Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (4): 920-939.DOI: 10.12211/2096-8280.2025-056
• Invited Review • Previous Articles Next Articles
Extracellular multi-enzyme assembly and biocatalytic cascade: advances and prospects
MA Muqing, WU Yan, QU Maohua, LU Xiafeng, CAO Min, DU Feng, JI Rongtao, DONG Leichi, LUO Zhibo
- Hangzhou Weiyuan Biotechnology Co. ,Ltd. ,Hangzhou 310024,Zhejiang,China
-
Received:2025-06-06Revised:2025-06-30Online:2025-09-03Published:2025-08-31 -
Contact:LUO Zhibo
体外多酶组装与生物级联催化:进展与展望
马牧青, 吴彦, 曲茂华, 卢夏锋, 曹敏, 杜峰, 季荣涛, 董磊迟, 罗志波
- 杭州微远生物科技有限公司,浙江 杭州 310024
-
通讯作者:罗志波 -
作者简介:马牧青 (1993—),女,博士,工程师。研究方向为天然产物合成生物学。E-mail:mqma@wybio.cc吴彦 (1982—),男,工程师。研究方向为合成生物学与药物化学。E-mail:ywu@wybio.cc罗志波 (1991—),男,博士,高级工程师,副研究员。研究方向为合成生物学、药物化学、酶分子工程与工业生物催化等,在合成生物学、新药创制及工程转化领域取得系列产业化成果。E-mail:zhiboluo@126.com
第一联系人:共同第一作者 -
基金资助:杭州市农业与社会发展领域公益性科研引导项目(20241029Y103)
CLC Number:
Cite this article
MA Muqing, WU Yan, QU Maohua, LU Xiafeng, CAO Min, DU Feng, JI Rongtao, DONG Leichi, LUO Zhibo. Extracellular multi-enzyme assembly and biocatalytic cascade: advances and prospects[J]. Synthetic Biology Journal, 2025, 6(4): 920-939.
马牧青, 吴彦, 曲茂华, 卢夏锋, 曹敏, 杜峰, 季荣涛, 董磊迟, 罗志波. 体外多酶组装与生物级联催化:进展与展望[J]. 合成生物学, 2025, 6(4): 920-939.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-056
| 应用领域 | 酶系统组成 | 设计思路 | 性能指标 | 优势 | 参考文献 |
|---|---|---|---|---|---|
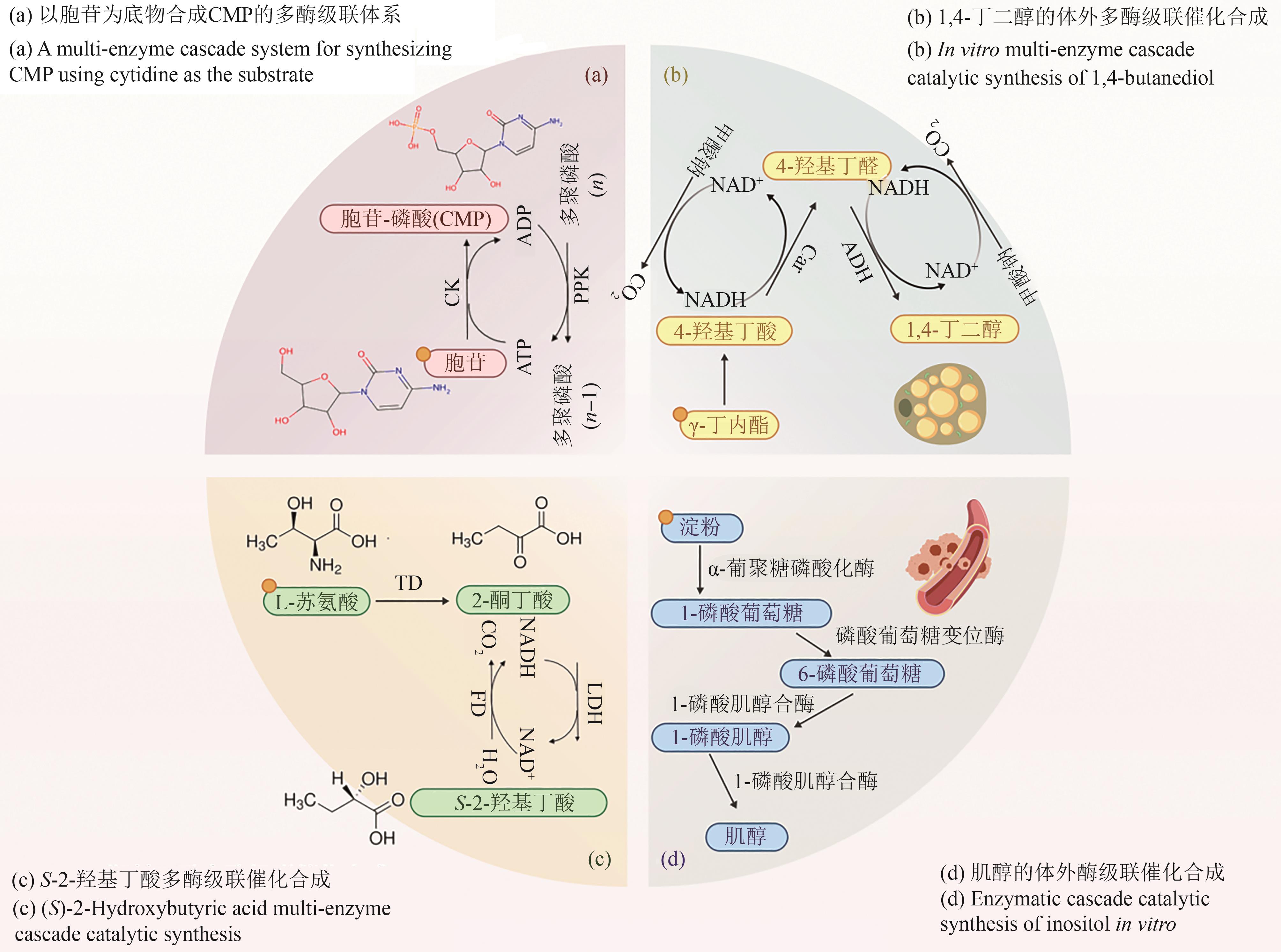
| 1,4-丁二醇合成 | 己内酯水解酶+羧酸还原酶+醇脱氢酶 | 三酶级联简化化学路线 辅酶循环(FDH) | 替代高压化学法 减少有毒试剂使用 | 产量2.41 g/L 反应步骤从9步减至3步 | [ |
| S-2-羟基丁酸 | 苏氨酸脱氨酶+乳酸脱氢酶+甲酸脱氢酶 | 动态动力学拆分 NADH循环系统 | 高转化率(97%) 光学纯度>99% | 产量143 g/L 无需外源辅酶 | [ |
| 索磷布韦中间体 | 转氨酶+亚胺还原酶 | 适配NADPH再生系统 选择热稳定性突变体 | 产物e.e.值>99.5% 高转化率(>90%) | 产率提升至92% 半衰期延长至120 h | [ |
| 手性胺合成 | 转氨酶+甲酸脱氢酶 | 热力学耦合设计 辅酶NADH原位再生 | 原子经济性>98% 产物e.e.值>99.9% | 时空产率8.3 g/(L·h) 辅酶周转数12000 | [ |
| 肌醇合成 | 淀粉磷酸化酶+肌醇-1-磷酸合酶等四酶 | 以淀粉为廉价底物 仿生代谢路径重构 | 转化率84.6% 成本较化学法低70% | 产量42.3 g/L 4步反应“一锅法” | [ |
| D-甘露醇生产 | 甘露糖脱氢酶+葡萄糖脱氢酶 | 双酶辅因子循环 底物通道效应优化 | 摩尔转化率81.9% 避免化学还原步骤 | 产量81.9 g/L 反应条件温和 | [ |
Table 1 Comparative analysis table of application cases of in vitro multi-enzyme catalysis
| 应用领域 | 酶系统组成 | 设计思路 | 性能指标 | 优势 | 参考文献 |
|---|---|---|---|---|---|
| 1,4-丁二醇合成 | 己内酯水解酶+羧酸还原酶+醇脱氢酶 | 三酶级联简化化学路线 辅酶循环(FDH) | 替代高压化学法 减少有毒试剂使用 | 产量2.41 g/L 反应步骤从9步减至3步 | [ |
| S-2-羟基丁酸 | 苏氨酸脱氨酶+乳酸脱氢酶+甲酸脱氢酶 | 动态动力学拆分 NADH循环系统 | 高转化率(97%) 光学纯度>99% | 产量143 g/L 无需外源辅酶 | [ |
| 索磷布韦中间体 | 转氨酶+亚胺还原酶 | 适配NADPH再生系统 选择热稳定性突变体 | 产物e.e.值>99.5% 高转化率(>90%) | 产率提升至92% 半衰期延长至120 h | [ |
| 手性胺合成 | 转氨酶+甲酸脱氢酶 | 热力学耦合设计 辅酶NADH原位再生 | 原子经济性>98% 产物e.e.值>99.9% | 时空产率8.3 g/(L·h) 辅酶周转数12000 | [ |
| 肌醇合成 | 淀粉磷酸化酶+肌醇-1-磷酸合酶等四酶 | 以淀粉为廉价底物 仿生代谢路径重构 | 转化率84.6% 成本较化学法低70% | 产量42.3 g/L 4步反应“一锅法” | [ |
| D-甘露醇生产 | 甘露糖脱氢酶+葡萄糖脱氢酶 | 双酶辅因子循环 底物通道效应优化 | 摩尔转化率81.9% 避免化学还原步骤 | 产量81.9 g/L 反应条件温和 | [ |
| [1] | WATARI T, HATA S, NAKAJIMA K, et al. Limited quantity and quality of steel supply in a zero-emission future[J]. Nature Sustainability, 2023, 6(3): 336-343. |
| [2] | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2): 801-838. |
| [3] | SCHRITTWIESER J H, VELIKOGNE S, HALL M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules[J]. Chemical Reviews, 2018, 118(1): 270-348. |
| [4] | XIAO Y Q, FENG C, FU J, et al. Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation[J]. Nature Catalysis, 2020, 3(11): 932-940. |
