Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (2): 294-309.DOI: 10.12211/2096-8280.2023-071
• Invited Review • Previous Articles Next Articles
Applications of vector vaccines developed through T-cell immune responses in preventing and treating human diseases
JIANG Shasha1, WANG Chen1, LU Ran2, LIU Fengjun3, LI Jun1, WANG Bin1,3
- 1.Department of Pathogenic Biology,School of Basic Medicine,Qingdao University,Qingdao 266000,Shandong,China
2.Microbiological Laboratory,Chaoyang District Center for Disease Control and Prevention,Beijing 100021,China
3.Department of Special Medicine,School of Basic Medicine,Qingdao University,Qingdao 266000,Shandong,China
-
Received:2023-10-07Revised:2024-03-12Online:2024-04-28Published:2024-04-30 -
Contact:WANG Bin
T细胞免疫反应载体疫苗在人类疾病预防和治疗中的应用
江莎莎1, 王晨1, 路冉2, 刘俸君3, 李俊1, 王斌1,3
- 1.青岛大学基础医学院,病原生物学系,山东 青岛 266000
2.北京市朝阳区疾病预防控制中心,微生物检验科,北京 100021
3.青岛大学基础医学院,特种医学系,山东 青岛 266000
-
通讯作者:王斌 -
作者简介:江莎莎 (1995—),女,博士研究生。研究方向为人巨细胞病毒疫苗的设计和研发。E-mail:15844207055@163.com王晨 (1998—),男,硕士研究生。研究方向为人巨细胞病毒疫苗的设计和研发。E-mail:13546694969@163.com王斌 (1962—),男,教授,博士生导师。研究方向为人类巨细胞病毒致神经损伤的分子机制和免疫学机制。E-mail:wangbin532@126.com -
基金资助:国家重点研发计划(2018YFA0900802);山东省重点研发计划(2019JZZY011009);青岛市自然科学基金(20-2-3-4-nsh)
CLC Number:
Cite this article
JIANG Shasha, WANG Chen, LU Ran, LIU Fengjun, LI Jun, WANG Bin. Applications of vector vaccines developed through T-cell immune responses in preventing and treating human diseases[J]. Synthetic Biology Journal, 2024, 5(2): 294-309.
江莎莎, 王晨, 路冉, 刘俸君, 李俊, 王斌. T细胞免疫反应载体疫苗在人类疾病预防和治疗中的应用[J]. 合成生物学, 2024, 5(2): 294-309.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-071
| 1 | CROTTY S. Follicular helper CD4 T cells (TFH)[J]. Annual Review of Immunology, 2011, 29: 621-663. |
| 2 | ZHU J F, YAMANE H, PAUL W E. Differentiation of effector CD4 T cell populations*[J]. Annual Review of Immunology, 2010, 28: 445-489. |
| 3 | SALLUSTO F, LANZAVECCHIA A, ARAKI K, et al. From vaccines to memory and back[J]. Immunity, 2010, 33(4): 451-463. |
| 4 | LEVIN M J, OXMAN M N, ZHANG J H, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine[J]. The Journal of Infectious Diseases, 2008, 197(6): 825-835. |
| 5 | MCELHANEY J E, XIE D X, HAGER W D, et al. T cell responses are better correlates of vaccine protection in the elderly[J]. Journal of Immunology, 2006, 176(10): 6333-6339. |
| 6 | PANTALEO G, KOUP R A. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know[J]. Nature Medicine, 2004, 10(8): 806-810. |
| 7 | BUNDE T, KIRCHNER A, HOFFMEISTER B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells[J]. The Journal of Experimental Medicine, 2005, 201(7): 1031-1036. |
| 8 | HOFT D F. Tuberculosis vaccine development: goals, immunological design, and evaluation[J]. Lancet, 2008, 372(9633): 164-175. |
| 9 | REYES-SANDOVAL A, PEARSON F E, TODRYK S, et al. Potency assays for novel T-cell-inducing vaccines against malaria[J]. Current Opinion in Molecular Therapeutics, 2009, 11(1): 72-80. |
| 10 | REINA-CAMPOS M, SCHARPING N E, GOLDRATH A W. CD8+ T cell metabolism in infection and cancer[J]. Nature Reviews Immunology, 2021, 21(11): 718-738. |
| 11 | CHAPMAN N M, BOOTHBY M R, CHI H B. Metabolic coordination of T cell quiescence and activation[J]. Nature Reviews Immunology, 2020, 20(1): 55-70. |
| 12 | SADELAIN M, RIVIÈRE I, RIDDELL S. Therapeutic T cell engineering[J]. Nature, 2017, 545(7655): 423-431. |
