Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (6): 1109-1125.DOI: 10.12211/2096-8280.2022-029
• Invited Review • Previous Articles Next Articles
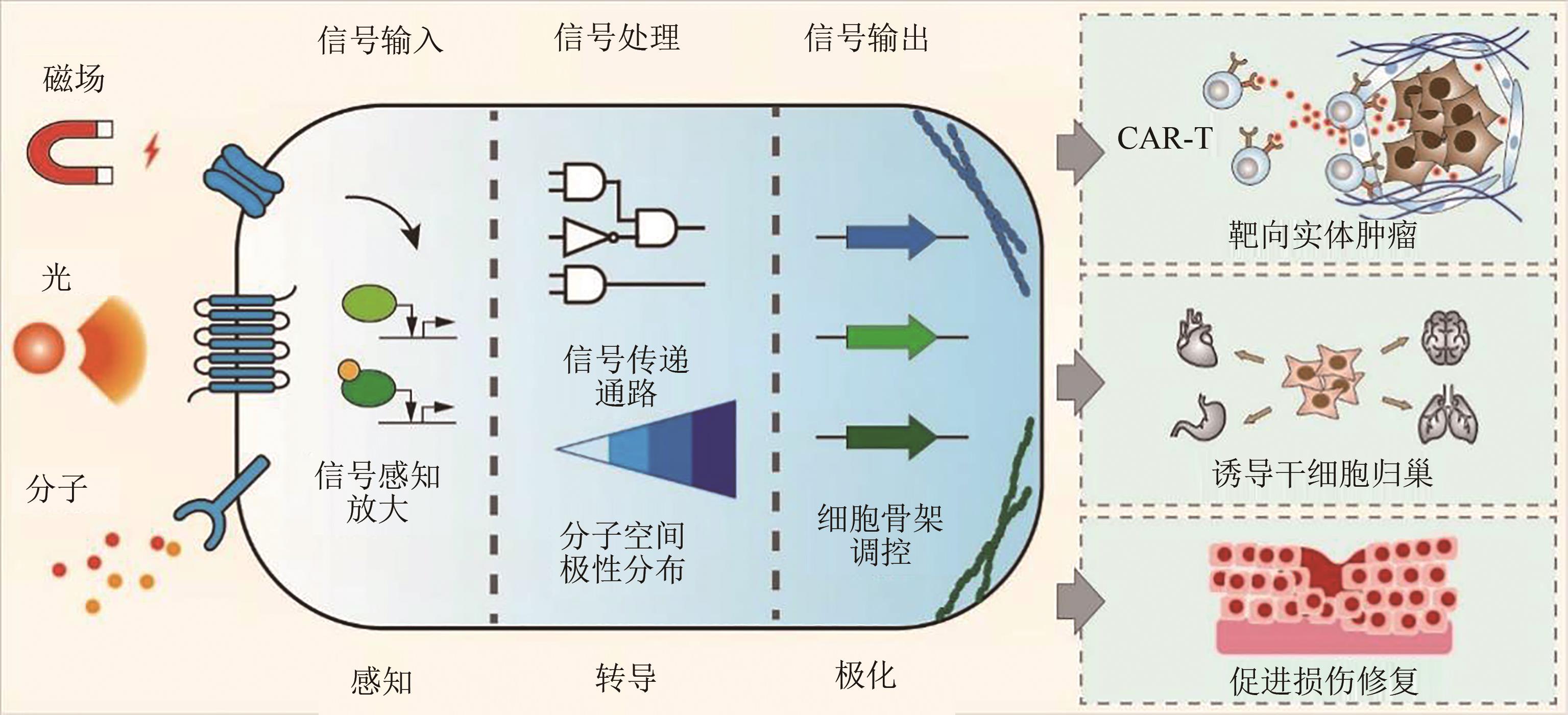
Artificial control of mammalian cell chemotaxis and motility
GUO Wei, FU Yuhao, FAN Yingying, ZHOU Jialing, LI Xin, WEI Ping
- CAS Key Laboratory of Quantitative Engineering Biology,Cell and Gene Circuit Design Center,Shenzhen Institute of Synthetic Biology,Shenzhen Institute of Advanced Technology,Chinese Academy of Science,Shenzhen 518055,Guangdong,China
-
Received:2022-05-23Revised:2022-09-05Online:2023-01-17Published:2022-12-31 -
Contact:WEI Ping
哺乳动物细胞的趋化迁移及人工控制
郭伟, 付禹豪, 范盈盈, 周佳铃, 李鑫, 魏平
- 中国科学院深圳先进技术研究院,深圳合成生物学创新研究院,细胞与基因线路设计中心,中国科学院定量工程生物学重点实验室,广东 深圳 518055
-
通讯作者:魏平 -
作者简介:郭伟 (1987—),男,博士后。研究方向为基于合成生物学的蛋白质工程、免疫细胞趋化线路的设计与开发。E-mail:wei.guo@siat.ac.cn魏平 (1980—),男,研究员,博士生导师。研究方向为生物网络的人工设计合成,基于合成生物学的细胞信息处理机制研究,以及免疫细胞工程化设计等。E-mail:ping.wei@siat.ac.cn -
基金资助:国家重点研发计划(2018YFA0902800);国家自然科学基金(31622022)
CLC Number:
Cite this article
GUO Wei, FU Yuhao, FAN Yingying, ZHOU Jialing, LI Xin, WEI Ping. Artificial control of mammalian cell chemotaxis and motility[J]. Synthetic Biology Journal, 2022, 3(6): 1109-1125.