| [5] | ROSA R, SPINELLI R, NERI P, et al. Life cycle assessment of chemical vs enzymatic-assisted extraction of proteins from black soldier fly prepupae for the preparation of biomaterials for potential agricultural use[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14752-14764. |
| [6] | 国家统计局社会科技和文化产业统计司. 中国高技术产业统计年鉴[M]. 北京: 中国统计出版社, 2023. |
| Department of Social Science, Technology and Cultural Industry Statistics, National Bureau of Statistics. Statistical yearbook of China’s high-tech industries[M]. Beijing: China Statistics Press, 2023. | |
| [7] | WU B, WANG Y W, DAI Y H, et al. Current status and future prospective of bio-ethanol industry in China[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111079. |
| [8] | DENG W P, YAN L F, WANG B J, et al. Efficient catalysts for the green synthesis of adipic acid from biomass[J]. Angewandte Chemie International Edition, 2021, 60(9): 4712-4719. |
| [9] | LI M, ZHANG Z J, KONG X D, et al. Engineering Streptomyces coelicolor carbonyl reductase for efficient atorvastatin precursor synthesis[J]. Applied and Environmental Microbiology, 2017, 83(12): e00603-17. |
| [10] | DARGÓ G, KIS D, RÁDULY A, et al. Furandicarboxylic acid (FDCA): electrosynthesis and its facile recovery from polyethylene furanoate (PEF) via depolymerization[J]. ChemSusChem, 2025, 18(3): e202401190. |
| [11] | MOKALE KOGNOU A L, SHRESTHA S, JIANG Z H, et al. High-fructose corn syrup production and its new applications for 5-hydroxymethylfurfural and value-added furan derivatives: promises and challenges[J]. Journal of Bioresources and Bioproducts, 2022, 7(3): 148-160. |
| [12] | 李举谋, 石焜, 张志钧, 等. 多酶级联反应的构建及其在双官能团功能化学品合成中的应用[J]. 生物工程学报, 2023, 39(6): 2158-2189. |
| LI J M, SHI K, ZHANG Z J, et al. Construction of multi-enzyme cascade reactions and its application in the synthesis of bifunctional chemicals[J]. Chinese Journal of Biotechnology, 2023, 39(6): 2158-2189. | |
| [13] | LOPEZ-GALLEGO F, SCHMIDT-DANNERT C. Multi-enzymatic synthesis[J]. Current Opinion in Chemical Biology, 2010, 14(2): 174-183. |
| [14] | KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux[J]. Nature Communications, 2019, 10: 4248. |
| [15] | ABIDIN M Z, SARAVANAN T, ZHANG J L, et al. Modular enzymatic cascade synthesis of vitamin B5 and its derivatives[J]. Chemistry-A European Journal, 2018, 24(66): 17434-17438. |
| [16] | DONG H R, GUO N X, HU D C, et al. Chemoenzymatic total synthesis of alchivemycin A[J]. Nature Synthesis, 2024, 3(9): 1124-1133. |
| [17] | SHI Q C, ZHANG B Y, WU Z H, et al. Cascade catalytic systems for converting CO2 into C2+ products[J]. ChemSusChem, 2025, 18(7): e202401916. |
| [18] | 郭华, 张蕾, 董旭, 等. 固定化多酶级联反应器[J]. 化学进展, 2020, 32(4): 392-405. |
| GUO H, ZHANG L, DONG X, et al. Immobilized multi-enzyme cascade reactor[J]. Progress in Chemistry, 2020, 32(4): 392-405. | |
| [19] | JIANG S, LI H Q, ZHANG L, et al. Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics[J]. Nucleic Acids Research, 2025, 53(D1): D1670-D1676. |
| [20] | GAO Y, LI F, LUO Z S, et al. Modular assembly of an artificially concise biocatalytic cascade for the manufacture of phenethylisoquinoline alkaloids[J]. Nature Communications, 2024, 15: 30. |
