Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (2): 267-280.DOI: 10.12211/2096-8280.2023-078
• Invited Review • Previous Articles Next Articles
New strategies for engineering influenza viruses and their applications
GUO Xiya, CHEN Ji, DONG Mingxin
- Department of Medicinal Chemistry,College of Pharmacy,Qingdao University,Qingdao 266000,Shandong,China
-
Received:2023-11-09Revised:2024-02-22Online:2024-04-28Published:2024-04-30 -
Contact:DONG Mingxin
流感病毒改造新策略及其应用
郭茜亚, 陈积, 董铭心
- 青岛大学药学院药物化学系,山东 青岛 266000
-
通讯作者:董铭心 -
作者简介:郭茜亚 (1997—),女,博士研究生。研究方向为溶瘤病毒的研发策略及应用。E-mail:guoxiya132@163.com董铭心 (1978—),男,教授,博士生导师。研究方向为抗病毒和神经系统小分子药物及疫苗。E-mail:Mxdong64@qdu.edu.cn -
基金资助:国家重点研发计划“合成生物学”重点专项(2018YFA0900804)
CLC Number:
Cite this article
GUO Xiya, CHEN Ji, DONG Mingxin. New strategies for engineering influenza viruses and their applications[J]. Synthetic Biology Journal, 2024, 5(2): 267-280.
郭茜亚, 陈积, 董铭心. 流感病毒改造新策略及其应用[J]. 合成生物学, 2024, 5(2): 267-280.
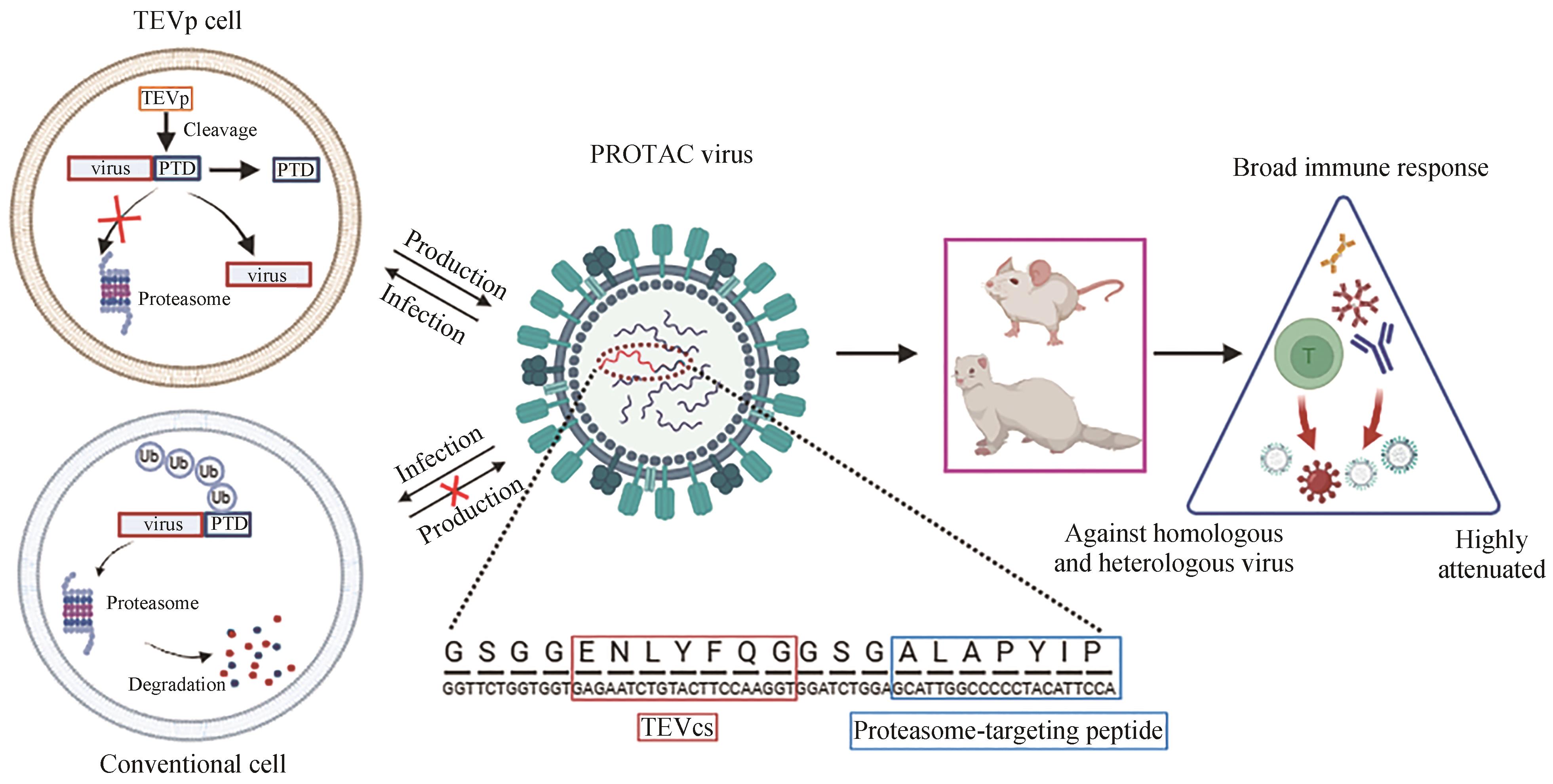
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-078
| 1 | WOOLHOUSE M E J, BRIERLEY L. Epidemiological characteristics of human-infective RNA viruses[J]. Scientific Data, 2018, 5: 180017. |
| 2 | GOUNDER A P, BOON A C M. Influenza pathogenesis: the effect of host factors on severity of disease[J]. Journal of Immunology, 2019, 202(2): 341-350. |
| 3 | FUKUYAMA S, KAWAOKA Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors[J]. Current Opinion in Immunology, 2011, 23(4): 481-486. |
| 4 | BELSHE R B, EDWARDS K M, VESIKARI T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children[J]. The New England Journal of Medicine, 2007, 356(7): 685-696. |
| 5 | BELSHE R B, NEWMAN F K, WILKINS K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults[J]. Vaccine, 2007, 25(37-38): 6755-6763. |
| 6 | GORSE G J, BELSHE R B, MUNN N J. Superiority of live attenuated compared with inactivated influenza A virus vaccines in older, chronically ill adults[J]. Chest, 1991, 100(4): 977-984. |
| 7 | PICA N, PALESE P. Toward a universal influenza virus vaccine: prospects and challenges[J]. Annual Review of Medicine, 2013, 64: 189-202. |
