Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (4): 703-719.DOI: 10.12211/2096-8280.2022-054
• Invited Review • Previous Articles Next Articles
Application and prospect of CRISPR-Cas9 system in tumor biology
MA Mengdan1,2,3, SHANG Mengyu1,2,4, LIU Yuchen1,2
- 1.The first Affiliated Hospital of Shenzhen University,Shenzhen Second People′s Hospital,Shenzhen Institute of Translational Medicine,Shenzhen 518035,Guangdong,China
2.Guangdong Provincial Key Laboratory of Systems Biology and Synthetic Biology for Urogenital Tumors,Shenzhen 518035,Guangdong,China
3.Shantou University Medical College,Shantou 515041,Guangdong,China
4.School of Basic Medical Sciences,Shenzhen University Health Science Center,Shenzhen 518060,China
-
Received:2022-09-30Revised:2023-02-01Online:2023-09-14Published:2023-08-31 -
Contact:LIU Yuchen
CRISPR-Cas9系统在肿瘤生物学中的应用及前景
马孟丹1,2,3, 尚梦宇1,2,4, 刘宇辰1,2
- 1.深圳大学第一附属医院,深圳市第二人民医院,深圳转化医学研究院,广东 深圳 518035
2.广东省泌尿生殖肿瘤系统生物学与合成生物学重点实验室,广东 深圳 518035
3.汕头大学医学院,广东 汕头 515041
4.深圳大学医学部基础医学院,广东 深圳 518060
-
通讯作者:刘宇辰 -
作者简介:马孟丹 (1997—),女,硕士研究生。研究方向为医学合成生物学。E-mail:mamengdan7@163.com刘宇辰 (1988—),男,副研究员,研究生导师,博士后合作导师。研究方向为(1)肿瘤生物治疗和医学合成生物学;(2)创新肿瘤治疗新方法;(3)构建人工基因线路,肿瘤精准治疗。E-mail:liuyuchenmdcg@163.com -
基金资助:国家重点研发计划(2021YFA0911600)
CLC Number:
Cite this article
MA Mengdan, SHANG Mengyu, LIU Yuchen. Application and prospect of CRISPR-Cas9 system in tumor biology[J]. Synthetic Biology Journal, 2023, 4(4): 703-719.
马孟丹, 尚梦宇, 刘宇辰. CRISPR-Cas9系统在肿瘤生物学中的应用及前景[J]. 合成生物学, 2023, 4(4): 703-719.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-054
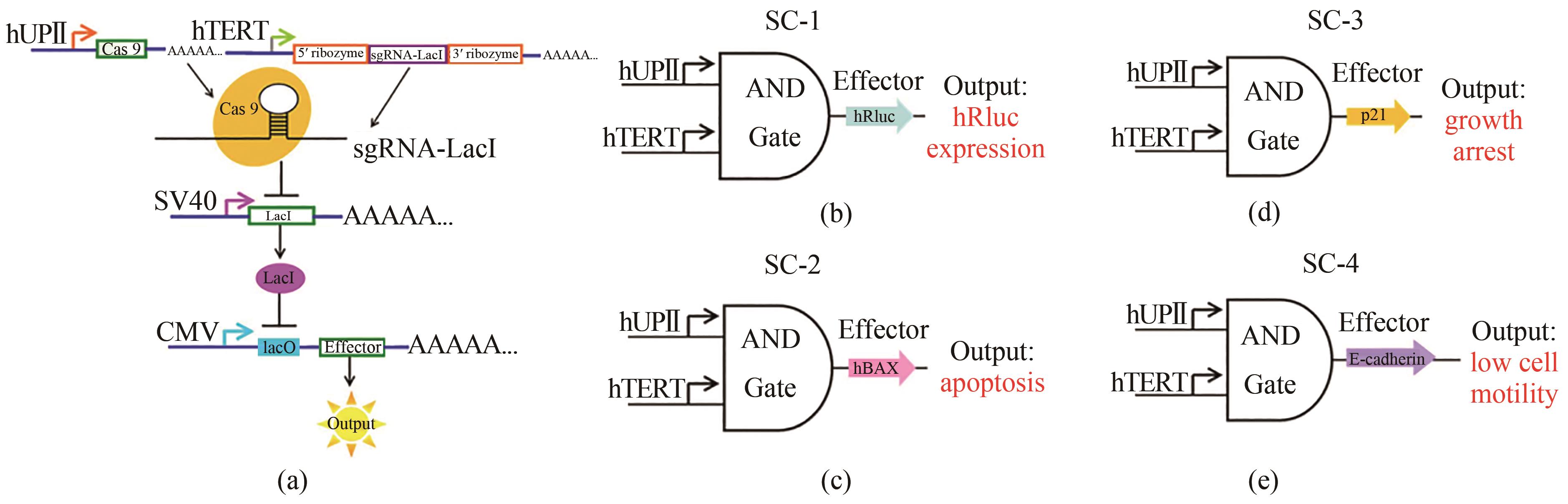
Fig. 2 Design and construction of the AND gate genetic circuits[50](a) The hUPⅡ and hTERT promoters are the inputs to the circuit. The hUPⅡ promoter drives the transcription of Cas9 mRNA and the hTERT promoter is linked to the transcription of sgRNA targeting LacI. The output gene is driven by a LacI-controlled CMV promoter. The effector can be expressed only when both Cas9 protein and sgRNA are presented. (b)~(e) are schematic representations of the synthetic circuits (SC-1, SC-2, SC-3 and SC-4). The output genes were hRluc, hBAX, p21 and E-cadherin.