| [21] | KHOBRAGADE T P, SARAK S, PAGAR A D, et al. Synthesis of sitagliptin intermediate by a multi-enzymatic cascade system using lipase and transaminase with benzylamine as an amino donor[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 757062. |
| [22] | SIEGEL J B, ZANGHELLINI A, LOVICK H M, et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction[J]. Science, 2010, 329(5989): 309-313. |
| [23] | WANG C, ZHANG H Y, WANG Y, et al. A general strategy for the synthesis of hierarchically ordered metal-organic frameworks with tunable macro-, meso-, and micro-pores[J]. Small, 2023, 19(3): 2206116. |
| [24] | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| [25] | RAUTER M, NIETZ D, KUNZE G. Cutinase ACut2 from blastobotrysraffinosifermentans for the selective desymmetrization of the symmetric diester diethyl adipate to the monoester monoethyl adipate[J]. Microorganisms, 2022, 10(7): 1316. |
| [26] | WANG F H, QI H B, LI H M, et al. State-of-the-art strategies and research advances for the biosynthesis of D-amino acids[J]. Critical Reviews in Biotechnology, 2024, 44(4): 495-513. |
| [27] | SUN Y P, SHU T, MA J X, et al. Rational design of ZIF-8 for constructing luminescent biosensors with glucose oxidase and AIE-type gold nanoclusters[J]. Analytical Chemistry, 2022, 94(7): 3408-3417. |
| [28] | ENGEL J, BORNSCHEUER U T, KARA S. Kinetics modeling of a convergent cascade catalyzed by monooxygenase-alcohol dehydrogenase coupled enzymes[J]. Organic Process Research & Development, 2021, 25(3): 411-420. |
| [29] | ZHANG D P, JING X R, ZHANG W L, et al. Highly selective synthesis of D-amino acids from readily available L-amino acids by a one-pot biocatalytic stereoinversion cascade[J]. RSC Advances, 2019, 9(51): 29927-29935. |
| [30] | LI S F, ZHANG W, ZHANG W, et al. Recent advances in the synthesis and analysis of atorvastatin and its intermediates[J]. Current Medicinal Chemistry, 2024, 31(37): 6063-6083. |
| [31] | TIBREWAL N, TANG Y. Biocatalysts for natural product biosynthesis[J]. Annual Review of Chemical and Biomolecular Engineering, 2014, 5: 347-366. |
| [32] | MONTERREY D T, AZCONA L, REVUELTA J, et al. Polyphosphate kinase from Burkholderia cenocepacia, one enzyme catalyzing a two-step cascade reaction to synthesize ATP from AMP[J]. International Journal of Molecular Sciences, 2024, 25(23): 12995. |
| [33] | XIAO W L, HUANG T E, ZHOU J, et al. Inhibition of MAT2A impairs skeletal muscle repair function[J]. Biomolecules, 2024, 14(9): 1098. |
| [34] | RODRIGUEZ-ABETXUKO A, REIFS A, SÁNCHEZ-DEALCÁZAR D, et al. A versatile chemoenzymatic nanoreactor that mimics NAD(P)H oxidase for the in situ regeneration of cofactors[J]. Angewandte Chemie International Edition, 2022, 61(39): e202206926. |
| [35] | SHI J F, WU Y Z, ZHANG S H, et al. Bioinspired construction of multi-enzyme catalytic systems[J]. Chemical Society Reviews, 2018, 47(12): 4295-4313. |
| [36] | PENG T, TIAN J, ZHAO Y Y, et al. Multienzyme redox system with cofactor regeneration for cyclic deracemization of sulfoxides[J]. Angewandte Chemie International Edition, 2022, 61(37): e202209272. |
| [37] | GHIMIRE N, OH T J. Cell-free system for one-pot production of protocatechuate via a two-enzyme cascade with coenzyme regeneration[J]. International Journal of Biological Macromolecules, 2025, 306: 141269. |