| 8 | BUONAGURIO D A, BECHERT T M, YANG C F, et al. Genetic stability of live, cold-adapted influenza virus components of the FluMist/CAIV-T vaccine throughout the manufacturing process[J]. Vaccine, 2006, 24(12): 2151-2160. |
| 9 | AMBROSE C S, LUKE C, COELINGH K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza[J]. Influenza and Other Respiratory Viruses, 2008, 2(6): 193-202. |
| 10 | DE VILLIERS P J, STEELE A D, HIEMSTRA L A, et al. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older[J]. Vaccine, 2009, 28(1): 228-234. |
| 11 | KRUG R M. Functions of the influenza A virus NS1 protein in antiviral defense[J]. Current Opinion in Virology, 2015, 12: 1-6. |
| 12 | AYLLON J, GARCÍA-SASTRE A. The NS1 protein: a multitasking virulence factor[J]. Current Topics in Microbiology and Immunology, 2015, 386: 73-107. |
| 13 | FERNANDEZ-SESMA A, MARUKIAN S, EBERSOLE B J, et al. Influenza virus evades innate and adaptive immunity via the NS1 protein[J]. Journal of Virology, 2006, 80(13): 6295-6304. |
| 14 | GEISS G K, SALVATORE M, TUMPEY T M, et al. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(16): 10736-10741. |
| 15 | RICHT J A, GARCÍA-SASTRE A. Attenuated influenza virus vaccines with modified NS1 proteins[J]. Current Topics in Microbiology and Immunology, 2009, 333: 177-195. |
| 16 | MUELLER S N, LANGLEY W A, CARNERO E, et al. Immunization with live attenuated influenza viruses that express altered NS1 proteins results in potent and protective memory CD8+ T-cell responses[J]. Journal of Virology, 2010, 84(4): 1847-1855. |
| 17 | PICA N, LANGLOIS R A, KRAMMER F, et al. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge[J]. Journal of Virology, 2012, 86(19): 10293-10301. |
| 18 | EGOROV A, BRANDT S, SEREINIG S, et al. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells[J]. Journal of Virology, 1998, 72(8): 6437-6441. |
| 19 | SI L L, SHEN Q, LI J, et al. Generation of a live attenuated influenza A vaccine by proteolysis targeting[J]. Nature Biotechnology, 2022, 40(9): 1370-1377. |
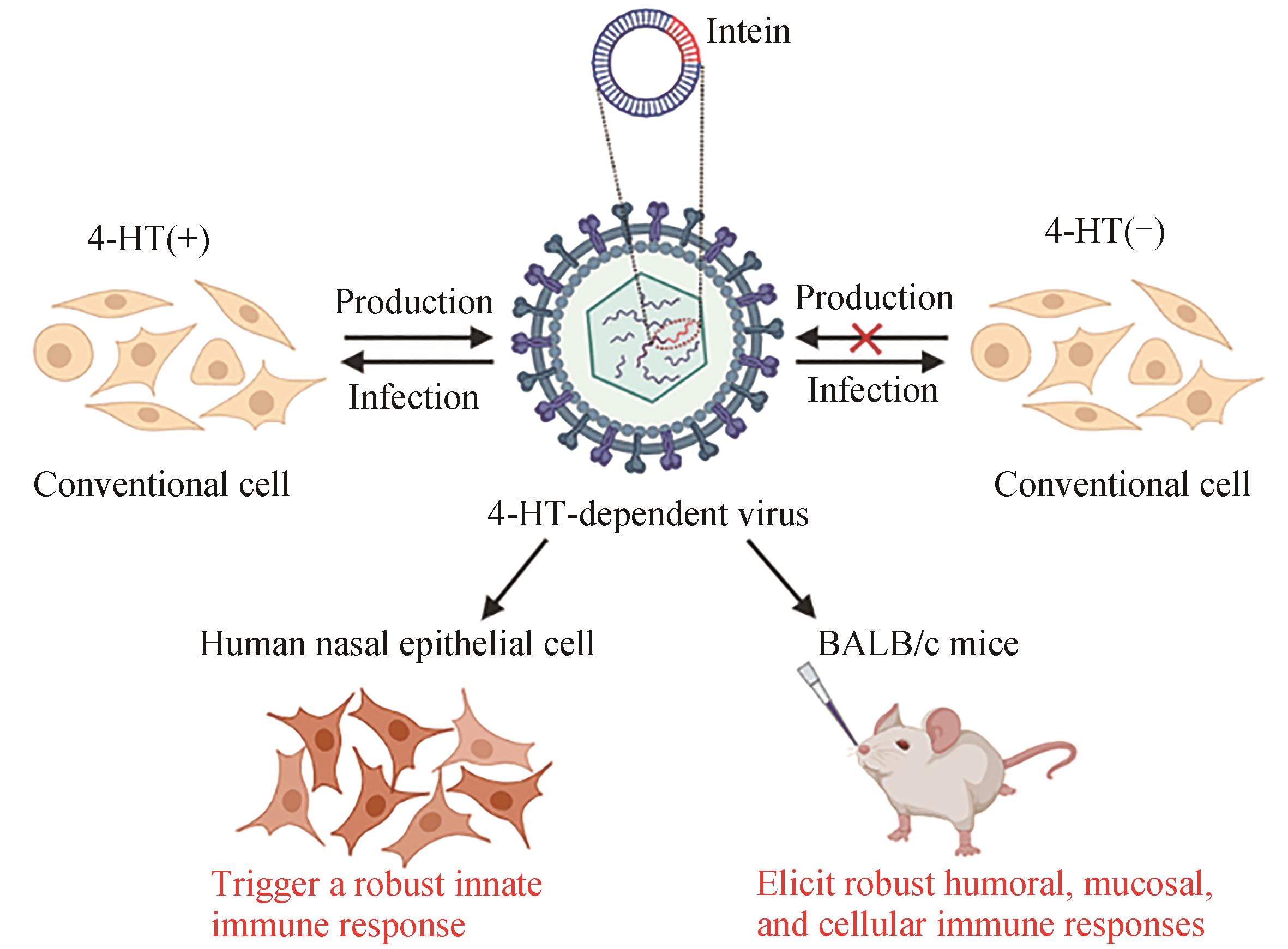
| 20 | CHEN J, WANG J Y, ZHU H Y, et al. Generation of a live attenuated influenza A vaccine using chemical-triggered intein[J]. ACS Synthetic Biology, 2023, 12(6): 1686-1695. |