郭伟, 付禹豪, 范盈盈, 周佳铃, 李鑫, 魏平. 哺乳动物细胞的趋化迁移及人工控制[J]. 合成生物学, 2022, 3(6): 1109-1125.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-029
| 1 | SENGUPTA S, PARENT C A, BEAR J E. The principles of directed cell migration[J]. Nature Reviews Molecular Cell Biology, 2021, 22(8): 529-547. |
| 2 | LI D, SUN F F, YANG Y H, et al. Gradients of PI(4, 5)P2 and PI(3, 5)P2 jointly participate in shaping the back state of dictyostelium cells[J]. Frontiers in Cell and Developmental Biology, 2022, 10: 835185. |
| 3 | MUYLAERT D E, FLEDDERUS J O, BOUTEN C V, et al. Combining tissue repair and tissue engineering; bioactivating implantable cell-free vascular scaffolds[J]. Heart, 2014, 100(23): 1825-1830. |
| 4 | MARTINEZ M, MOON E K. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment[J]. Frontiers in Immunology, 2019, 10: 128. |
| 5 | HUANG C H, TANG M, SHI C J, et al. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration[J]. Nature Cell Biology, 2013, 15(11): 1307-1316. |
| 6 | CARLIER M F, LE CLAINCHE C, WIESNER S, et al. Actin-based motility: from molecules to movement[J]. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 2003, 25(4): 336-345. |
| 7 | BORRELL V. Recent advances in understanding neocortical development[J]. F1000Research, 2019, 8(F1000FacultyRev-F1000Faculty): 1791. |
| 8 | LOCASCIO A, NIETO M A. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration[J]. Current Opinion in Genetics & Development, 2001, 11(4): 464-469. |
| 9 | FRANZE K. The mechanical control of nervous system development[J]. Development 2013, 140(15): 3069-3077. |
| 10 | SCARPA E, MAYOR R. Collective cell migration in development[J]. The Journal of Cell Biology, 2016, 212(2): 143-155. |
| 11 | JANSSEN E, GEHA R S. Primary immunodeficiencies caused by mutations in actin regulatory proteins[J]. Immunological Reviews, 2019, 287(1): 121-134. |
| 12 | MRASS P, WENINGER W. Immune cell migration as a means to control immune privilege: lessons from the CNS and tumors[J]. Immunological Reviews, 2006, 213(1): 195-212. |
| 13 | Migration GUNZER M., cell–cell interaction and adhesion in the immune system[C]// Baier G, Schraven B, Zügel U, von Bonin A ed. Sparking Signals. Ernst Schering Foundation Symposium Proceedings. Berlin, Heidelberg: Springer, 2007, 3(3): 97-137. |
| 14 | LI L, JIANG J X. Regulatory factors of mesenchymal stem cell migration into injured tissues and their signal transduction mechanisms[J]. Frontiers of Medicine, 2011, 5(1): 33-39. |
| 15 | ZHAO M. Electrical fields in wound healing—an overriding signal that directs cell migration[J]. Seminars in Cell & Developmental Biology, 2009, 20(6): 674-682. |
| 16 | ABREU-BLANCO M T, WATTS J J, VERBOON J M, et al. Cytoskeleton responses in wound repair[J]. Cellular and Molecular Life Sciences, 2012, 69(15): 2469-2483. |
| 17 | GARCÍA-CUESTA E M, SANTIAGO C A, VALLEJO-DÍAZ J, et al. The role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases[J]. Frontiers in Endocrinology, 2019, 10: 585. |
| 18 | GRIFFITH J W, LUSTER A D. Targeting cells in motion: migrating toward improved therapies[J]. European Journal of Immunology, 2013, 43(6): 1430-1435. |
| 19 | ZERNECKE A, WEBER C. Chemokines in the vascular inflammatory response of atherosclerosis[J]. Cardiovascular Research, 2010, 86(2): 192-201. |
| 20 | NOVIKOV N M, ZOLOTARYOVA S Y, GAUTREAU A M, et al. Mutational drivers of cancer cell migration and invasion[J]. British Journal of Cancer, 2021, 124(1): 102-114. |
| 21 | WELLS A, GRAHOVAC J, WHEELER S, et al. Targeting tumor cell motility as a strategy against invasion and metastasis[J]. Trends in Pharmacological Sciences, 2013, 34(5): 283-289. |