| [38] | ZHOU M J, BOUAZZAOUI S, JONES L E, et al. Isolation and structural determination of non-racemic tertiary cathinone derivatives[J]. Organic & Biomolecular Chemistry, 2015, 13(37): 9629-9636. |
| [39] | DEL VECCHIO D. Modularity, context-dependence, and insulation in engineered biological circuits[J]. Trends in Biotechnology, 2015, 33(2): 111-119. |
| [40] | KIM Y C, YOO H W, PARK B G, et al. One-pot biocatalytic route from alkanes to α, ω-diamines by whole-cell consortia of engineered Yarrowia lipolytica and Escherichia coli [J]. ACS Synthetic Biology, 2024, 13(7): 2188-2198. |
| [41] | SAVILE C K, JANEY J M, MUNDORFF E C, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture[J]. Science, 2010, 329(5989): 305-309. |
| [42] | HUANG X Q, FENG J Q, CUI J W, et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition[J]. Nature Catalysis, 2022, 5(7): 586-593. |
| [43] | NGUYEN L T, YANG K L. Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions[J]. Enzyme and Microbial Technology, 2017, 100: 52-59. |
| [44] | FERNANDES C G, SAWANT S C, MULE T A, et al. Enhancing cellulases through synergistic β-glucosidases for intensifying cellulose hydrolysis[J]. Process Biochemistry, 2022, 120: 202-212. |
| [45] | YU X, CHEN X Y, YU H L, et al. Regio- and stereo-selective amination of fatty acids tod-amino acids by a three-step one-pot cascade[J]. Green Chemistry, 2023, 25(9): 3469-3474. |
| [46] | CUI J D, QIU J Q, FAN X W, et al. Biotechnological production and applications of microbial phenylalanine ammonia lyase: a recent review[J]. Critical Reviews in Biotechnology, 2014, 34(3): 258-268. |
| [47] | 任杰, 曾安平. 基于二氧化碳的生物制造: 从基础研究到工业应用的挑战[J]. 合成生物学, 2021, 2(6): 854-862. |
| REN J, ZENG A P. CO2 based biomanufacturing: from basic research to industrial application[J]. Synthetic Biology Journal, 2021, 2(6): 854-862. | |
| [48] | NAM K H. Glucose isomerase: functions, structures, and applications[J]. Applied Sciences, 2022, 12(1): 428. |
| [49] | 徐铮, 徐恺, 陈昱金, 等. 异构酶在生物制造中的研究进展[J]. 食品与发酵工业, 2021, 47(5): 244-251. |
| XU Z, XU K, CHEN Y J, et al. Recent advances on isomerases for bio-manufacturing[J]. Food and Fermentation Industries, 2021, 47(5): 244-251. | |
| [50] | HAMMER S C, KUBIK G, WATKINS E, et al. Anti-Markovnikov alkene oxidation by metal-oxo-mediated enzyme catalysis[J]. Science, 2017, 358(6360): 215-218. |
| [51] | GUO J M, XU C Y, LI J, et al. Dual role of gluconic acid in the cascading saccharification of hemicellulose and cellulose from various lignocellulosic stuff[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(22): 8325-8339. |
| [52] | XU J, CEN Y X, SINGH W, et al. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters[J]. Journal of the American Chemical Society, 2019, 141(19): 7934-7945. |
| [53] | SUN S Z, NICHOLLS B T, BAIN D, et al. Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis[J]. Nature Catalysis, 2023, 7(1): 35-42. |
| [54] | LAUKO A, PELLOCK S J, SUMIDA K H, et al. Computational design of serine hydrolases[J]. Science, 388(6744): 24-54. |
| [55] | PAN Y J, LI G B, LIU R X, et al. Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acids[J]. Nature Communications, 2023, 14: 1669. |
| [56] | UTHARALA R, GRAB A, VAFAIZADEH V, et al. A microfluidic Braille valve platform for on-demand production, combinatorial screening and sorting of chemically distinct droplets[J]. Nature Protocols, 2022, 17(12): 2920-2965. |