| 21 | DU Y S, XIN L, SHI Y, et al. Genome-wide identification of interferon-sensitive mutations enables influenza vaccine design[J]. Science, 2018, 359(6373): 290-296. |
| 22 | ALSAAB H O, SAU S, ALZHRANI R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome[J]. Frontiers in Pharmacology, 2017, 8: 561. |
| 23 | MARIN-ACEVEDO J A, DHOLARIA B, SOYANO A E, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges[J]. Journal of Hematology & Oncology, 2018, 11(1): 39. |
| 24 | SCUDELLARI M. Protein-slaying drugs could be the next blockbuster therapies[J]. Nature, 2019, 567(7748): 298-300. |
| 25 | SALAMI J, CREWS C M. Waste disposal—an attractive strategy for cancer therapy[J]. Science, 2017, 355(6330): 1163-1167. |
| 26 | CROMM P M, CREWS C M. Targeted protein degradation: from chemical biology to drug discovery[J]. Cell Chemical Biology, 2017, 24(9): 1181-1190. |
| 27 | DESHAIES R J. Protein degradation: prime time for PROTACs[J]. Nature Chemical Biology, 2015, 11(9): 634-635. |
| 28 | YAU R, RAPE M. The increasing complexity of the ubiquitin code[J]. Nature Cell Biology, 2016, 18(6): 579-586. |
| 29 | FINLEY D. Recognition and processing of ubiquitin-protein conjugates by the proteasome[J]. Annual Review of Biochemistry, 2009, 78: 477-513. |
| 30 | HON W C, WILSON M I, HARLOS K, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL[J]. Nature, 2002, 417(6892): 975-978. |
| 31 | JAAKKOLA P, MOLE D R, TIAN Y M, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2 - regulated prolyl hydroxylation[J]. Science, 2001, 292(5516): 468-472. |
| 32 | IVAN M, KONDO K, YANG H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing[J]. Science, 2001, 292(5516): 464-468. |
| 33 | GU S S, CUI D R, CHEN X Y, et al. PROTACs: an emerging targeting technique for protein degradation in drug discovery[J]. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 2018, 40(4): e1700247. |
| 34 | ZHANG C, PENG Z H, ZHU M L, et al. USP9X destabilizes pVHL and promotes cell proliferation[J]. Oncotarget, 2016, 7(37): 60519-60534. |
| 35 | IWAI K, YAMANAKA K, KAMURA T, et al. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(22): 12436-12441. |