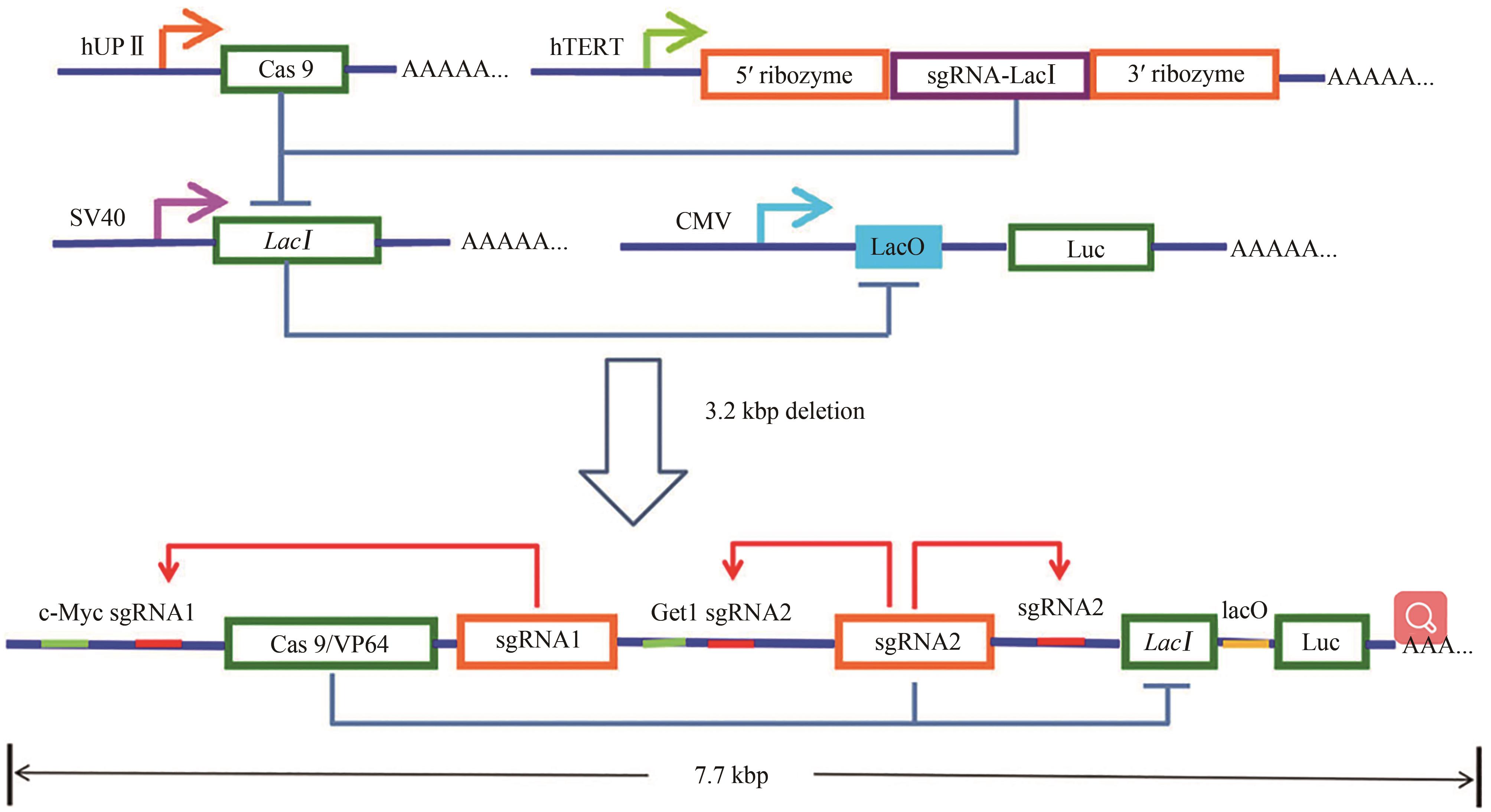
Fig. 3 Design and construction of the AND gate minigene circuits[51]The UPⅡ promoter drove the transcription of Cas9 mRNA, while the hTERT promoter was used to promote the transcription of sgRNA targeting LacI. The output Renilla luciferase gene was regulated by a LacI-controlled CMV promoter. The luciferase was expressed only when both UPⅡ promoter and TERT promoter were both activated. In the design of the minigene circuit, the UPⅡ and hTERT promoters were replaced by their respective transcription factor binding elements. Both c-Myc and Get1, only in bladder cancer cells, had a relative high expression level at the same time. After initial expression of sgRNA1 and sgRNA2, they could further bind upstream of their own transcription initiation sites and then amplify the transcription signals of c-Myc and Get1 through the positive feedback mechanism to amplify their corresponding downstream genes transcription, respectively. Furthermore, the LacI gene was knocked out by sgRNA2, and luciferase reporter gene was activated by transcription. In normal bladder epithelial cells, luciferase could not be effectively transcribed and was further silenced by trace amounts of LacI expressed at the background level
| 1 | KNOTT G J, DOUDNA J A. CRISPR-Cas guides the future of genetic engineering[J]. Science, 2018, 361(6405): 866-869. |
| 2 | YIN H, XUE W, ANDERSON D G. CRISPR-Cas: a tool for cancer research and therapeutics[J]. Nature Reviews Clinical Oncology, 2019, 16(5): 281-295. |
| 3 | RAY S K, MUKHERJEE S. Genome editing with CRISPR-Cas9: a budding biological contrivance for colorectal carcinoma research and its perspective in molecular medicine[J]. Current Molecular Medicine, 2021, 21(6): 462-475. |
| 4 | HAN K, JENG E E, HESS G T, et al. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions[J]. Nature Biotechnology, 2017, 35(5): 463-474. |
| 5 | KONG X J, KUILMAN T, SHAHRABI A, et al. Cancer drug addiction is relayed by an ERK2-dependent phenotype switch[J]. Nature, 2017, 550(7675): 270-274. |
| 6 | KURATA M, YAMAMOTO K, MORIARITY B S, et al. CRISPR/Cas9 library screening for drug target discovery[J]. Journal of Human Genetics, 2018, 63(2): 179-186. |
| 7 | LIU D, ZHAO X, TANG A Q, et al. CRISPR screen in mechanism and target discovery for cancer immunotherapy[J]. Biochimica et Biophysica Acta Reviews on Cancer, 2020, 1874(1): 188378. |
| 8 | SONG X R, LIU C, WANG N, et al. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy[J]. Advanced Drug Delivery Reviews, 2021, 168: 158-180. |
| 9 | MORENO A M, FU X, ZHU J, et al. In situ gene therapy via AAV-CRISPR-Cas9-mediated targeted gene regulation[J]. Molecular Therapy, 2018, 26(7): 1818-1827. |
| 10 | MANGUSO R T, POPE H W, ZIMMER M D, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target[J]. Nature, 2017, 547(7664): 413-418. |
| 11 | DONG M B, WANG G C, CHOW R D, et al. Systematic immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells[J]. Cell, 2019, 178(5): 1189-1204.e23. |