| 13 | SUN L N, SU Y H, JIAO A J, et al. T cells in health and disease[J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 235. |
| 14 | KUMAR B V, CONNORS T J, FARBER D L. Human T cell development, localization, and function throughout life[J]. Immunity, 2018, 48(2): 202-213. |
| 15 | CHEN L P, FLIES D B. Molecular mechanisms of T cell co-stimulation and co-inhibition[J]. Nature Reviews Immunology, 2013, 13(4): 227-242. |
| 16 | HENRY C J, ORNELLES D A, MITCHELL L M, et al. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17[J]. Journal of Immunology, 2008, 181(12): 8576-8584. |
| 17 | DÍAZ-MONTERO C M, NAGGAR S EL, KHAMI A AL, et al. Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8+CD62Lhi cells and results in superior anti-tumor activity in a tolerogenic murine model[J]. Cancer Immunology, Immunotherapy, 2008, 57(4): 563-572. |
| 18 | RAMOS H J, DAVIS A M, COLE A G, et al. Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates[J]. Blood, 2009, 113(22): 5516-5525. |
| 19 | REDEKER A, WELTEN S P, BAERT M R, et al. The quantity of autocrine IL-2 governs the expansion potential of CD8+ T cells[J]. Journal of Immunology, 2015, 195(10): 4792-4801. |
| 20 | GETT A V, HODGKIN P D. A cellular calculus for signal integration by T cells[J]. Nature Immunology, 2000, 1(3): 239-244. |
| 21 | BORST J, AHRENDS T, BĄBAŁA N, et al. CD4+ T cell help in cancer immunology and immunotherapy[J]. Nature Reviews Immunology, 2018, 18(10): 635-647. |
| 22 | RUTERBUSCH M, PRUNER K B, SHEHATA L, et al. In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm[J]. Annual Review of Immunology, 2020, 38: 705-725. |
| 23 | MARTIN M D, BADOVINAC V P, GRIFFITH T S. CD4 T cell responses and the sepsis-induced immunoparalysis state[J]. Frontiers in Immunology, 2020, 11: 1364. |
| 24 | LIU M A. Gene-based vaccines: recent developments[J]. Current Opinion in Molecular Therapeutics, 2010, 12(1): 86-93. |
| 25 | ROBINSON H L, AMARA R R. T cell vaccines for microbial infections[J]. Nature Medicine, 2005, 11(4 ): S25-S32. |
| 26 | NIDETZ N F, MCGEE M C, TSE L V, et al. Adeno-associated viral vector-mediated immune responses: understanding barriers to gene delivery[J]. Pharmacology & Therapeutics, 2020, 207: 107453. |
| 27 | XU Z L, MIZUGUCHI H, SAKURAI F, et al. Approaches to improving the kinetics of adenovirus-delivered genes and gene products[J]. Advanced Drug Delivery Reviews, 2005, 57(5): 781-802. |
| 28 | MAYS L E, WILSON J M. The complex and evolving story of T cell activation to AAV vector-encoded transgene products[J]. Molecular Therapy, 2011, 19(1): 16-27. |
| 29 | HASANPOURGHADI M, NOVIKOV M, ERTL H C J. COVID-19 vaccines based on adenovirus vectors[J]. Trends in Biochemical Sciences, 2021, 46(5): 429-430. |
| 30 | KOGER-PEASE C, PERERA D J, NDAO M. Recent advances in the development of adenovirus-vectored vaccines for parasitic infections[J]. Pharmaceuticals, 2023, 16(3): 334. |
| 31 | LASARO M O, ERTL H C J. New insights on adenovirus as vaccine vectors[J]. Molecular Therapy, 2009, 17(8): 1333-1339. |
| 32 | ZHOU X Y, XIANG Z Q, ERTL H C J. Vaccine design: replication-defective adenovirus vectors[J]. Methods in Molecular Biology, 2016, 1404: 329-349. |
| 33 | MACNEIL K M, DODGE M J, EVANS A M, et al. Adenoviruses in medicine: innocuous pathogen, predator, or partner[J]. Trends in Molecular Medicine, 2023, 29(1): 4-19. |