| 36 | LATIF F, TORY K, GNARRA J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene[J]. Science, 1993, 260(5112): 1317-1320. |
| 37 | LOS M, JANSEN G H, KAELIN W G, et al. Expression pattern of the von Hippel-Lindau protein in human tissues[J]. Laboratory Investigation, 1996, 75(2): 231-238. |
| 38 | PING J H, LOPES T J S, NEUMANN G, et al. Development of high-yield influenza B virus vaccine viruses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(51): E8296-E8305. |
| 39 | MUELLER S, COLEMAN J R, PAPAMICHAIL D, et al. Live attenuated influenza virus vaccines by computer-aided rational design[J]. Nature Biotechnology, 2010, 28(7): 723-726. |
| 40 | WEI C J, CRANK M C, SHIVER J, et al. Next-generation influenza vaccines: opportunities and challenges[J]. Nature Reviews Drug Discovery, 2020, 19(4): 239-252. |
| 41 | BANIK S M, PEDRAM K, WISNOVSKY S, et al. Lysosome-targeting chimaeras for degradation of extracellular proteins[J]. Nature, 2020, 584(7820): 291-297. |
| 42 | TAKAHASHI D, MORIYAMA J, NAKAMURA T, et al. AUTACs: cargo-specific degraders using selective autophagy[J]. Molecular Cell, 2019, 76(5): 797-810.e10. |
| 43 | LI Z Y, WANG C, WANG Z Y, et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds[J]. Nature, 2019, 575(7781): 203-209. |
| 44 | BUSKIRK A R, ONG Y C, GARTNER Z J, et al. Directed evolution of ligand dependence: small-molecule-activated protein splicing[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(29): 10505-10510. |
| 45 | YUEN C M, RODDA S J, VOKES S A, et al. Control of transcription factor activity and osteoblast differentiation in mammalian cells using an evolved small-molecule-dependent intein[J]. Journal of the American Chemical Society, 2006, 128(27): 8939-8946. |
| 46 | PECK S H, CHEN I, LIU D R. Directed evolution of a small-molecule-triggered intein with improved splicing properties in mammalian cells[J]. Chemistry & Biology, 2011, 18(5): 619-630. |
| 47 | LO C Y, TANG Y S, SHAW P C. Structure and function of influenza virus ribonucleoprotein[J]. Sub-Cellular Biochemistry, 2018, 88: 95-128. |