| [57] | HOMMA F, HUANG J, VAN DER HOORN R A L. AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface[J]. Nature Communications, 2023, 14: 6040. |
| [58] | JANG T, SHIN S J, LIM H K, et al. DFT-CES2: quantum mechanics based embedding for mean-field QM/MM of solid-liquid interfaces[J]. JACS Au, 2025, 5(4): 2047-2058. |
| [59] | WU X L, YANG C, GE J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents[J]. Bioresources and Bioprocessing, 2017, 4(1): 24. |
| [60] | 董玲玲, 李斐煊, 雷航彬, 等. 仿生分区室固定化多酶体系[J]. 合成生物学, 2024, 5(6): 1518-1529. |
| DONG L L, LI F X, LEI H B, et al. Biomimetic compartmentalization immobilization of multi-enzyme system[J]. Synthetic Biology Journal, 2024, 5(6): 1518-1529. | |
| [61] | 毕春元, 任婷月, 张金玲, 等. 离子交换树脂共固定葡萄糖氧化酶-过氧化氢酶[J]. 食品与发酵工业, 2015, 41(7): 13-18. |
| BI C Y, REN T Y, ZHANG J L, et al. Co-immobilization of glucose oxidase and catalase on ion exchange resin[J]. Food and Fermentation Industries, 2015, 41(7): 13-18. | |
| [62] | WILLIAMS V, CUI Y X, JIANG X J, et al. Co-immobilized multienzyme system for the cofactor-driven cascade synthesis of (R)-2-amino-3-(2-bromophenyl)propanoic acid: a model reaction[J]. Organic Process Research & Development, 2022, 26(11): 3024-3033. |
| [63] | KIM M, LEE C, JEON K, et al. Harnessing a paper-folding mechanism for reconfigurable DNA origami[J]. Nature, 2023, 619(7968): 78-86. |
| [64] | WANG Y, SELIVANOVITCH E, DOUGLAS T. Enhancing multistep reactions: biomimetic design of substrate channeling using P22 virus-like particles[J]. Advanced Science, 2023, 10(13): 2206906. |
| [65] | YANG L, YUAN Q Y, LI T T, et al. Recent developments and applications of pH-responsive polymers[J]. Textile Research Journal, 2025: 00405175241305543. |
| [66] | GODOY-GALLARDO M, LABAY C, TRIKALITIS V D, et al. Multicompartment artificial organelles conducting enzymatic cascade reactions inside cells[J]. ACS Applied Materials & Interfaces, 2017, 9(19): 15907-15921. |
| [67] | YE J J, CHU T S, CHU J L, et al. A versatile approach for enzyme immobilization using chemically modified 3D-printed scaffolds[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(21): 18048-18054. |
| [68] | 苏枫. 微生物脂肪酶的固定化新技术及在生物柴油制备中的应用[D]. 武汉: 华中科技大学, 2017. |
| SU F. Improving performance of microbial lipases via new immobilization technology and application in biodiesel preparation[D]. Wuhan: Huazhong University of Science and Technology, 2017. | |
| [69] | 陈坚, Omasa Takeshi, Katakura Yoshio, 等. 固定化酶-离子交换组合系统进行青霉素G水解生产6-APA的模型化研究[J]. 生物工程学报, 1995, 11(4): 343-349. |
| CHEN J, OMASA T, KATAKURA Y, et al. Modeling of penicillin G hydrolysis to 6-APA in an immobilized enzyme-ion exchange system[J]. Chinese Journal of Biotechnology, 1995, 11(4): 343-349. | |
| [70] | 万娟娟, 刘旭峰, 戎海波, 等. 酶固定化技术及固定化酶应用的研究进展[J]. 现代化工, 2024, 44(S1): 73-79. |
| WAN J J, LIU X F, RONG H B, et al. An overview of enzyme immobilizing technologies and application of immobilized enzymes[J]. Modern Chemical Industry, 2024, 44(S1): 73-79. | |
| [71] | 尚红岩, 翁绮纹, 练文妃, 等. 纤维素酶固定化工艺条件优化及在甘蔗渣酶解中的应用[J]. 甘蔗糖业, 2024, 53(5): 42-48. |
| SHANG H Y, WENG Q W, LIAN W F, et al. Optimization of cellulase immobilization process conditions and its application in sugarcane bagasse enzymatic hydrolysis[J]. Sugarcane and Canesugar, 2024, 53(5): 42-48. | |