| 34 | TATSIS N, ERTL H C J. Adenoviruses as vaccine vectors[J]. Molecular Therapy, 2004, 10(4): 616-629. |
| 35 | MIZUGUCHI H, KAY M A, HAYAKAWA T. Approaches for generating recombinant adenovirus vectors[J]. Advanced Drug Delivery Reviews, 2001, 52(3): 165-176. |
| 36 | KRATZER R F, KREPPEL F. Production, purification, and titration of first-generation adenovirus vectors[J]. Methods in Molecular Biology, 2017, 1654: 377-388. |
| 37 | KAMEN A, HENRY O. Development and optimization of an adenovirus production process[J]. The Journal of Gene Medicine, 2004, 6(): S184-S192. |
| 38 | CHAN J F, KOK K H, ZHU Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan[J]. Emerging Microbes & Infections, 2020, 9(1): 221-236. |
| 39 | 张倩, 胡倩倩, 王富珍, 等. 腺病毒载体疫苗研发进展[J]. 中国疫苗和免疫, 2020, 26(4): 484-492. |
| ZHANG Q, HU Q Q, WANG F Z, et al. Research and development of adenovirus vectored vaccines[J]. Chinese Journal of Vaccines and Immunization, 2020, 26(4): 484-492. | |
| 40 | HALPERIN S A, YE L Y, MACKINNON-CAMERON D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial[J]. Lancet, 2022, 399(10321): 237-248. |
| 41 | PARRINO J, GRAHAM B S. Smallpox vaccines: past, present, and future[J]. The Journal of Allergy and Clinical Immunology, 2006, 118(6): 1320-1326. |
| 42 | MACKETT M, SMITH G L, MOSS B. Vaccinia virus: a selectable eukaryotic cloning and expression vector[J]. Proceedings of the National Academy of Sciences of the United States of America, 1982, 79(23): 7415-7419. |
| 43 | PANICALI D, PAOLETTI E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus[J]. Proceedings of the National Academy of Sciences of the United States of America, 1982, 79(16): 4927-4931. |
| 44 | PUISSANT B, COMBADIÈRE B. Keeping the memory of smallpox virus[J]. Cellular and Molecular Life Sciences, 2006, 63(19-20): 2249-2259. |
| 45 | MOSS B. Vaccinia virus: a tool for research and vaccine development[J]. Science, 1991, 252(5013): 1662-1667. |
| 46 | NORBURY C C, MALIDE D, GIBBS J S, et al. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo [J]. Nature Immunology, 2002, 3(3): 265-271. |
| 47 | VAN STIPDONK M J B, HARDENBERG G, BIJKER M S, et al. Dynamic programming of CD8+ T lymphocyte responses[J]. Nature Immunology, 2003, 4(4): 361-365. |
| 48 | GÓMEZ C E, PERDIGUERO B, GARCIA-ARRIAZA J, et al. Poxvirus vectors as HIV/AIDS vaccines in humans[J]. Human Vaccines & Immunotherapeutics, 2012, 8(9): 1192-1207. |
| 49 | SÁNCHEZ-SAMPEDRO L, PERDIGUERO B, MEJÍAS-PÉREZ E, et al. The evolution of poxvirus vaccines[J]. Viruses, 2015, 7(4): 1726-1803. |
| 50 | GUO Z S, LU B F, GUO Z B, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics[J]. Journal for Immunotherapy of Cancer, 2019, 7(1): 6. |
| 51 | SUTTER G, MOSS B. Nonreplicating vaccinia vector efficiently expresses recombinant genes[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(22): 10847-10851. |
| 52 | SUTER M, MEISINGER-HENSCHEL C, TZATZARIS M, et al. Modified vaccinia Ankara strains with identical coding sequences actually represent complex mixtures of viruses that determine the biological properties of each strain[J]. Vaccine, 2009, 27(52): 7442-7450. |
| 53 | VON KREMPELHUBER A, VOLLMAR J, POKORNY R, et al. A randomized, double-blind, dose-finding phase Ⅱ study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE[J]. Vaccine, 2010, 28(5): 1209-1216. |