| 12 | DUFVA O, KOSKI J, MALINIEMI P, et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity[J]. Blood, 2020, 135(9): 597-609. |
| 13 | GEORGE S L, LORENZI F, KING D, et al. Therapeutic vulnerabilities in the DNA damage response for the treatment of ATRX mutant neuroblastoma[J]. eBioMedicine, 2020, 59: 102971. |
| 14 | GELLER D S, GORLICK R. Osteosarcoma: a review of diagnosis, management, and treatment strategies[J]. Clinical Advances in Hematology & Oncology, 2010, 8(10): 705-718. |
| 15 | FENG Y, SASSI S, SHEN J K, et al. Targeting CDK11 in osteosarcoma cells using the CRISPR-Cas9 system[J]. Journal of Orthopaedic Research, 2015, 33(2): 199-207. |
| 16 | LEHMANN R, LEE C M, SHUGART E C, et al. Human organoids: a new dimension in cell biology[J]. Molecular Biology of the Cell, 2019, 30(10): 1129-1137. |
| 17 | OSTROM Q T, GITTLEMAN H, XU J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009—2013[J]. Neuro-Oncology, 2016, 18(): v1-v75. |
| 18 | BIAN S, REPIC M, GUO Z M, et al. Genetically engineered cerebral organoids model brain tumor formation[J]. Nature Methods, 2018, 15(8): 631-639. |
| 19 | DEKKERS J F, WHITTLE J R, VAILLANT F, et al. Modeling breast cancer using CRISPR-Cas9-mediated engineering of human breast organoids[J]. Journal of the National Cancer Institute, 2020, 112(5): 540-544. |
| 20 | RATH D, AMLINGER L, RATH A, et al. The CRISPR-Cas immune system: biology, mechanisms and applications[J]. Biochimie, 2015, 117: 119-128. |
| 21 | YANG H, WANG H Y, SHIVALILA C S, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering[J]. Cell, 2013, 154(6): 1370-1379. |
| 22 | XUE W, CHEN S D, YIN H, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver[J]. Nature, 2014, 514(7522): 380-384. |
| 23 | WANG X, HAYES J E, XU X, et al. Validation of prostate cancer risk variants rs10993994 and rs7098889 by CRISPR/Cas9 mediated genome editing[J]. Gene, 2021, 768: 145265. |
| 24 | ZHU C B, KROS J M, CHENG C, et al. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies[J]. Neuro-Oncology, 2017, 19(11): 1435-1446. |
| 25 | MUOIO M G, TALIA M, LAPPANO R, et al. Activation of the S100A7/RAGE pathway by IGF-1 contributes to angiogenesis in breast cancer[J]. Cancers, 2021, 13(4): 621. |
| 26 | HE M Y, HALFORD M M, LIU R F, et al. Three-dimensional CRISPR screening reveals epigenetic interaction with anti-angiogenic therapy[J]. Communications Biology, 2021, 4: 878. |
| 27 | LO Y H, KOLAHI K S, DU Y H, et al. A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation[J]. Cancer Discovery, 2021, 11(6): 1562-1581. |
| 28 | CUELLA-MARTIN R, HAYWARD S B, FAN X, et al. Functional interrogation of DNA damage response variants with base editing screens[J]. Cell, 2021, 184(4): 1081-1097.e19. |
| 29 | ZHANG Y Y, ZHANG Z M. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications[J]. Cellular & Molecular Immunology, 2020, 17(8): 807-821. |
| 30 | MIZUKOSHI E, KANEKO S. Immune cell therapy for hepatocellular carcinoma[J]. Journal of Hematology & Oncology, 2019, 12(1): 52. |
| 31 | JUNE C H, O'CONNOR R S, KAWALEKAR O U, et al. CAR T cell immunotherapy for human cancer[J]. Science, 2018, 359(6382): 1361-1365. |
