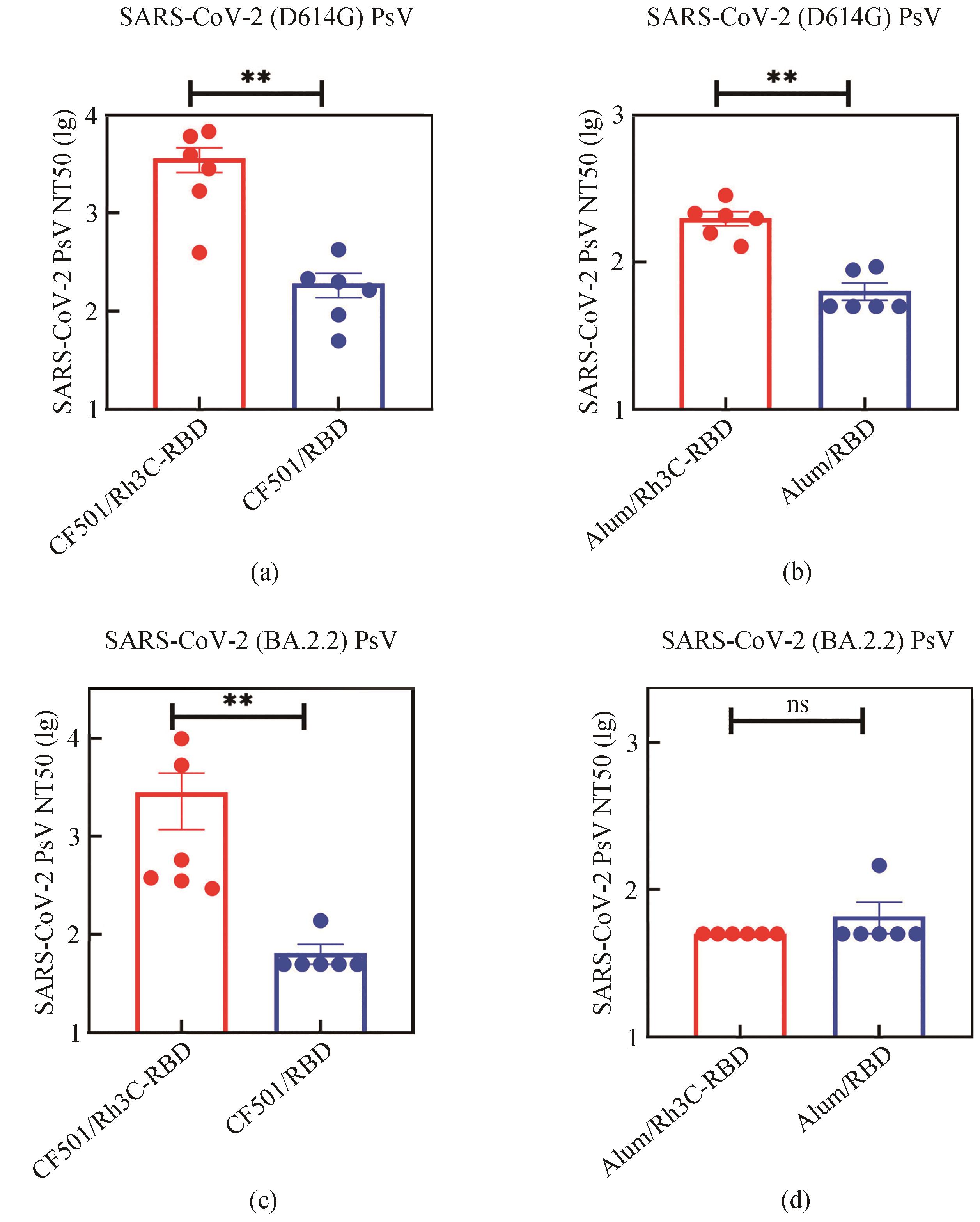
| 1 |
DU L Y, HE Y X, ZHOU Y S, et al. The spike protein of SARS-CoV—a target for vaccine and therapeutic development[J]. Nature Reviews Microbiology, 2009, 7(3): 226-236.
|
| 2 |
XIA S A, LIU M Q, WANG C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion[J]. Cell Research, 2020, 30(4): 343-355.
|
| 3 |
ZHOU J, LIU Z Z, ZHANG G X, et al. Development of variant-proof severe acute respiratory syndrome coronavirus 2, pan-sarbecovirus, and pan-β-coronavirus vaccines[J]. Journal of Medical Virology, 2023, 95(1): e28172.
|
| 4 |
LIU Z Z, XU W, XIA S, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response[J]. Signal Transduction and Targeted Therapy, 2020, 5: 282.
|
| 5 |
WRAPP D, WANG N S, CORBETT K S, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation[J]. Science, 2020, 367(6483): 1260-1263.
|
| 6 |
ZHANG N R, JI Q T, LIU Z Z, et al. Effect of different adjuvants on immune responses elicited by protein-based subunit vaccines against SARS-CoV-2 and its delta variant[J]. Viruses, 2022, 14(3): 501.
|
| 7 |
ROUTHU N K, CHEEDARLA N, BOLLIMPELLI V S, et al. SARS-CoV-2 RBD trimer protein adjuvanted with Alum-3M-052 protects from SARS-CoV-2 infection and immune pathology in the lung[J]. Nature Communications, 2021, 12: 3587.
|
| 8 |
YAMAYOSHI S, KAWAOKA Y. Current and future influenza vaccines[J]. Nature Medicine, 2019, 25(2): 212-220.