| 48 | LIU Q, YANG Y J, TAN X F, et al. Plasmodium parasite as an effective hepatocellular carcinoma antigen glypican-3 delivery vector[J]. Oncotarget, 2017, 8(15): 24785-24796. |
| 49 | YU R R, ZHU B, CHEN D G. Type Ⅰ interferon-mediated tumor immunity and its role in immunotherapy[J]. Cellular and Molecular Life Sciences, 2022, 79(3): 191. |
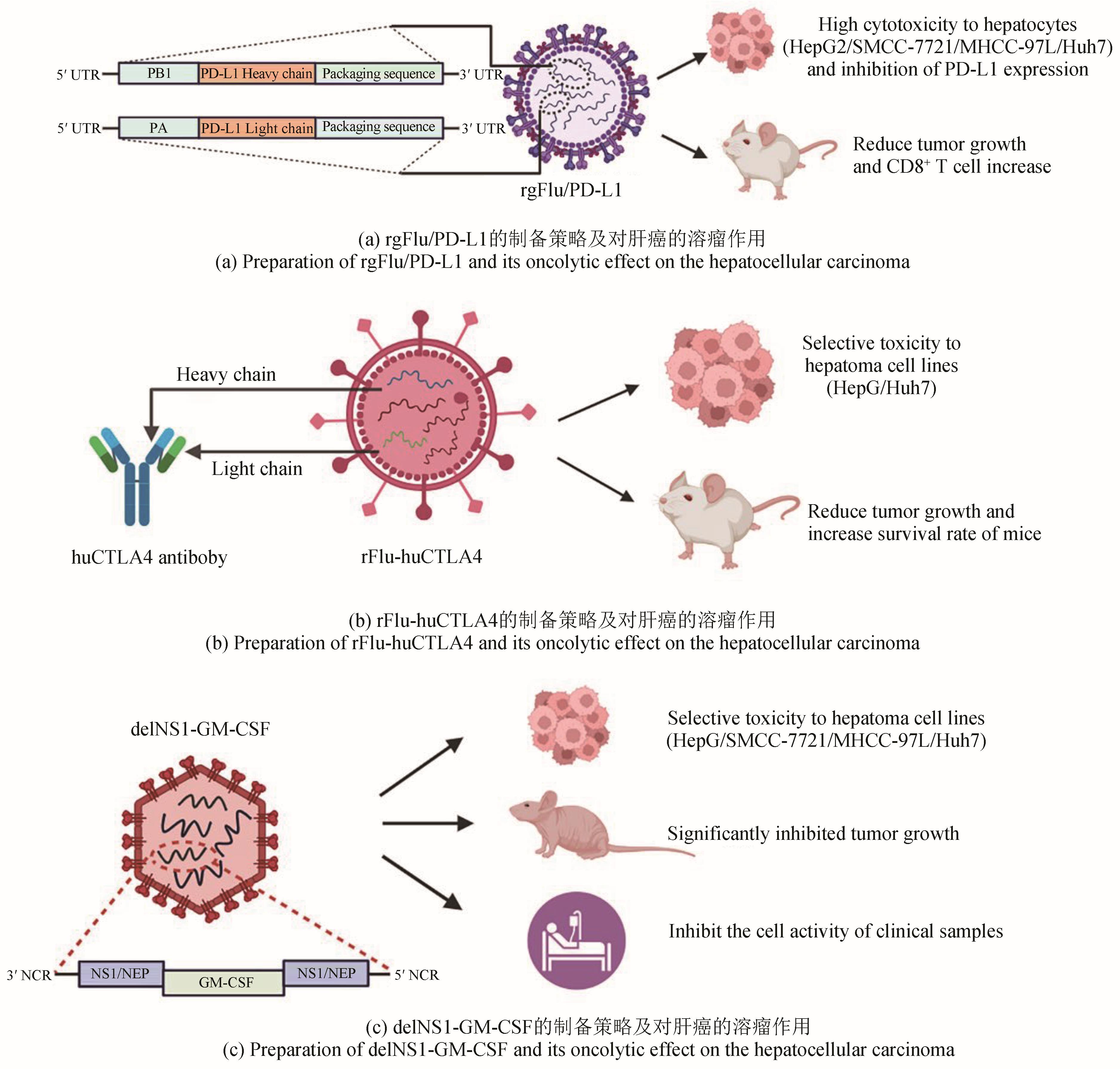
| 50 | WU N C, OLSON C A, DU Y S, et al. Functional constraint profiling of a viral protein reveals discordance of evolutionary conservation and functionality[J]. PLoS Genetics, 2015, 11(7): e1005310. |
| 51 | DU Y S, ZHANG T H, DAI L, et al. Effects of mutations on replicative fitness and major histocompatibility complex class Ⅰ binding affinity are among the determinants underlying cytotoxic-T-lymphocyte escape of HIV-1 gag epitopes[J]. mBio, 2017, 8(6): e01050-17. |
| 52 | HOFFMANN E, NEUMANN G, KAWAOKA Y, et al. A DNA transfection system for generation of influenza A virus from eight plasmids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(11): 6108-6113. |
| 53 | 夏忠军,常建华,张力. 基因工程腺病毒(H101)瘤内注射联合化疗治疗头颈部及食管鳞癌的Ⅲ期临床研究[J]. 癌症, 2004, 23(12): 1666-1670. |
| XIA Z J, CHANG J H, ZHANG L, et al. Phase Ⅲ randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus[J]. Chinese Journal of Cancer, 2004, 23(12): 1666-1670. | |
| 54 | GARBER K. China approves world’s first oncolytic virus therapy for cancer treatment[J]. Journal of the National Cancer Institute, 2006, 98(5): 298-300. |
| 55 | FERRUCCI P F, PALA L, CONFORTI F, et al. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma[J]. Cancers, 2021, 13(6): 1383. |
| 56 | WANG T, ZHANG J J, WANG Y L, et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs[J]. Nature Immunology, 2023, 24(3): 423-438. |
| 57 | PASTORINO U. The development of an international registry[J]. Journal of Thoracic Oncology, 2010, 5(S2): S196-S197. |
| 58 | SITNIK S, MASEMANN D, LEITE DANTAS R, et al. PD-1 IC inhibition synergistically improves influenza A virus-mediated oncolysis of metastatic pulmonary melanoma[J]. Molecular Therapy Oncolytics, 2020, 17: 190-204. |
| 59 | ANDTBACKA R H I, KAUFMAN H L, COLLICHIO F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma[J]. Journal of Clinical Oncology, 2015, 33(25): 2780-2788. |
