Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (4): 651-675.DOI: 10.12211/2096-8280.2022-056
• Invited Review • Previous Articles Next Articles
Recent advances in photoenzymatic synthesis
MING Yang, CHEN Bin, HUANG Xiaoqiang
- State Key Laboratory of Coordination Chemistry,Chemistry and Biomedicine Innovation Center,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210023,Jiangsu,China
-
Received:2022-10-10Revised:2022-12-06Online:2023-09-14Published:2023-08-31 -
Contact:HUANG Xiaoqiang
光酶催化合成进展
明阳, 陈彬, 黄小强
- 南京大学化学化工学院,南京大学化学与生物医药创新研究院,配位化学国家重点实验室,江苏 南京 210023
-
通讯作者:黄小强 -
作者简介:明阳 (1999—),女,博士研究生。研究方向为光酶催化不对称生物合成。E-mail:yang222ming@163.com黄小强 (1991—),男,特聘研究员,博士生导师。研究方向为交叉融合生物合成与化学合成。E-mail:huangx513@nju.edu.cn -
基金资助:国家自然科学基金面上项目(22277053);科技部重点研发计划(2022YFA0913000);江苏省青年基金(BK20220760)
CLC Number:
Cite this article
MING Yang, CHEN Bin, HUANG Xiaoqiang. Recent advances in