| 22 | POLACHECK W J, ZERVANTONAKIS I K, KAMM R D. Tumor cell migration in complex microenvironments[J]. Cellular and Molecular Life Sciences, 2013, 70(8): 1335-1356. |
| 23 | SHELLARD A, MAYOR R. All roads lead to directional cell migration[J]. Trends in Cell Biology, 2020, 30(11): 852-868. |
| 24 | LADOUX B, MÈGE R M. Mechanobiology of collective cell behaviours[J]. Nature Reviews Molecular Cell Biology, 2017, 18(12): 743-757. |
| 25 | ARMSTRONG J P K, STEVENS M M. Using remote fields for complex tissue engineering[J]. Trends in Biotechnology, 2020, 38(3): 254-263. |
| 26 | STRÖMBLAD S. Cancer biology: hypoxia-induced talin tail-docking Sparks cancer metastasis[J]. Current Biology: CB, 2022, 32(2): R79-R81. |
| 27 | GRAZIANI V, RODRIGUEZ-HERNANDEZ I, MAIQUES O, et al. The amoeboid state as part of the epithelial-to-mesenchymal transition programme[J]. Trends in Cell Biology, 2022, 32(3): 228-242. |
| 28 | AOUN L, FARUTIN A, GARCIA-SEYDA N, et al. Amoeboid swimming is propelled by molecular paddling in lymphocytes[J]. Biophysical Journal, 2020, 119(6): 1157-1177. |
| 29 | LIU Y J, LE BERRE M, LAUTENSCHLAEGER F, et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells[J]. Cell, 2015, 160(4): 659-672. |
| 30 | OAKES P W. Balancing forces in migration[J]. Current Opinion in Cell Biology, 2018, 54: 43-49. |
| 31 | BOEKHORST V TE, PREZIOSI L, FRIEDL P. Plasticity of cell migration in vivo and in silico[J]. Annual Review of Cell and Developmental Biology, 2016, 32: 491-526. |
| 32 | SCHUMANN K, LÄMMERMANN T, BRUCKNER M, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells[J]. Immunity, 2010, 32(5): 703-713. |
| 33 | YAMADA K M, SIXT M. Mechanisms of 3D cell migration[J]. Nature Reviews Molecular Cell Biology, 2019, 20(12): 738-752. |
| 34 | JIAO H F, JIANG D, HU X Y, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process[J]. Cell, 2021, 184(11): 2896-2910.e13. |
| 35 | GRAY A L, PUN N, RIDLEY A J L, et al. Role of extracellular matrix proteoglycans in immune cell recruitment[J]. International Journal of Experimental Pathology, 2022, 103(2): 34-43. |
| 36 | SCHWARTZ M A. Integrins and extracellular matrix in mechanotransduction[J]. Cold Spring Harbor Perspectives in Biology, 2010, 2(12): a005066. |
| 37 | ESPINA J A, MARCHANT C L, BARRIGA E H. Durotaxis: the mechanical control of directed cell migration[J]. The FEBS Journal, 2022, 289(10): 2736-2754. |
| 38 | SEETHARAMAN S, ETIENNE-MANNEVILLE S. Integrin diversity brings specificity in mechanotransduction[J]. Biology of the Cell, 2018, 110(3): 49-64. |
| 39 | LOU H Y, ZHAO W T, ZENG Y P, et al. The role of membrane curvature in nanoscale topography-induced intracellular signaling[J]. Accounts of Chemical Research, 2018, 51(5): 1046-1053. |
| 40 | HOTARY K B, ROBINSON K R. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption[J]. Developmental Biology, 1994, 166(2): 789-800. |
| 41 | SONG B, GU Y, JIANG W K, et al. Electric signals counterbalanced posterior vs anterior PTEN signaling in directed migration of Dictyostelium[J]. Cell & Bioscience, 2021, 11(1): 111. |
| 42 | PAL D S, LI X G, BANERJEE T, et al. The excitable signal transduction networks: movers and shapers of eukaryotic cell migration[J]. The International Journal of Developmental Biology, 2019, 63(8/9): 407-416. |
| 43 | RIDLEY A J, SCHWARTZ M A, BURRIDGE K, et al. Cell migration: integrating signals from front to back[J]. Science, 2003, 302(5651): 1704-1709. |
