173. |
| [10] | TAO Fei, SUN Tao, WANG Yu, WEI Ting, NI Jun, XU Ping. Challenges and opportunities in the research of Synechococcus chassis under the context of carbon peak and neutrality [J]. Synthetic Biology Journal, 2022, 3(5): 932-952. |
| [11] | DONG Zhengxin, SUN Tao, CHEN Lei, ZHANG Weiwen. Applications of regulatory engineering in photosynthetic cyanobacteria [J]. Synthetic Biology Journal, 2022, 3(5): 966-984. |
| [12] | CUI Jinyu, ZHANG Aidi, LUAN Guodong, LYU Xuefeng. Engineering microalgae for photosynthetic biosynthesis: progress and prospect [J]. Synthetic Biology Journal, 2022, 3(5): 884-900. |
| [13] | BI Jiacheng, TIAN Zhigang. Synthetic immunology and future NK cell immunotherapy [J]. Synthetic Biology Journal, 2022, 3(1): 22-34. |
| [14] | REN Shichao, SUN Qiuyan, FENG Xudong, LI Chun. Biosynthesis of pentacyclic triterpenoid saponins in microbial cell factories [J]. Synthetic Biology Journal, 2022, 3(1): 168-183. |
| [15] | CHEN Jiuzhou, WANG Yu, PU Wei, ZHENG Ping, SUN Jibin. Advances and perspective on bioproduction of 5-aminolevulinic acid [J]. Synthetic Biology Journal, 2021, 2(6): 1000-1016. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||