| 60 | ANDTBACKA R H, ROSS M, PUZANOV I, et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase Ⅲ clinical trial[J]. Annals of Surgical Oncology, 2016, 23(13): 4169-4177. |
| 61 | BOMMAREDDY P K, PATEL A, HOSSAIN S, et al. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma[J]. American Journal of Clinical Dermatology, 2017, 18(1): 1-15. |
| 62 | JOHNSON D B, PUZANOV I, KELLEY M C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma[J]. Immunotherapy, 2015, 7(6): 611-619. |
| 63 | ALCAZER V, BONAVENTURA P, TONON L, et al. Neoepitopes-based vaccines: challenges and perspectives[J]. European Journal of Cancer, 2019, 108: 55-60. |
| 64 | BLASS E, OTT P A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines[J]. Nature Reviews Clinical Oncology, 2021, 18(4): 215-229. |
| 65 | YARCHOAN M, JOHNSON B A 3 RD, LUTZ E R,et al. Targeting neoantigens to augment antitumour immunity[J]. Nature Reviews Cancer, 2017, 17(9): 569. |
| 66 | TWUMASI-BOATENG K, PETTIGREW J L, EUNICE KWOK Y Y E, et al. Oncolytic viruses as engineering platforms for combination immunotherapy[J]. Nature Reviews Cancer, 2018, 18(7): 419-432. |
| 67 | GERLACH T, ELBAHESH H, SALETTI G, et al. Recombinant influenza A viruses as vaccine vectors[J]. Expert Review of Vaccines, 2019, 18(4): 379-392. |
| 68 | JI D Z, ZHANG Y J, SUN J Q, et al. An engineered influenza virus to deliver antigens for lung cancer vaccination[J/OL]. Nature Biotechnology, 2023[2023-12-01]. . |
| 69 | SHARMA P, ALLISON J P. The future of immune checkpoint therapy[J]. Science, 2015, 348(6230): 56-61. |
| 70 | WEI S C, DUFFY C R, ALLISON J P. Fundamental mechanisms of immune checkpoint blockade therapy[J]. Cancer Discovery, 2018, 8(9): 1069-1086. |
| 71 | WANG G, KANG X, CHEN K S, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses[J]. Nature Communications, 2020, 11(1): 1395. |
| 72 | RIBAS A, DUMMER R, PUZANOV I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy[J]. Cell, 2017, 170 (6): 1109-1119 e10. |
| 73 | HILDNER K, EDELSON B T, PURTHA W E, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity[J]. Science, 2008, 322(5904): 1097-1100. |