| 44 | FRITZ R D, PERTZ O. The dynamics of spatio-temporal Rho GTPase signaling: formation of signaling patterns[J]. F1000Research, 2016, 5(F1000FacultyRev): 749. |
| 45 | BEMENT W M, MILLER A L, VON DASSOW G. Rho GTPase activity zones and transient contractile arrays[J]. BioEssays, 2006, 28(10): 983-993. |
| 46 | MAÑES S, GÓMEZ-MOUTÓN C, LACALLE R A, et al. Mastering time and space: Immune cell polarization and chemotaxis[J]. Seminars in Immunology, 2005, 17(1): 77-86. |
| 47 | WANG Y Q, KU C J, ZHANG E R, et al. Identifying network motifs that buffer front-to-back signaling in polarized neutrophils[J]. Cell Reports, 2013, 3(5): 1607-1616. |
| 48 | BISHOP A L, HALL A. Rho GTPases and their effector proteins[J]. The Biochemical Journal, 2000, 348(Pt 2): 241-255. |
| 49 | MENG X T, AROCENA M, PENNINGER J, et al. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors[J]. Experimental Neurology, 2011, 227(1): 210-217. |
| 50 | MIAO Y C, BHATTACHARYA S, EDWARDS M, et al. Altering the threshold of an excitable signal transduction network changes cell migratory modes[J]. Nature Cell Biology, 2017, 19(4): 329-340. |
| 51 | KUROKAWA K, NAKAMURA T, AOKI K, et al. Mechanism and role of localized activation of Rho-family GTPases in growth factor-stimulated fibroblasts and neuronal cells[J]. Biochemical Society Transactions, 2005, 33(Pt 4): 631-634. |
| 52 | BOS J L, REHMANN H, WITTINGHOFER A. GEFs and GAPs: critical elements in the control of small G proteins[J]. Cell, 2007, 129(5): 865-877. |
| 53 | GERMENA G, HIRSCH E. PI3Ks and small GTPases in neutrophil migration: Two sides of the same coin[J]. Molecular Immunology, 2013, 55(1): 83-86. |
| 54 | VICKER M G. F-actin assembly in Dictyostelium cell locomotion and shape oscillations propagates as a self-organized reaction-diffusion wave[J]. FEBS Letters, 2002, 510(1/2): 5-9. |
| 55 | VAN HAASTERT P J, KEIZER-GUNNINK I, KORTHOLT A. Coupled excitable Ras and F-actin activation mediates spontaneous pseudopod formation and directed cell movement[J]. Molecular Biology of the Cell, 2017, 28(7): 922-934. |
| 56 | WEINER O D, MARGANSKI W A, WU L F, et al. An actin-based wave generator organizes cell motility[J]. PLoS Biology, 2007, 5(9): e221. |
| 57 | TANG M, WANG M J, SHI C J, et al. Evolutionarily conserved coupling of adaptive and excitable networks mediates eukaryotic chemotaxis[J]. Nature Communications, 2014, 5: 5175. |
| 58 | MIAO Y C, BHATTACHARYA S, BANERJEE T, et al. Wave patterns organize cellular protrusions and control cortical dynamics[J]. Molecular Systems Biology, 2019, 15(3): e8585. |
| 59 | KAMIMURA Y, XIONG Y, IGLESIAS P A, et al. PIP3-independent activation of TorC2 and PKB at the cell's leading edge mediates chemotaxis[J]. Current Biology, 2008, 18(14): 1034-1043. |
| 60 | FETS L, NICHOLS J M E, KAY R R. A PIP5 kinase essential for efficient chemotactic signaling[J]. Current Biology, 2014, 24(4): 415-421. |
| 61 | CHAREST P G, SHEN Z X, LAKODUK A, et al. A ras signaling complex controls the RasC-TORC2 pathway and directed cell migration[J]. Developmental Cell, 2010, 18(5): 737-749. |
| 62 | PIPATHSOUK A, BRUNETTI R M, TOWN J P, et al. WAVE complex self-organization templates lamellipodial formation[J]. bioRxiv, 2019, DOI:10.1101/836585 . |
| 63 | BRUNETTI R M, KOCKELKOREN G, RAGHAVAN P, et al. WASP integrates substrate topology and cell polarity to guide neutrophil migration[J]. The Journal of Cell Biology, 2022, 221(2): e202104046. |
| 64 | GRAZIANO B R, GONG D, ANDERSON K E, et al. A module for Rac temporal signal integration revealed with optogenetics[J]. The Journal of Cell Biology, 2017, 216(8): 2515-2531. |