| 54 | PITTMAN P R, HAHN M, LEE H S, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox[J]. The New England Journal of Medicine, 2019, 381(20): 1897-1908. |
| 55 | MUNIER C M L, ANDERSEN C R, KELLEHER A D. HIV vaccines: progress to date[J]. Drugs, 2011, 71(4): 387-414. |
| 56 | COTTINGHAM M G, CARROLL M W. Recombinant MVA vaccines: dispelling the myths[J]. Vaccine, 2013, 31(39): 4247-4251. |
| 57 | ZHOU Y, SULLIVAN N J. Immunology and evolvement of the adenovirus prime, MVA boost Ebola virus vaccine[J]. Current Opinion in Immunology, 2015, 35: 131-136. |
| 58 | 曾谷城. 埃博拉病毒研究进展[J]. 中山大学学报(医学科学版), 2015, 36(2): 161-166. |
| ZENG G C. Progresses and problems in Ebola virus research[J]. Journal of Sun Yat-Sen University (Medical Sciences), 2015, 36(2): 161-166. | |
| 59 | WU L H, ZHANG Z, GAO H N, et al. Open-label phase Ⅰclinical trial of Ad5-EBOV in Africans in China[J]. Human Vaccines & Immunotherapeutics, 2017, 13(9): 2078-2085. |
| 60 | MILLIGAN I D, GIBANI M M, SEWELL R, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial[J]. JAMA, 2016, 315(15): 1610-1623. |
| 61 | LICHTY B D, POWER A T, STOJDL D F, et al. Vesicular stomatitis virus: re-inventing the bullet[J]. Trends in Molecular Medicine, 2004, 10(5): 210-216. |
| 62 | FRECHA C, LÉVY C, COSSET F L, et al. Advances in the field of lentivector-based transduction of T and B lymphocytes for gene therapy[J]. Molecular Therapy, 2010, 18(10): 1748-1757. |
| 63 | SASSO E, D’ALISE A M, ZAMBRANO N, et al. New viral vectors for infectious diseases and cancer[J]. Seminars in Immunology, 2020, 50: 101430. |
| 64 | BARBER G N. VSV-tumor selective replication and protein translation[J]. Oncogene, 2005, 24(52): 7710-7719. |
| 65 | DHAMA K, KARTHIK K, KHANDIA R, et al. Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus[J]. Frontiers in Immunology, 2018, 9: 1803. |
| 66 | LI H Y, ZHAO C Y, ZHANG Y H, et al. Establishment of replication-competent vesicular stomatitis virus-based recombinant viruses suitable for SARS-CoV-2 entry and neutralization assays[J]. Emerging Microbes & Infections, 2020, 9(1): 2269-2277. |
| 67 | SCHNELL M J, BUONOCORE L, KRETZSCHMAR E, et al. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(21): 11359-11365. |
| 68 | CLARKE D K, HENDRY R M, SINGH V, et al. Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: standardized template with key considerations for a risk/benefit assessment[J]. Vaccine, 2016, 34(51): 6597-6609. |
| 69 | GEISBERT T W, FELDMANN H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections[J]. The Journal of Infectious Diseases, 2011, 204(S3): S1075-S1081. |
| 70 | 卢建博, 郑文铝, 余云舟, 等. 重组水疱性口炎病毒载体病毒包装体系的建立及优化[J]. 生物技术通讯, 2018, 29(2): 183-188. |
| LU J B, ZHENG W L, YU Y Z, et al. Establishment and optimization of recombinant VSV packaging system[J]. Letters in Biotechnology, 2018, 29(2): 183-188. | |
| 71 | MONATH T P, NICHOLS R, TUSSEY L, et al. Recombinant vesicular stomatitis vaccine against Nipah virus has a favorable safety profile: model for assessment of live vaccines with neurotropic potential[J]. PLoS Pathogens, 2022, 18(6): e1010658. |
| 72 | JOHNSON J E, NASAR F, COLEMAN J W, et al. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human Primates[J]. Virology, 2007, 360(1): 36-49. |
| 73 | LAI L L, DAVEY R, BECK A, et al. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick[J]. JAMA, 2015, 313(12): 1249-1255. |