| [72] | 徐惠东, 尤扬, 游颖欣, 等. 一种高耐热乳糖酶的异源表达、固定化及酶学性质研究[J]. 食品与发酵工业, 2024, 50(21): 1-8. |
| XU H D, YOU Y, YOU Y X, et al. Heterologous expression and enzymatic characterization of a highly thermostable lactase and its immobilized enzyme[J]. Food and Fermentation Industries, 2024, 50(21): 1-8. | |
| [73] | TANG C D, ZHANG Z H, SHI H L, et al. Directed evolution of formate dehydrogenase and its application in the biosynthesis of L-phenylglycine from phenylglyoxylic acid[J]. Molecular Catalysis, 2021, 513: 111666. |
| [74] | GORAN J M, FAVELA C A, STEVENSON K J. Investigating the electrocatalytic oxidation of dihydronicotinamide adenine dinucleotide at nitrogen-doped carbon nanotube electrodes: implications to electrochemically measuring dehydrogenase enzyme kinetics[J]. ACS Catalysis, 2014, 4(9): 2969-2976. |
| [75] | LIU T, YIN Y X, YANG Y, et al. Layer-by-layer engineered all-liquid microfluidic chips for enzyme immobilization[J]. Advanced Materials, 2022, 34(5): 2105386. |
| [76] | LI G R, MA W D, YANG Y X, et al. Nanoscale covalent organic frameworks with donor-acceptor structures as highly efficient light-responsive oxidase-like mimics for colorimetric detection of glutathione[J]. ACS Applied Materials & Interfaces, 2021, 13(41): 49482-49489. |
| [77] | CHEN Y, TAO K, JI W, et al. Histidine as a key modulator of molecular self-assembly: peptide-based supramolecular materials inspired by biological systems[J]. Materials Today, 2022, 60: 106-127. |
| [78] | KIGHTLINGER W, DUNCKER K E, RAMESH A, et al. A cell-free biosynthesis platform for modular construction of protein glycosylation pathways[J]. Nature Communications, 2019, 10: 5404. |
| [79] | SU H H, GUO Z W, WU X L, et al. Efficient bioconversion of sucrose to high-value-added glucaric acid by in vitro metabolic engineering[J]. ChemSusChem, 2019, 12(10): 2278-2285. |
| [80] | SUN Z B, XU J L, LU X, et al. Directed mutation of β-glucanases from probiotics to enhance enzymatic activity, thermal and pH stability[J]. Archives of Microbiology, 2020, 202(7): 1749-1756. |
| [81] | BACHOSZ K, ZDARTA J, BILAL M, et al. Enzymatic cofactor regeneration systems: a new perspective on efficiency assessment[J]. Science of the Total Environment, 2023, 868: 161630. |
| [82] | HOLZMAN D C. The carbon footprint of biofuels: can we shrink it down to size in time?[J]. Environmental Health Perspectives, 2008, 116(6): A246-A252. |
| [83] | KHOBRAGADE T P, PAGAR A D, GIRI P, et al. Biocatalytic cascade for synthesis of sitagliptin intermediate employing coupled transaminase[J]. Biotechnology and Bioprocess Engineering, 2023, 28(2): 300-309. |
| [84] | 曹熙. 一种固定化脂肪酶的方法及其在生物柴油反应中的应用[D]. 北京: 北京化工大学, 2015. |
| CAO X. A method for fixing lipase and its application in bio-diesel reactions[D]. Beijing: Beijing University of Chemical Technology, 2015. | |
| [85] | TING W-W, NISHIKAWA S, YU W-C, et al. Chemo-enzymatic synthesis of coenzyme a using copurified enzymes from probiotic Escherichia coli Nissle[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(27): 10068-10074. |
| [86] | PAN Y J, LIU Y F, PHAN T L, et al. Biomanufacturing of inositol from corn stover with biological pretreatment by an in vitro synthetic biology platform[J]. ACS Sustainable Chemistry & Engineering, 2025, 13(1): 436-446. |