| 32 | MURTY S, HAILE S T, BEINAT C, et al. Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model[J]. OncoImmunology, 2020, 9(1): 1757360. |
| 33 | LYNN R C, WEBER E W, SOTILLO E, et al. C-Jun overexpression in CAR T cells induces exhaustion resistance[J]. Nature, 2019, 576(7786): 293-300. |
| 34 | HONG M H, CLUBB J D, CHEN Y Y. Engineering CAR-T cells for next-generation cancer therapy[J]. Cancer Cell, 2020, 38(4): 473-488. |
| 35 | QIN H Y, YANG L L, CHUKINAS J A, et al. Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design[J]. Journal for Immunotherapy of Cancer, 2021, 9(9): e003149. |
| 36 | RABILLOUD T, POTIER D, PANKAEW S, et al. Single-cell profiling identifies pre-existing CD19-negative subclones in a B-ALL patient with CD19-negative relapse after CAR-T therapy[J]. Nature Communications, 2021, 12: 865. |
| 37 | ZHANG J Q, HU Y X, YANG J X, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL[J]. Nature, 2022, 609(7926): 369-374. |
| 38 | LARSON R C, KANN M C, BAILEY S R, et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours[J]. Nature, 2022, 604(7906): 563-570. |
| 39 | RAMOS C A, GROVER N S, BEAVEN A W, et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma[J]. Journal of Clinical Oncology, 2020, 38(32): 3794-3804. |
| 40 | ZHAO L J, CAO Y J. Engineered T cell therapy for cancer in the clinic[J]. Frontiers in Immunology, 2019, 10: 2250. |
| 41 | STADTMAUER E A, FRAIETTA J A, DAVIS M M, et al. CRISPR-engineered T cells in patients with refractory cancer[J]. Science, 2020, 367(6481): eaba7365. |
| 42 | EYQUEM J, MANSILLA-SOTO J, GIAVRIDIS T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection[J]. Nature, 2017, 543(7643): 113-117. |
| 43 | LU Y, XUE J X, DENG T, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer[J]. Nature Medicine, 2020, 26(5): 732-740. |
| 44 | SI J W, SHI X J, SUN S H, et al. Hematopoietic progenitor Kinase1 (HPK1) mediates T cell dysfunction and is a druggable target for T cell-based immunotherapies[J]. Cancer Cell, 2020, 38(4): 551-566.e11. |
| 45 | XIONG J F, TAN S W, YU L, et al. E7-targeted nanotherapeutics for key HPV afflicted cervical lesions by employing CRISPR/Cas9 and poly (beta-amino ester)[J]. International Journal of Nanomedicine, 2021, 16: 7609-7622. |
| 46 | SONG J, ZHANG X C, GE Q Y, et al. CRISPR/Cas9-mediated knockout of HBsAg inhibits proliferation and tumorigenicity of HBV-positive hepatocellular carcinoma cells[J]. Journal of Cellular Biochemistry, 2018, 119(10): 8419-8431. |
| 47 | YU Y H, WU X, GUAN N Z, et al. Engineering a far-red light-activated split-Cas9 system for remote-controlled genome editing of internal organs and tumors[J]. Science Advances, 2020, 6(28): eabb1777. |
| 48 | LIU Y C, HAN J H, CHEN Z C, et al. Engineering cell signaling using tunable CRISPR-Cpf1-based transcription factors[J]. Nature Communications, 2017, 8: 2095. |
| 49 | LIU Y C, ZHAN Y H, CHEN Z C, et al. Directing cellular information flow via CRISPR signal conductors[J]. Nature Methods, 2016, 13(11): 938-944. |