| 74 | ROSE N F, MARX P A, LUCKAY A, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants[J]. Cell, 2001, 106(5): 539-549. |
| 75 | GIRARD-GAGNEPAIN A, AMIRACHE F, COSTA C, et al. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs[J]. Blood, 2014, 124(8): 1221-1231. |
| 76 | FELICIANO D, OTT C M, ESPINOSA-MEDINA I, et al. YAP1 nuclear efflux and transcriptional reprograming follow membrane diminution upon VSV-G-induced cell fusion[J]. Nature Communications, 2021, 12(1): 4502. |
| 77 | NIKOLIC J, BELOT L, RAUX H, et al. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein[J]. Nature Communications, 2018, 9(1): 1029. |
| 78 | CASE J B, ROTHLAUF P W, CHEN R E, et al. Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis in mice[J]. Cell Host & Microbe, 2020, 28(3): 465-474.e4. |
| 79 | SHEN W W, PATNAIK M M, RUIZ A, et al. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia[J]. Blood, 2016, 127(11): 1449-1458. |
| 80 | MOROZOV I, MONATH T P, MEEKINS D A, et al. High dose of vesicular stomatitis virus-vectored Ebola virus vaccine causes vesicular disease in swine without horizontal transmission[J]. Emerging Microbes & Infections, 2021, 10(1): 651-663. |
| 81 | ROEDIGER E K, KUGATHASAN K, ZHANG X Z, et al. Heterologous boosting of recombinant adenoviral prime immunization with a novel vesicular stomatitis virus-vectored tuberculosis vaccine[J]. Molecular Therapy, 2008, 16(6): 1161-1169. |
| 82 | MARZI A, FELDMANN F, GEISBERT T W, et al. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses[J]. Emerging Infectious Diseases, 2015, 21(2): 305-307. |
| 83 | IMAZU S, NAKAGAWA S, NAKANISHI T, et al. A novel nonviral vector based on vesicular stomatitis virus[J]. Journal of Controlled Release, 2000, 68(2): 187-194. |
| 84 | CLARKE D K, COOPER D, EGAN M A, et al. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector[J]. Springer Seminars in Immunopathology, 2006, 28(3): 239-253. |
| 85 | WITKO S E, JOHNSON J E, KALYAN N K, et al. Refined methods for propagating vesicular stomatitis virus vectors that are defective for G protein expression[J]. Journal of Virological Methods, 2010, 164(1-2): 43-50. |
| 86 | FUCHS J, FRANK I, KOCHAR N, et al. First-in-human phase Ⅰ clinical trial of a recombinant vesicular stomatitis virus (rVSV)-based preventive HIV-1 vaccine[J]. Retrovirology, 2012, 9(2): P134. |
| 87 | FUCHS J D, FRANK I, ELIZAGA M L, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant vesicular stomatitis virus human immunodeficiency virus-1 gag vaccine (HVTN 090)[J]. Open Forum Infectious Diseases, 2015, 2(3): ofv082. |
| 88 | WILSON S R, WILSON J H, BUONOCORE L, et al. Intranasal immunization with recombinant vesicular stomatitis virus expressing murine cytomegalovirus glycoprotein B induces humoral and cellular immunity[J]. Comparative Medicine, 2008, 58(2): 129-139. |
| 89 | BRANDSMA J L, SHYLANKEVICH M, SU Y H, et al. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus[J]. Journal of Virology, 2007, 81(11): 5749-5758. |
| 90 | BRIDLE B W, BOUDREAU J E, LICHTY B D, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus[J]. Molecular Therapy, 2009, 17(10): 1814-1821. |
| 91 | HUANG H, WANG Y J, WHITE A P, et al. Salmonella expressing a T-cell epitope from Sendai virus are able to induce anti-infection immunity[J]. Journal of Medical Microbiology, 2009, 58(Pt 9): 1236-1242. |