| 65 | ALLEN T M, CULLIS P R. Liposomal drug delivery systems: From concept to clinical applications[J]. Advanced Drug Delivery Reviews, 2013, 65(1): 36-48. |
| 66 | PAUL C D, HUNG W C, WIRTZ D, et al. Engineered models of confined cell migration[J]. Annual Review of Biomedical Engineering, 2016, 18: 159-180. |
| 67 | ULLAH M, LIU D D, THAKOR A S. Mesenchymal stromal cell homing: Mechanisms and strategies for improvement[J]. iScience, 2019, 15: 421-438. |
| 68 | WON Y W, PATEL A N, BULL D A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient[J]. Biomaterials, 2014, 35(21): 5627-5635. |
| 69 | SASAKI T, FUKAZAWA R, OGAWA S, et al. Stromal cell-derived factor-1α improves infarcted heart function through angiogenesis in mice[J]. Pediatrics International, 2007, 49(6): 966-971. |
| 70 | SEGERS V F M, REVIN V, WU W T, et al. Protease-resistant stromal cell-derived factor-1 for the treatment of experimental peripheral artery disease[J]. Circulation, 2011, 123(12): 1306-1315. |
| 71 | FUJII H, LI S H, WU J, et al. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair[J]. European Heart Journal, 2010, 32(16): 2075-2084. |
| 72 | KIMURA Y, TABATA Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis[J]. Journal of Biomaterials Science, Polymer Edition, 2010, 21(1): 37-51. |
| 73 | CONKLIN B R, HSIAO E C, CLAEYSEN S, et al. Engineering GPCR signaling pathways with RASSLs[J]. Nature Methods, 2008, 5(8): 673-678. |
| 74 | PARK J S, RHAU B, HERMANN A, et al. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(16): 5896-5901. |
| 75 | XU Y, HYUN Y M, LIM K, et al. Optogenetic control of chemokine receptor signal and T-cell migration[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(17): 6371-6376. |
| 76 | WU Y I, FREY D, LUNGU O I, et al. A genetically encoded photoactivatable Rac controls the motility of living cells[J]. Nature, 2009, 461(7260): 104-108. |
| 77 | DAGLIYAN O, DOKHOLYAN N V, HAHN K M. Engineering proteins for allosteric control by light or ligands[J]. Nature Protocols, 2019, 14(6): 1863-1883. |
| 78 | O'NEILL P R, GAUTAM N. Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration[J]. Molecular Biology of the Cell, 2014, 25(15): 2305-2314. |
| 79 | KARUNARATHNE W K A, GIRI L, PATEL A K, et al. Optical control demonstrates switch-like PIP3 dynamics underlying the initiation of immune cell migration[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(17): E1575-E1583. |
| 80 | BERLEW E E, KUZNETSOV I A, YAMADA K, et al. Single-component optogenetic tools for inducible RhoA GTPase signaling[J]. Advanced Biology, 2021, 5(9): e2100810. |
| 81 | BERLEW E E, KUZNETSOV I A, YAMADA K, et al. Optogenetic Rac1 engineered from membrane lipid-binding RGS-LOV for inducible lamellipodia formation[J]. Photochemical & Photobiological Sciences, 2020, 19(3): 353-361. |
| 82 | HANNANTA-ANAN P, GLANTZ S T, CHOW B Y. Optically inducible membrane recruitment and signaling systems[J]. Current Opinion in Structural Biology, 2019, 57: 84-92. |
| 83 | ARBAB A S, JORDAN E K, WILSON L B, et al. In vivo trafficking and targeted delivery of magnetically labeled stem cells[J]. Human Gene Therapy, 2004, 15(4): 351-360. |
| 84 | KOBAYASHI T, OCHI M, YANADA S, et al. Augmentation of degenerated human cartilage in vitro using magnetically labeled mesenchymal stem cells and an external magnetic device[J]. Arthroscopy: the Journal of Arthroscopic & Related Surgery, 2009, 25(12): 1435-1441. |
| 85 | YUN W S, CHOI J S, JU H M, et al. Enhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse models[J]. International Journal of Molecular Sciences, 2018, 19(5): 1376. |