|
| 9 |
GÜTHE S, KAPINOS L, MÖGLICH A, et al. Very fast folding and association of a trimerization domain from bacteriophage T4 fibritin[J]. Journal of Molecular Biology, 2004, 337(4): 905-915.
|
| 10 |
TAO Y Z, STRELKOV S V, MESYANZHINOV V V, et al. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain[J]. Structure, 1997, 5(6): 789-798.
|
| 11 |
LI T T, ZHANG Z Y, ZHANG Z Q, et al. Characterization of native-like HIV-1 gp140 glycoprotein expressed in insect cells[J]. Vaccine, 2019, 37(11): 1418-1427.
|
| 12 |
RINGE R P, YASMEEN A, OZOROWSKI G, et al. Influences on the design and purification of soluble, recombinant native-like HIV-1 envelope glycoprotein trimers[J]. Journal of Virology, 2015, 89(23): 12189-12210.
|
| 13 |
YANG X Z, LEE J, MAHONY E M, et al. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin[J]. Journal of Virology, 2002, 76(9): 4634-4642.
|
| 14 |
AHN J Y, LEE J, SUH Y S, et al. Safety and immunogenicity of two recombinant DNA COVID-19 vaccines containing the coding regions of the spike or spike and nucleocapsid proteins: an interim analysis of two open-label, non-randomised, phase 1 trials in healthy adults[J]. The Lancet Microbe, 2022, 3(3): e173-e183.
|
| 15 |
VOGEL A B, KANEVSKY I, CHE Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2[J]. Nature, 2021, 592(7853): 283-289.
|
| 16 |
SLIEPEN K, VAN MONTFORT T, MELCHERS M, et al. Immunosilencing a highly immunogenic protein trimerization domain[J]. The Journal of Biological Chemistry, 2015, 290(12): 7436-7442.
|
| 17 |
CAO Y L, JIAN F C, WANG J, et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution[J]. Nature, 2023, 614(7948): 521-529.
|
| 18 |
LI H, YOU S, YANG X, et al. Injectable recombinant human collagen-derived material with high cell adhesion activity limits adverse remodelling and improves pelvic floor function in pelvic floor dysfunction rats[J]. Biomaterials Advances, 2022, 134: 112715.
|
| 19 |
GORDON M K, HAHN R A. Collagens[J]. Cell and Tissue Research, 2010, 339(1): 247-257.
|
| 20 |
LIU X, WU H, BYRNE M, et al. Type Ⅲ collagen is crucial for collagen Ⅰ fibrillogenesis and for normal cardiovascular development[J]. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(5): 1852-1856.
|
| 21 |
HUA C, ZHU Y, XU W, et al. Characterization by high-resolution crystal structure analysis of a triple-helix region of human collagen type Ⅲ with potent cell adhesion activity[J]. Biochemical and Biophysical Research Communications, 2019, 508(4): 1018-1023.
|
| 22 |
LIU Z Z, ZHOU J, WANG X L, et al. A pan-sarbecovirus vaccine based on RBD of SARS-CoV-2 original strain elicits potent neutralizing antibodies against XBB in non-human primates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(11): e2221713120.
|
| 23 |
LIU Z Z, ZHOU J, XU W, et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs[J]. Cell Research, 2022, 32(3): 269-287.
|
| 24 |
JUMPER J, EVANS R, PRITZEL A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589.
|
| 25 |
LIU Z Z, CHAN J F W, ZHOU J, et al. A pan-sarbecovirus vaccine induces highly potent and durable neutralizing antibody responses in non-human primates against SARS-CoV-2 Omicron variant[J]. Cell Research, 2022, 32(5): 495-497.
|
| 26 |
RAINHO-TOMKO J N, PAVOT V, KISHKO M, et al. Immunogenicity and protective efficacy of RSV G central conserved domain vaccine with a prefusion nanoparticle[J]. NPJ Vaccines, 2022, 7: 74.
|
| 27 |
DAI L P, ZHENG T Y, XU K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS[J]. Cell, 2020, 182(3): 722-733. e11.
|
| 28 |
YOU S, LIU S B, DONG X J, et al. Intravaginal administration of human type Ⅲ collagen-derived biomaterial with high cell-adhesion activity to treat vaginal atrophy in rats[J]. ACS Biomaterials Science & Engineering, 2020, 6(4): 1977-1988.
|
| 29 |
YANG L, WU H S, LU L, et al. A tailored extracellular matrix (ECM)-Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type Ⅲ collagen[J]. Biomaterials, 2021, 276: 121055.
|
| 30 |
HU C, LIU W Q, LONG L Y, et al. Microenvironment-responsive multifunctional hydrogels with spatiotemporal sequential release of tailored recombinant human collagen type Ⅲ for the rapid repair of infected chronic diabetic wounds[J]. Journal of Materials Chemistry B, 2021, 9(47): 9684-9699.
|
| 31 |
LONG L Y, LIU W Q, LI L, et al. Dissolving microneedle-encapsulated drug-loaded nanoparticles and recombinant humanized collagen type Ⅲ for the treatment of chronic wound via anti-inflammation and enhanced cell proliferation and angiogenesis[J]. Nanoscale, 2022, 14(4): 1285-1295.
|
| 32 |
WANG J, QIU H, XU Y, et al. The biological effect of recombinant humanized collagen on damaged skin induced by UV-photoaging: an in vivo study[J]. Bioactive Materials, 2021, 11: 154-165.
|
| 33 |
YOU S, ZHU Y, LI H, et al. Recombinant humanized collagen remodels endometrial immune microenvironment of chronic endometritis through macrophage immunomodulation[J]. Regenerative Biomaterials, 2023, 10: rbad033.
|