| 92 | FENNELLY G J, KHAN S A, ABADI M A, et al. Mucosal DNA vaccine immunization against measles with a highly attenuated Shigella flexneri vector[J]. Journal of Immunology, 1999, 162(3): 1603-1610. |
| 93 | FERRARI G, KOSTYU D D, COX J, et al. Identification of highly conserved and broadly cross-reactive HIV type 1 cytotoxic T lymphocyte epitopes as candidate immunogens for inclusion in Mycobacterium bovis BCG-vectored HIV vaccines[J]. AIDS Research and Human Retroviruses, 2000, 16(14): 1433-1443. |
| 94 | GAHAN M E, WEBSTER D E, WESSELINGH S L, et al. Bacterial antigen expression is an important component in inducing an immune response to orally administered Salmonella-delivered DNA vaccines[J]. PLoS One, 2009, 4(6): e6062. |
| 95 | ZHANG X L, JEZA V T, PAN Q. Salmonella typhi: from a human pathogen to a vaccine vector[J]. Cellular & Molecular Immunology, 2008, 5(2): 91-97. |
| 96 | MITAMURA T, HIGASHIYAMA S, TANIGUCHI N, et al. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity[J]. The Journal of Biological Chemistry, 1995, 270(3): 1015-1019. |
| 97 | BUZZI S, RUBBOLI D, BUZZI G, et al. CRM197 (nontoxic diphtheria toxin): effects on advanced cancer patients[J]. Cancer Immunology, Immunotherapy, 2004, 53(11): 1041-1048. |
| 98 | SHINEFIELD H R. Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use[J]. Vaccine, 2010, 28(27): 4335-4339. |
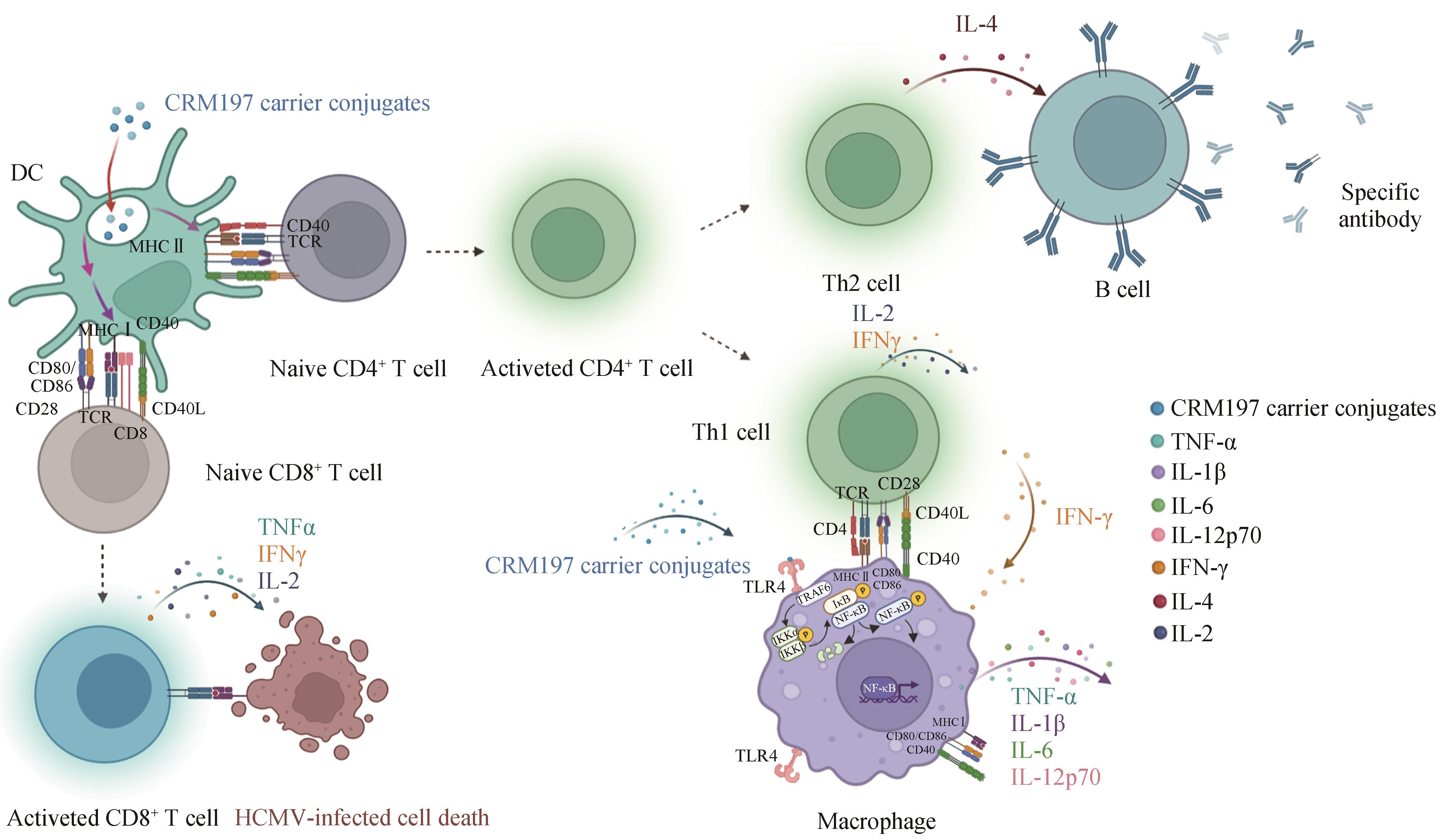
| 99 | JIANG S S, NAN F L, ZHANG S Y, et al. CRM197-conjugated multi antigen dominant epitope for effective human cytomegalovirus vaccine development[J]. International Journal of Biological Macromolecules, 2023, 224: 79-93. |
| 100 | ZHANG S Y, NAN F L, JIANG S S, et al. CRM197-conjugated peptides vaccine of HCMV pp65 and gH induce maturation of DC and effective viral-specific T cell responses[J]. Virulence, 2023, 14(1): 2169488. |
| 101 | HOEBE K, JANSSEN E, BEUTLER B. The interface between innate and adaptive immunity[J]. Nature Immunology, 2004, 5(10): 971-974. |
| 102 | SCHOEN C, STRITZKER J, GOEBEL W, et al. Bacteria as DNA vaccine carriers for genetic immunization[J]. International Journal of Medical Microbiology, 2004, 294(5): 319-335. |
| 103 | DUNHAM S P. The application of nucleic acid vaccines in veterinary medicine[J]. Research in Veterinary Science, 2002, 73(1): 9-16. |
| 104 | DERTZBAUGH M T. Genetically engineered vaccines: an overview[J]. Plasmid, 1998, 39(2): 100-113. |
| 105 | MEKONNEN Z A, GRUBOR-BAUK B, MASAVULI M G, et al. Toward DNA-based T-cell mediated vaccines to target HIV-1 and hepatitis C virus: approaches to elicit localized immunity for protection[J]. Frontiers in Cellular and Infection Microbiology, 2019, 9: 91. |
| 106 | PAGLIARI S, DEMA B, SANCHEZ-MARTINEZ A, et al. DNA vaccines: history, molecular mechanisms and future perspectives[J]. Journal of Molecular Biology, 2023, 435(23): 168297. |
| 107 | TIRIVEEDHI V, TUCKER N, HERNDON J, et al. Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer[J]. Clinical Cancer Research, 2014, 20(23): 5964-5975. |
| 108 | BRENNER S, JACOB F, MESELSON M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis[J]. Nature, 1961, 190: 576-581. |
| 109 | XU S Q, YANG K P, LI R, et al. mRNA vaccine era-mechanisms, drug platform and clinical prospection[J]. International Journal of Molecular Sciences, 2020, 21(18): 6582. |
| 110 | WOLFF J A, MALONE R W, WILLIAMS P, et al. Direct gene transfer into mouse muscle in vivo [J]. Science, 1990, 247(4949 Pt 1): 1465-1468. |