| 50 | LIU Y C, ZENG Y Y, LIU L, et al. Synthesizing AND gate genetic circuits based on CRISPR-Cas9 for identification of bladder cancer cells[J]. Nature Communications, 2014, 5: 5393. |
| 51 | LIU Y C, HUANG W R, CAI Z M. Synthesizing AND gate minigene circuits based on CRISPReader for identification of bladder cancer cells[J]. Nature Communications, 2020, 11: 5486. |
| 52 | KURT I C, ZHOU R H, IYER S, et al. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells[J]. Nature Biotechnology, 2021, 39(1): 41-46. |
| 53 | ZHAO D D, LI J, LI S W, et al. Glycosylase base editors enable C-to-A and C-to-G base changes[J]. Nature Biotechnology, 2021, 39(1): 35-40. |
| 54 | JEONG Y K, LEE S, HWANG G H, et al. Adenine base editor engineering reduces editing of bystander cytosines[J]. Nature Biotechnology, 2021, 39(11): 1426-1433. |
| 55 | JIN S, FEI H Y, ZHU Z X, et al. Rationally designed APOBEC3B cytosine base editors with improved specificity[J]. Molecular Cell, 2020, 79(5): 728-740.e6. |
| 56 | ANZALONE A V, RANDOLPH P B, DAVIS J R, et al. Search-and-replace genome editing without double-strand breaks or donor DNA[J]. Nature, 2019, 576(7785): 149-157. |
| 57 | KIM D Y, MOON S B, KO J H, et al. Unbiased investigation of specificities of prime editing systems in human cells[J]. Nucleic Acids Research, 2020, 48(18): 10576-10589. |
| 58 | KANTOR A, MCCLEMENTS M E, MACLAREN R E. CRISPR-Cas9 DNA base-editing and prime-editing[J]. International Journal of Molecular Sciences, 2020, 21(17): 6240. |
| 59 | HU Z T, OTT P A, WU C J. Towards personalized, tumour-specific, therapeutic vaccines for cancer[J]. Nature Reviews Immunology, 2018, 18(3): 168-182. |
| 60 | SAHIN U, TÜRECI Ö. Personalized vaccines for cancer immunotherapy[J]. Science, 2018, 359(6382): 1355-1360. |
| 61 | BLASS E, OTT P A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines[J]. Nature Reviews Clinical Oncology, 2021, 18(4): 215-229. |
| 62 | HU Z T, LEET D E, ALLESØE R L, et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma[J]. Nature Medicine, 2021, 27(3): 515-525. |
| 63 | OTT P A, HU Z T, KESKIN D B, et al. An immunogenic personal neoantigen vaccine for patients with melanoma[J]. Nature, 2017, 547(7662): 217-221. |
| 64 | RAVINDRANATHAN S, NGUYEN K G, KURTZ S L, et al. Tumor-derived granulocyte colony-stimulating factor diminishes efficacy of breast tumor cell vaccines[J]. Breast Cancer Research, 2018, 20(1): 126. |
| 65 | GUO L P, XIE H, ZHANG Z, et al. Fusion protein vaccine based on Ag85B and STEAP1 induces a protective immune response against prostate cancer[J]. Vaccines, 2021, 9(7): 786. |
| 66 | MOFFETT H F, HARMS C K, FITZPATRICK K S, et al. B cells engineered to express pathogen-specific antibodies protect against infection[J]. Science Immunology, 2019, 4(35): eaax0644. |
| 67 | BIANCUR D E, KAPNER K S, YAMAMOTO K, et al. Functional genomics identifies metabolic vulnerabilities in pancreatic cancer[J]. Cell Metabolism, 2021, 33(1): 199-210.e8. |
| 68 | KAWASAKI K, TOSHIMITSU K, MATANO M, et al. An organoid biobank of neuroendocrine neoplasms enables genotype-phenotype mapping[J]. Cell, 2020, 183(5): 1420-1435.e21. |