| 74 | BOMMAREDDY P K, SHETTIGAR M, KAUFMAN H L. Integrating oncolytic viruses in combination cancer immunotherapy[J]. Nature Reviews Immunology, 2018, 18(8): 498-513. |
| 75 | RUSSELL S J, BARBER G N. Oncolytic viruses as antigen-agnostic cancer vaccines[J]. Cancer Cell, 2018, 33(4): 599-605. |
| 76 | SUN F, XU Y, DENG Z Y, et al. A recombinant oncolytic influenza virus expressing a PD-L1 antibody induces CD8+ T-cell activation via the cGas-STING pathway in mice with hepatocellular carcinoma[J]. International Immunopharmacology, 2023, 120: 110323. |
| 77 | HOSSEINI A, GHARIBI T, MAROFI F, et al. CTLA-4: from mechanism to autoimmune therapy[J]. International Immunopharmacology, 2020, 80: 106221. |
| 78 | YANG H, LEI G L, SUN F, et al. Oncolytic activity of a chimeric influenza A virus carrying a human CTLA4 antibody in hepatocellular carcinoma[J]. Frontiers in Oncology, 2022, 12: 875525. |
| 79 | LEI G L, WANG L P, DONG S H, et al. A recombinant influenza virus with a CTLA4-specific scFv inhibits tumor growth in a mouse model[J]. Cell Biology International, 2021, 45(6): 1202-1210. |
| 80 | HAMILTON J R, VIJAYAKUMAR G, PALESE P. A recombinant antibody-expressing influenza virus delays tumor growth in a mouse model[J]. Cell Reports, 2018, 22(1): 1-7. |
| 81 | BURDACH S E, MÜSCHENICH M, JOSEPHS W, et al. Granulocyte-macrophage-colony stimulating factor for prevention of neutropenia and infections in children and adolescents with solid tumors. Results of a prospective randomized study[J]. Cancer, 1995, 76(3): 510-516. |
| 82 | JAHAN N, TALAT H, CURRY W T. Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells[J]. Neuro-Oncology, 2018, 20(1): 44-54. |
| 83 | YANG P H, SUN F, WANG R L, et al. Oncolytic activity of a novel influenza A virus carrying granulocyte-macrophage colony-stimulating factor in hepatocellular carcinoma[J]. Human Gene Therapy, 2019, 30(3): 330-338. |
| 84 | LIPKIN W I. Biocontainment in gain-of-function infectious disease research[J]. mBio, 2012, 3(5): e00290-12. |
| 85 | SEIDEL J A, OTSUKA A, KABASHIMA K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations[J]. Frontiers in Oncology, 2018, 8: 86. |
| [1] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||