| 111 | SHROFF R T, CHALASANI P, WEI R, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors[J]. Nature Medicine, 2021, 27(11): 2002-2011. |
| 112 | JACKSON L A, ANDERSON E J, ROUPHAEL N G, et al. An mRNA vaccine against SARS-CoV-2-preliminary report[J]. The New England Journal of Medicine, 2020, 383(20): 1920-1931. |
| 113 | HEMMI H, TAKEUCHI O, KAWAI T, et al. A Toll-like receptor recognizes bacterial DNA[J]. Nature, 2000, 408(6813): 740-745. |
| 114 | KARIKÓ K, BUCKSTEIN M, NI H P, et al. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA[J]. Immunity, 2005, 23(2): 165-175. |
| 115 | PARDI N, HOGAN M J, PORTER F W, et al. mRNA vaccines - a new era in vaccinology[J]. Nature Reviews Drug Discovery, 2018, 17(4): 261-279. |
| 116 | FELDMAN R A, FUHR R, SMOLENOV I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials[J]. Vaccine, 2019, 37(25): 3326-3334. |
| 117 | JONG W, LEAL L, BUYZE J, et al. Therapeutic vaccine in chronically HIV-1-infected patients: a randomized, double-blind, placebo-controlled phase Ⅱa trial with HTI-TriMix[J]. Vaccines, 2019, 7(4): 209. |
| 118 | ZHANG N N, LI X F, DENG Y Q, et al. A thermostable mRNA vaccine against COVID-19[J]. Cell, 2020, 182(5): 1271-1283.e16. |
| 119 | RICHNER J M, HIMANSU S, DOWD K A, et al. Modified mRNA vaccines protect against Zika virus infection[J]. Cell, 2017, 168(6): 1114-1125.e10. |
| 120 | XUE T, STAVROPOULOS E, YANG M, et al. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection[J]. Infection and Immunity, 2004, 72(11): 6324-6329. |
| 121 | PASTOR F, BERRAONDO P, ETXEBERRIA I, et al. An RNA toolbox for cancer immunotherapy[J]. Nature Reviews Drug Discovery, 2018, 17(10): 751-767. |
| 122 | TOMBÁCZ I, WEISSMAN D, PARDI N. Vaccination with messenger RNA: a promising alternative to DNA vaccination[J]. Methods in Molecular Biology, 2021, 2197: 13-31. |
| 123 | LINARES-FERNÁNDEZ S, LACROIX C, EXPOSITO J Y, et al. Tailoring mRNA vaccine to balance innate/adaptive immune response[J]. Trends in Molecular Medicine, 2020, 26(3): 311-323. |
| 124 | FAGHFURI E, POURFARZI F, FAGHFOURI A H, et al. Recent developments of RNA-based vaccines in cancer immunotherapy[J]. Expert Opinion on Biological Therapy, 2021, 21(2): 201-218. |
| 125 | VAN NUFFEL A M, WILGENHOF S, THIELEMANS K, et al. Overcoming HLA restriction in clinical trials: immune monitoring of mRNA-loaded DC therapy[J]. Oncoimmunology, 2012, 1(8): 1392-1394. |
| 126 | IAVARONE C, O’HAGAN D T, YU D, et al. Mechanism of action of mRNA-based vaccines[J]. Expert Review of Vaccines, 2017, 16(9): 871-881. |
| 127 | SAXENA M, VAN DER BURG S H, MELIEF C J M, et al. Therapeutic cancer vaccines[J]. Nature Reviews Cancer, 2021, 21(6): 360-378. |
| 128 | JIANG T, ZHOU C C, REN S X. Role of IL-2 in cancer immunotherapy[J]. Oncoimmunology, 2016, 5(6): e1163462. |
| 129 | BECK J D, REIDENBACH D, SALOMON N, et al. mRNA therapeutics in cancer immunotherapy[J]. Molecular Cancer, 2021, 20(1): 69. |
| 130 | SAHIN U, MUIK A, DERHOVANESSIAN E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses[J]. Nature, 2020, 586(7830): 594-599. |
| 131 | KRAMMER F. SARS-CoV-2 vaccines in development[J]. Nature, 2020, 586(7830): 516-527. |
| 132 | EBERHARDT C S, SIEGRIST C A. Is there a role for childhood vaccination against COVID-19?[J]. Pediatric Allergy and Immunology, 2021, 32(1): 9-16. |