| 69 | GONÇALVES E, SEGURA-CABRERA A, PACINI C, et al. Drug mechanism-of-action discovery through the integration of pharmacological and CRISPR screens[J]. Molecular Systems Biology, 2020, 16(7): e9405. |
| 70 | FELLMANN C, GOWEN B G, LIN P C, et al. Cornerstones of CRISPR-Cas in drug discovery and therapy[J]. Nature Reviews Drug Discovery, 2017, 16(2): 89-100. |
| 71 | NGUYEN T T, RAMSAY L, AHANFESHAR-ADAMS M, et al. Mutations in the IFNγ-JAK-STAT pathway causing resistance to immune checkpoint inhibitors in melanoma increase sensitivity to oncolytic virus treatment[J]. Clinical Cancer Research, 2021, 27(12): 3432-3442. |
| 72 | NEEB A, HERRANZ N, ARCE-GALLEGO S, et al. Advanced prostate cancer with ATM loss: PARP and ATR inhibitors[J]. European Urology, 2021, 79(2): 200-211. |
| 73 | OUYANG Q Y, LIU Y J, TAN J Q, et al. Loss of ZNF587B and SULF1 contributed to cisplatin resistance in ovarian cancer cell lines based on Genome-scale CRISPR/Cas9 screening[J]. American Journal of Cancer Research, 2019, 9(5): 988-998. |
| 74 | FENG J R, LU H, MA W H, et al. Genome-wide CRISPR screen identifies synthetic lethality between DOCK1 inhibition and metformin in liver cancer[J]. Protein & Cell, 2022, 13(11): 825-841. |
| 75 | FOMICHEVA M, MACARA I G. Genome-wide CRISPR screen identifies noncanonical NF-κB signaling as a regulator of density-dependent proliferation[J]. eLife, 2020, 9: e63603. |
| 76 | VAN DER WEYDEN L, HARLE V, TURNER G, et al. CRISPR activation screen in mice identifies novel membrane proteins enhancing pulmonary metastatic colonisation[J]. Communications Biology, 2021, 4: 395. |
| 77 | FENG X, TANG M F, DEDE M, et al. Genome-wide CRISPR screens using isogenic cells reveal vulnerabilities conferred by loss of tumor suppressors[J]. Science Advances, 2022, 8(19): eabm6638. |
| 78 | WANG D R, PRAGER B C, GIMPLE R C, et al. CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies[J]. Cancer Discovery, 2021, 11(5): 1192-1211. |
| 79 | LI J Y, YUAN S, NORGARD R J, et al. Epigenetic and transcriptional control of the epidermal growth factor receptor regulates the tumor immune microenvironment in pancreatic cancer[J]. Cancer Discovery, 2021, 11(3): 736-753. |
| 80 | JOUNG J, KIRCHGATTERER P C, SINGH A, et al. CRISPR activation screen identifies BCL-2 proteins and B3GNT2 as drivers of cancer resistance to T cell-mediated cytotoxicity[J]. Nature Communications, 2022, 13(1): 1606. |
| 81 | QUINN J J, JONES M G, OKIMOTO R A, et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts[J]. Science, 2021, 371(6532): eabc1944. |
| 82 | SIMEONOV K P, BYRNS C N, CLARK M L, et al. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states[J]. Cancer Cell, 2021, 39(8): 1150-1162.e9. |
| 83 | YANG D, JONES M G, NARANJO S, et al. Lineage tracing reveals the phylodynamics, plasticity, and paths of tumor evolution [J]. Cell, 2022, 185(11): 1905-1923.e25. |
| 84 | GILBERT L A, LARSON M H, MORSUT L, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes[J]. Cell, 2013, 154(2): 442-451. |