| [87] | LI Z L, NING X, ZHAO Y R, et al. Efficient one-pot synthesis of cytidine 5'-monophosphate using an extremophilic enzyme cascade system[J]. Journal of Agricultural and Food Chemistry, 2020, 68(34): 9188-9194. |
| [88] | FERREIRA S, BALOLA A, SVESHNIKOVA A, et al. Computer-aided design and implementation of efficient biosynthetic pathways to produce high added-value products derived from tyrosine in Escherichia coli [J]. Frontiers in Bioengineering and Biotechnology, 2024, 12: 1360740. |
| [89] | IMAM H T, MARR P C, MARR A C. Enzyme entrapment, biocatalyst immobilization without covalent attachment[J]. Green Chemistry, 2021, 23(14): 4980-5005. |
| [90] | YU T H, CUI H Y, LI J C, et al. Enzyme function prediction using contrastive learning[J]. Science, 2023, 379(6639): 1358-1363. |
| [91] | LI R F, WIJMA H J, SONG L, et al. Computational redesign of enzymes for regio- and enantioselective hydroamination[J]. Nature Chemical Biology, 2018, 14(7): 664-670. |
| [92] | JIAO Y F, WANG H Y, WANG H, et al. A DNA origami-based enzymatic cascade nanoreactor for chemodynamic cancer therapy and activation of antitumor immunity[J]. Science Advances, 2025, 11(2): eadr9196. |
| [93] | XU K Q, CHATZITAKIS A, BACKE P H, et al. In situ cofactor regeneration enables selective CO2 reduction in a stable and efficient enzymatic photoelectrochemical cell[J]. Applied Catalysis B: Environmental, 2021, 296: 120349. |
| [94] | 支睿, 李国辉, 毛银, 等. 己二酸生物合成的途径改造以及发酵条件优化[J]. 食品与发酵工业, 2024, 50(3): 38-44. |
| ZHI R, LI G H, MAO Y, et al. Metabolic pathway and fermentation optimization of the biosynthesis of adipic acid[J]. Food and Fermentation Industries, 2024, 50(3): 38-44. | |
| [95] | WANG F, ZHAO J, LI Q, et al. One-pot biocatalytic route from cycloalkanes to α, ω‐dicarboxylic acids by designed Escherichia coli consortia[J]. Nature Communications, 2020, 11: 5035. |
| [96] | LI X Y, CAO Y F, LUO K, et al. Highly active enzyme-metal nanohybrids synthesized in protein-polymer conjugates[J]. Nature Catalysis, 2019, 2(8): 718-725. |
| [97] | 郭艺鸣, 姜君逸, 潘学玮, 等. 多酶级联反应催化γ-丁内酯生成1,4-丁二醇[J]. 应用与环境生物学报, 2024, 30(1): 167-175. |
| GUO Y M, JIANG J Y, PAN X W, et al. Multi-enzyme cascade reaction catalyzed γ-butyrolactone to 1,4-butanediol[J]. Chinese Journal of Applied and Environmental Biology, 2024, 30(1): 167-175. | |
| [98] | 姜君逸, 郭艺鸣, 杨套伟, 等. 代谢工程改造大肠杆菌从头合成1,4-丁二醇[J]. 生物工程学报, 2024, 40(9): 3142-3157. |
| JIANG J Y, GUO Y M, YANG T W, et al. Metabolic engineering of Escherichia coli for de novo synthesis of 1,4-butanediol[J]. Chinese Journal of Biotechnology, 2024, 40(9): 3142-3157. | |
| [99] | 田灵芝, 周俊平, 杨套伟, 等. 基于多酶级联协调表达策略高效催化合成(S)-2-羟基丁酸[J]. 生物工程学报, 2021, 37(12): 4231-4242. |
| TIAN L Z, ZHOU J P, YANG T W, et al. Efficient cascade biosynthesis of (S)-2-hydroxybutyric acid[J]. Chinese Journal of Biotechnology, 2021, 37(12): 4231-4242. | |
| [100] | BENÍTEZ-MATEOS A I, ROURA PADROSA D, PARADISI F. Multistep enzyme cascades as a route towards green and sustainable pharmaceutical syntheses[J]. Nature Chemistry, 2022, 14(5): 489-499. |
| [101] | LIU L, WANG D H, CHEN F F, et al. Development of an engineered thermostable amine dehydrogenase for the synthesis of structurally diverse chiral amines[J]. Catalysis Science & Technology, 2020, 10(8): 2353-2358. |