| 133 | 袁军鸿, 杨昭庆, 马绍辉. mRNA疫苗的研究进展[J]. 中国生物制品学杂志, 2022(6): 734-739. |
| YUAN J H, YANG Z Q, MA S H. Progress in research on mRNA vaccines[J]. Chinese Journal of Biologicals, 2022(6): 734-739. | |
| 134 | 胡佳欣, 章晓联. mRNA疫苗的原理、研究现状和展望[J]. 武汉大学学报(医学版), 2023, 44(9): 1033-1045. |
| HU J X, ZHANG X L. mRNA vaccines: principle, current status and perspectives[J]. Medical Journal of Wuhan University, 2023, 44(9): 1033-1045. | |
| 135 | SELBY L I, CORTEZ-JUGO C M, SUCH G K, et al. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles[J]. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology, 2017, 9(5): e1452. |
| 136 | CAGIGI A, LORÉ K. Immune responses induced by mRNA vaccination in mice, monkeys and humans[J]. Vaccines, 2021, 9(1): 61. |
| 137 | NATAMI M, GORGZADEH A, GHOLIPOUR A, et al. An overview on mRNA-based vaccines to prevent monkeypox infection[J]. Journal of Nanobiotechnology, 2024, 22(1): 86. |
| 138 | HOU F J, ZHANG Y T, LIU X H, et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge[J]. Nature Communications, 2023, 14(1): 5925. |
| 139 | WANG H, YIN P, ZHENG T T, et al. Rational design of a ‘two-in-one’ immunogen DAM drives potent immune response against mpox virus[J]. Nature Immunology, 2024, 25(2): 307-315. |
| [1] | SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs [J]. Synthetic Biology Journal, 2024, 5(3): 593-611. |
| [2] | TAN Zibin, LIANG Kang, CHEN Youhai. Applications of synthetic biology in developing microbial-vectored cancer vaccines [J]. Synthetic Biology Journal, 2024, 5(2): 221-238. |
| [3] | TU Huiyang, HAN Weidong, ZHANG Bin. Strategies for the design and optimization of tumor neoantigen vaccines [J]. Synthetic Biology Journal, 2024, 5(2): 254-266. |
| [4] | MA Xuejing, GUO Chang, HUA Zhaolin, HOU Baidong. Dawn of the rational design of nanoparticle vaccines aided by the advance of synthetic biology techniques [J]. Synthetic Biology Journal, 2024, 5(2): 353-368. |
| [5] | MENG Qian, YIN Cong, HUANG Weiren. Tumor organoids and their research progress in synthetic biology [J]. Synthetic Biology Journal, 2024, 5(1): 191-201. |
| [6] | Mengdan MA, Mengyu SHANG, Yuchen LIU. Application and prospect of CRISPR-Cas9 system in tumor biology [J]. Synthetic Biology Journal, 2023, 4(4): 703-719. |
| [7] | Jiawen CHEN, Jiandong HUANG, Haitao SUN. Current developments in the use of engineered bacteria for cancer therapy [J]. Synthetic Biology Journal, 2023, 4(4): 690-702. |
| [8] | Junhong XIE, Jingjing HE, Penghui ZHOU. Synthetic biology and engineered T cell therapy [J]. Synthetic Biology Journal, 2023, 4(2): 373-393. |
| [9] | Shilin XU, Haiyan XU. Progress of bispecific antibodies and nanotechnology in tumor immunotherapies [J]. Synthetic Biology Journal, 2022, 3(2): 352-368. |
| [10] | Fei SONG, Yuchen LIU, Zhiming CAI, Weiren HUANG. Construction of tumor gene circuits using CRISPR/Cas tool and their applications [J]. Synthetic Biology Journal, 2022, 3(1): 53-65. |
| [11] | Pan CHU, Jingwen ZHU, Wenqi HUANG, Chenli LIU, Xiongfei FU. Host-circuit coupling: toward a new framework for genetic circuit design [J]. Synthetic Biology Journal, 2021, 2(1): 91-105. |
| [12] | Lu PU, Yajia HUANG, Shuai YANG, Fan JIN. Applications of synthetic biology in the treatment and prevention of infectious diseases [J]. Synthetic Biology Journal, 2020, 1(2): 141-157. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||