| 86 | SONG Y S, KU J H. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging[J]. Neurourology and Urodynamics, 2007, 26(4): 584-593. |
| 87 | FENG Q, LEE S S, KORNMANN B. A toolbox for organelle mechanobiology research-current needs and challenges[J]. Micromachines, 2019, 10(8): 538. |
| 88 | ETOC F, LISSE D, BELLAICHE Y, et al. Subcellular control of Rac-GTPase signalling by magnetogenetic manipulation inside living cells[J]. Nature Nanotechnology, 2013, 8(3): 193-198. |
| 89 | MOSABBIR A A, TRUONG K. Genetically encoded circuit for remote regulation of cell migration by magnetic fields[J]. ACS Synthetic Biology, 2018, 7(2): 718-726. |
| 90 | MILLS E, TRUONG K. Engineering Ca2+/calmodulin-mediated modulation of protein translocation by overlapping binding and signaling peptide sequences[J]. Cell Calcium, 2010, 47(4): 369-377. |
| 91 | SENGUPTA S, PARENT C A, BEAR J E. The principles of directed cell migration[J]. Nature Reviews Molecular Cell Biology, 2021, 22(8): 529-547. |
| 92 | SCHULTZ G S, WYSOCKI A. Interactions between extracellular matrix and growth factors in wound healing[J]. Wound Repair and Regeneration, 2009, 17(2): 153-162. |
| 93 | MOSABBIR A A, TRUONG K. Light directed migration of a cluster of cells in the centimeter scale[J]. Small GTPases, 2020, 11(4): 301-307. |
| 94 | WEI F Y, LEUNG K S, LI G, et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing[J]. PLoS One, 2014, 9(9): e106722. |
| 95 | XIA P, SHI Y, WANG X J, et al. Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells[J]. Stem Cell Research & Therapy, 2022, 13(1): 214. |
| 96 | CHEN J L, JIANG J W, WANG W, et al. Low intensity pulsed ultrasound promotes the migration of bone marrow- derived mesenchymal stem cells via activating FAK-ERK1/2 signalling pathway[J]. Artificial Cells, Nanomedicine, and Biotechnology, 2019, 47(1): 3603-3613. |
| 97 | CHEN C, BAI X, DING Y H, et al. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering[J]. Biomaterials Research, 2019, 23: 25. |
| 98 | ROYBAL K T, LIM W A. Synthetic immunology: hacking immune cells to expand their therapeutic capabilities[J]. Annual Review of Immunology, 2017, 35: 229-253. |
| 99 | JIN L Y, CAO L, ZHU Y J, et al. Enhance anti-lung tumor efficacy of chimeric antigen receptor-T cells by ectopic expression of C-C motif chemokine receptor 6[J]. Science Bulletin, 2021, 66(8): 803-812. |
| 100 | MOON E K, CARPENITO C, SUN J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor[J]. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research, 2011, 17(14): 4719-4730. |
| 101 | DI STASI A, DE ANGELIS B, ROONEY C M, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model[J]. Blood, 2009, 113(25): 6392-6402. |
| 102 | SANTORELLI M, LAM C, MORSUT L. Synthetic development: Building mammalian multicellular structures with artificial genetic programs[J]. Current Opinion in Biotechnology, 2019, 59: 130-140. |
| 103 | TODA S, BRUNGER J M, LIM W A. Synthetic development: Learning to program multicellular self-organization[J]. Current Opinion in Systems Biology, 2019, 14: 41-49. |
| 104 | GEERING B, FUSSENEGGER M. Synthetic immunology: modulating the human immune system[J]. Trends in biotechnology, 2015, 33(2): 65-79. |
| [1] | TANG Yiming, YAO Yifei, YANG Zhongyuan, ZHOU Yun, WANG Zichao, WEI Guanghong. Pathological aggregation and liquid-liquid phase separation of proteins associated with neurodegenerative diseases [J]. Synthetic Biology Journal, 2023, 4(3): 590-610. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||