| [102] | 王高杨. 肌醇-1-磷酸合成酶的固定化及其在肌醇合成中应用[D]. 天津: 天津科技大学, 2021. |
| WANG G Y. Immobilization of inositol-1-phosphate synthase and its application in inositol synthesis[D]. Tianjin: Tianjin University of Science & Technology, 2021. | |
| [103] | 魏梓佳, 樊宇成, 张槿博, 等. 肌醇-1-磷酸合酶的重组表达及在多酶级联催化合成肌醇中的应用[J]. 食品与发酵工业, 2025, 51(4): 280-287. |
| WEI Z J, FAN Y C, ZHANG J B, et al. Recombinant expression of inositol-1-phosphate synthase and its application in multienzyme cascade catalytic synthesis of inositol[J]. Food and Fermentation Industries, 2025, 51(4): 280-287. | |
| [104] | 潘珊, 胡孟凯, 潘学玮, 等. 基于双酶级联协调表达策略高效催化合成D-甘露醇[J]. 生物工程学报, 2022, 38(7): 2549-2565. |
| PAN S, HU M K, PAN X W, et al. Efficient biosynthesis of D-mannitol by coordinated expression of a two-enzyme cascade[J]. Chinese Journal of Biotechnology, 2022, 38(7): 2549-2565. | |
| [105] | 张建志, 付立豪, 唐婷, 等. 基于合成生物学策略的酶蛋白元件规模化挖掘[J]. 合成生物学, 2020, 1(3): 319-336. |
| ZHANG J Z, FU L H, TANG T, et al. Scalable mining of proteins for biocatalysis via synthetic biology[J]. Synthetic Biology Journal, 2020, 1(3): 319-336. | |
| [106] | 曲戈, 朱彤, 蒋迎迎, 等. 蛋白质工程: 从定向进化到计算设计[J]. 生物工程学报, 2019, 35(10): 1843-1856. |
| QU G, ZHU T, JIANG Y Y, et al. Protein engineering: from directed evolution to computational design[J]. Chinese Journal of Biotechnology, 2019, 35(10): 1843-1856. | |
| [107] | KASHINATH K P, SANJAY L R, ASHOKBHAI M K, et al. Continuous manufacturing based paradigm shift in pharmaceuticals production and current regulatory framework[J]. Chemical Engineering Research and Design, 2025, 215: 1-22. |
| [108] | 石婷, 宋展, 宋世怡, 等. 体外生物转化(ivBT): 生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| SHI T, SONG Z, SONG S Y, et al. In vitro bio transformation(ivBT): a new frontier of industrial biomanufacturing[J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [1] | WANG Mingpeng, CHEN Lei, ZHAO Yiran, ZHANG Yimin, ZHENG Qifan, LIU Xinyang, WANG Yixue, WANG Qinhong. Halogenases in biocatalysis: advances in mechanism elucidation, directed evolution, and green manufacturing [J]. Synthetic Biology Journal, 2025, 6(4): 728-763. |
| [2] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [3] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [4] | KANG Liqi, TAN Pan, HONG Liang. Enzyme engineering in the age of artificial intelligence [J]. Synthetic Biology Journal, 2023, 4(3): 524-534. |
| [5] | CUI Xinyu, WU Ranran, WANG Yuanming, ZHU Zhiguang. Construction and enhancement of enzymatic bioelectrocatalytic systems [J]. Synthetic Biology Journal, 2022, 3(5): 1006-1030. |
| [6] | XIONG Liangbin, SONG Lu, ZHAO Yunqiu, LIU Kun, LIU Yongjun, WANG Fengqing, WEI Dongzhi. Green biomanufacturing of steroids: from biotransformation to de novo synthesis by microorganisms [J]. Synthetic Biology Journal, 2021, 2(6): 942-963. |
| [7] | ZHANG Yi-Heng. Remembering Professor Daniel I.C. Wang’s contribution to biorefining and my perspective on the progress [J]. Synthetic Biology Journal, 2021, 2(4): 497-508. |
| [8] | SHI Ran, JIANG Zhengqiang. Enzymatic synthesis of 2'-fucosyllactose: advances and perspectives [J]. Synthetic Biology Journal, 2020, 1(4): 481-494. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||