| 85 | BRAVO J P K, LIU M S, HIBSHMAN G N, et al. Structural basis for mismatch surveillance by CRISPR-Cas9[J]. Nature, 2022, 603(7900): 343-347. |
| 86 | RICHARDSON C D, RAY G J, DEWITT M A, et al. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA[J]. Nature Biotechnology, 2016, 34(3): 339-344. |
| 87 | HU J H, MILLER S M, GEURTS M H, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity[J]. Nature, 2018, 556(7699): 57-63. |
| 88 | KIM D Y, LEE J M, MOON S B, et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus[J]. Nature Biotechnology, 2022, 40(1): 94-102. |
| 89 | GUO L Y, BIAN J, DAVIS A E, et al. Multiplexed genome regulation in vivo with hyper-efficient Cas12a[J]. Nature Cell Biology, 2022, 24(4): 590-600. |
| 90 | TONG H W, HUANG J, XIAO Q Q, et al. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects[J]. Nature Biotechnology, 2023, 41(1): 108-119. |
| 91 | XU C L, ZHOU Y S, XIAO Q Q, et al. Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes[J]. Nature Methods, 2021, 18(5): 499-506. |
| 92 | AKCAKAYA P, BOBBIN M L, GUO J A, et al. In vivo CRISPR editing with no detectable genome-wide off-target mutations[J]. Nature, 2018, 561(7723): 416-419. |
| 93 | KLEINSTIVER B P, PATTANAYAK V, PREW M S, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects[J]. Nature, 2016, 529(7587): 490-495. |
| 94 | DEWRAN KOCAK D, JOSEPHS E A, BHANDARKAR V, et al. Increasing the specificity of CRISPR systems with engineered RNA secondary structures[J]. Nature Biotechnology, 2019, 37(6): 657-666. |
| 95 | ZUO E W, SUN Y D, WEI W, et al. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos[J]. Science, 2019, 364(6437): 289-292. |
| 96 | LEI Z X, MENG H W, LV Z C, et al. Detect-seq reveals out-of-protospacer editing and target-strand editing by cytosine base editors[J]. Nature Methods, 2021, 18(6): 643-651. |
| 97 | YIN J H, LU R S, XIN C C, et al. Cas9 exo-endonuclease eliminates chromosomal translocations during genome editing[J]. Nature Communications, 2022, 13: 1204. |
| 98 | XU X J, WAN T, XIN H H, et al. Delivery of CRISPR/Cas9 for therapeutic genome editing[J]. The Journal of Gene Medicine, 2019, 21(7): e3107. |
| 99 | WILBIE D, WALTHER J, MASTROBATTISTA E. Delivery aspects of CRISPR/cas for in vivo genome editing[J]. Accounts of Chemical Research, 2019, 52(6): 1555-1564. |
| 100 | LATTANZI A, MENEGHINI V, PAVANI G, et al. Optimization of CRISPR/Cas9 delivery to human hematopoietic stem and progenitor cells for therapeutic genomic rearrangements[J]. Molecular Therapy, 2019, 27(1): 137-150. |
| 101 | LIU S, CHENG Q, WEI T, et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing[J]. Nature Materials, 2021, 20(5): 701-710. |
| 102 | SEGEL M, LASH B, SONG J W, et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery[J]. Science, 2021, 373(6557): 882-889. |
| 103 | ZHAN H J, ZHOU Q, GAO Q J, et al. Multiplexed promoterless gene expression with CRISPReader[J]. Genome Biology, 2019, 20(1): 113. |
| 104 | ZHAN Y H, LI A L, CAO C C, et al. CRISPR signal conductor 2.0 for redirecting cellular information flow[J]. Cell Discovery, 2022, 8: 26. |
| 105 | HAAPANIEMI E, BOTLA S, PERSSON J, et al. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response[J]. Nature Medicine, 2018, 24(7): 927-930. |
| 106 | IHRY R J, WORRINGER K A, SALICK M R, et al. p53 inhibits CRISPR–Cas9 engineering in human pluripotent stem cells[J]. Nature Medicine, 2018, 24(7): 939-946. |
| 107 | KOSICKI M, TOMBERG K, BRADLEY A. Erratum: Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements[J]. Nature Biotechnology, 2018, 36(9): 899. |
| 108 | XU S X, KIM J, TANG Q S, et al. CAS9 is a genome mutator by directly disrupting DNA-PK dependent DNA repair pathway[J]. Protein & Cell, 2020, 11(5): 352-365. |
| 109 | ADIKUSUMA F, PILTZ S, CORBETT M A, et al. Large deletions induced by Cas9 cleavage[J]. Nature, 2018, 560(7717): E8-E9. |
| 110 | SONG Y N, LIU Z Q, ZHANG Y X, et al. Large-fragment deletions induced by Cas9 cleavage while not in the BEs system[J]. Molecular Therapy Nucleic Acids, 2020, 21: 523-526. |
| 111 | CHARLESWORTH C T, DESHPANDE P S, DEVER D P, et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans[J]. Nature Medicine, 2019, 25(2): 249-254. |
| 112 | CHARLESWORTH C T, DESHPANDE P S, DEVER D P, et al. Identification of pre-existing adaptive immunity to Cas9 proteins in humans[EB/OL]. bioRxiv, 2018: 243345[2022-09-01]. . |
| 113 | LEIBOWITZ M L, PAPATHANASIOU S, DOERFLER P A, et al. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing[J]. Nature Genetics, 2021, 53(6): 895-905. |
| 114 | MAHAS A, NEAL STEWART JR C, MAHFOUZ M M. Harnessing CRISPR/Cas systems for programmable transcriptional and post-transcriptional regulation[J]. Biotechnology Advances, 2018, 36(1): 295-310. |
| 115 | GILBERT L A, HORLBECK M A, ADAMSON B, et al. Genome-scale CRISPR-mediated control of gene repression and activation[J]. Cell, 2014, 159(3): 647-661. |
| 116 | LIU X S, WU H, JI X, et al. Editing DNA methylation in the mammalian genome[J]. Cell, 2016, 167(1): 233-247.e17. |
| 117 | NUÑEZ J K, CHEN J, POMMIER G C, et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing[J]. Cell, 2021, 184(9): 2503-2519.e17. |
| 118 | PULECIO J, VERMA N, MEJÍA-RAMÍREZ E, et al. CRISPR/Cas9-based engineering of the epigenome[J]. Cell Stem Cell, 2017, 21(4): 431-447. |
| 119 | LIU X, ZHANG Y, CHEN Y, et al. In situ capture of chromatin interactions by biotinylated dCas9[J]. Cell, 2017, 170(5): 1028-1043.e19. |
| 120 | ZAFRA M P, SCHATOFF E M, KATTI A, et al. Optimized base editors enable efficient editing in cells, organoids and mice[J]. Nature Biotechnology, 2018, 36(9): 888-893. |
| 121 | WORKMAN R E, PAMMI T, NGUYEN B T K, et al. A natural single-guide RNA repurposes Cas9 to autoregulate CRISPR-Cas expression[J]. Cell, 2021, 184(3): 675-688.e19. |
| 122 | XU X S, CHEMPARATHY A, ZENG L P, et al. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing[J]. Molecular Cell, 2021, 81(20): 4333-4345.e4. |
| [1] | Fei SONG, Yuchen LIU, Zhiming CAI, Weiren HUANG. Construction of tumor gene circuits using CRISPR/Cas tool and their applications [J]. Synthetic Biology Journal, 2022, 3(1): 53-65. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||