photoenzymatic synthesis[J]. Synthetic Biology Journal, 2023, 4(4): 651-675.
明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-056
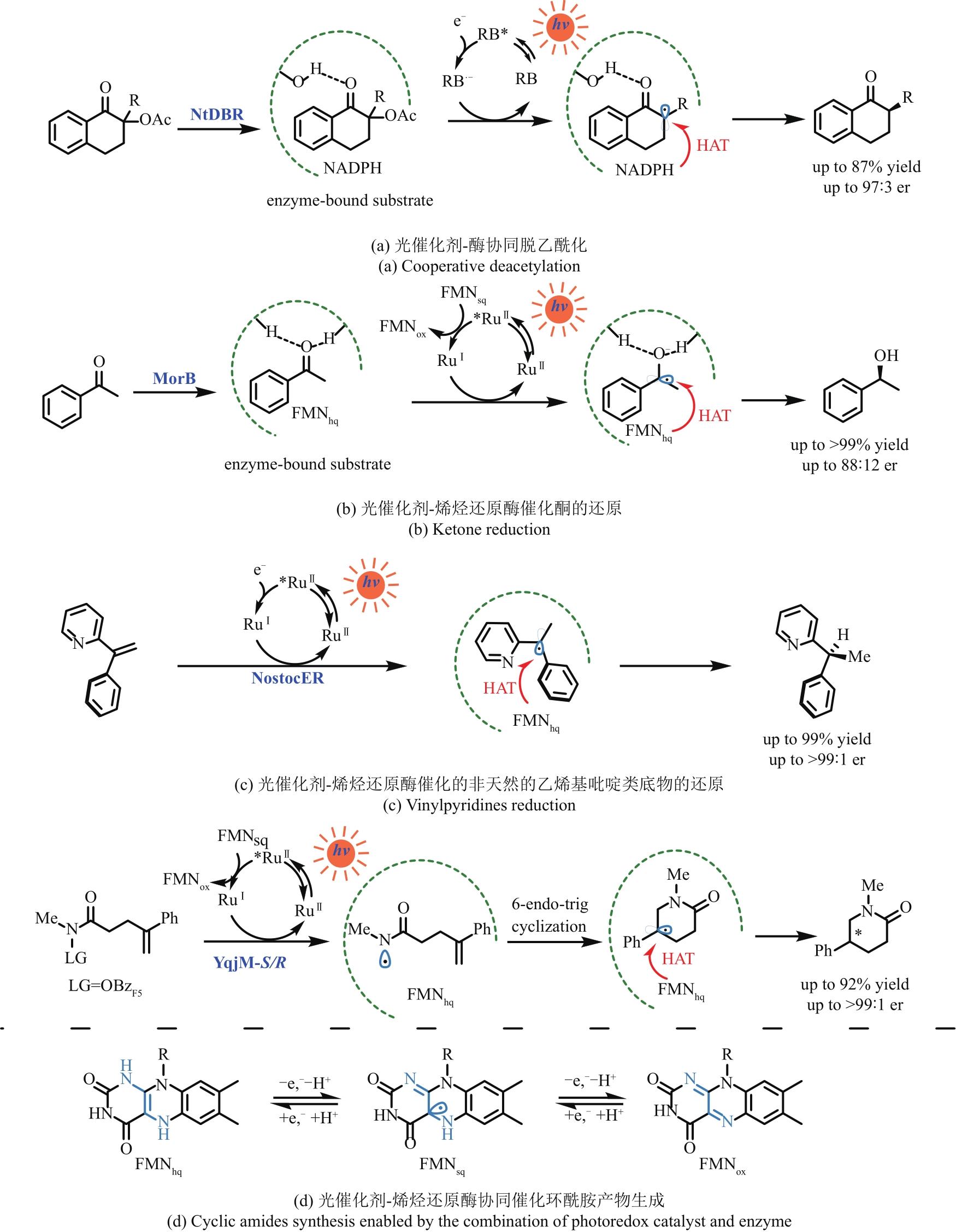
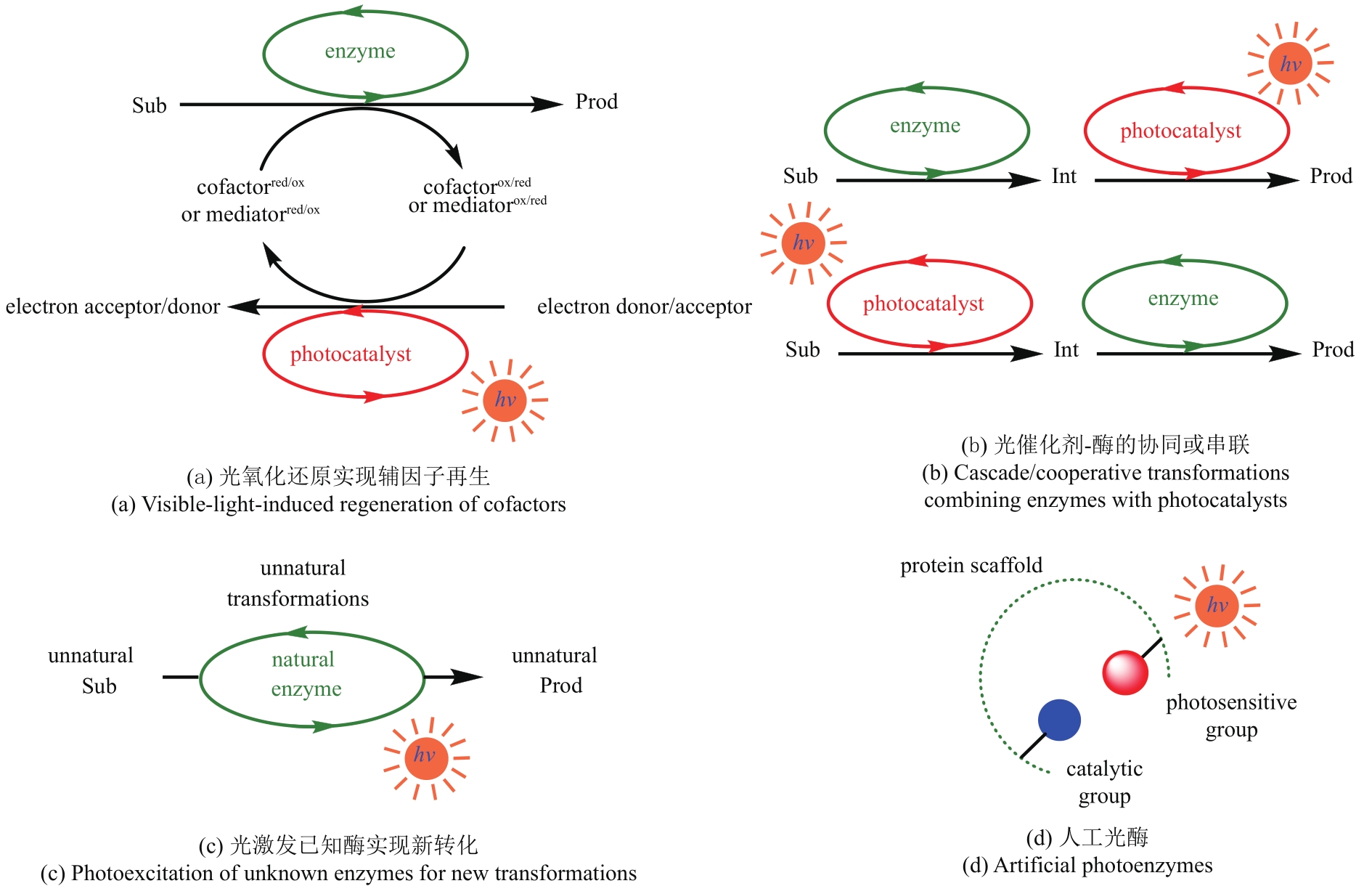
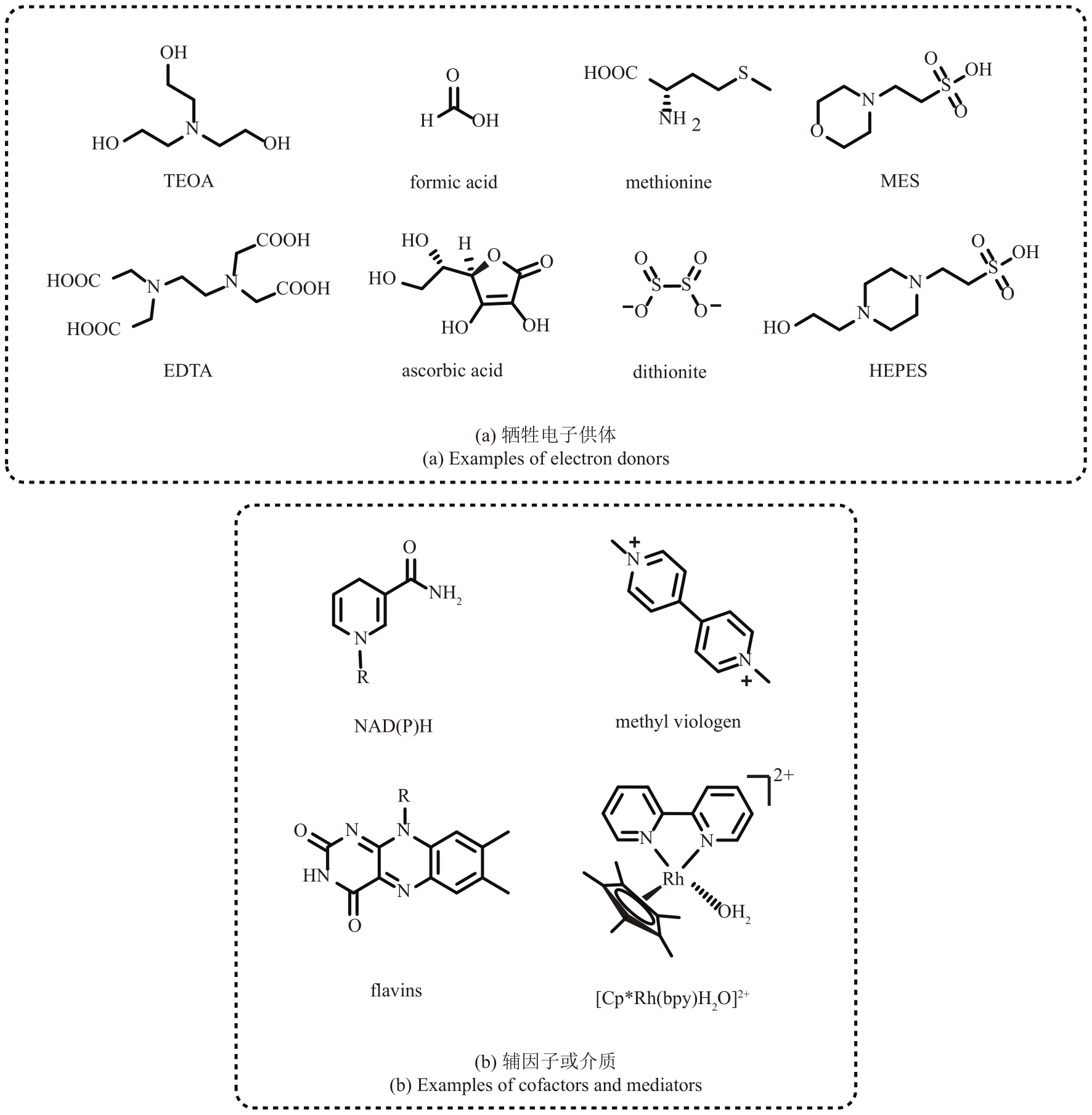
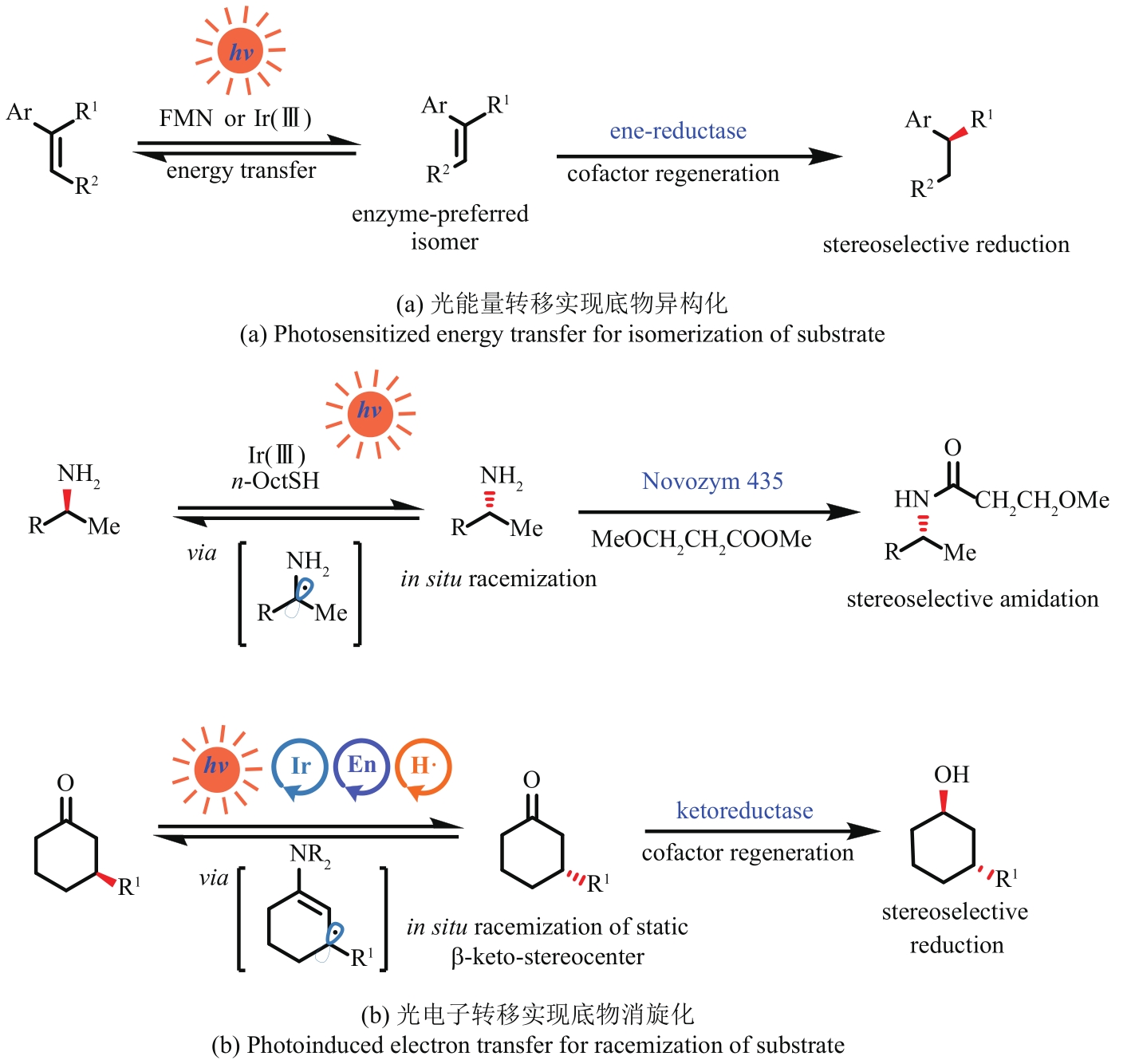
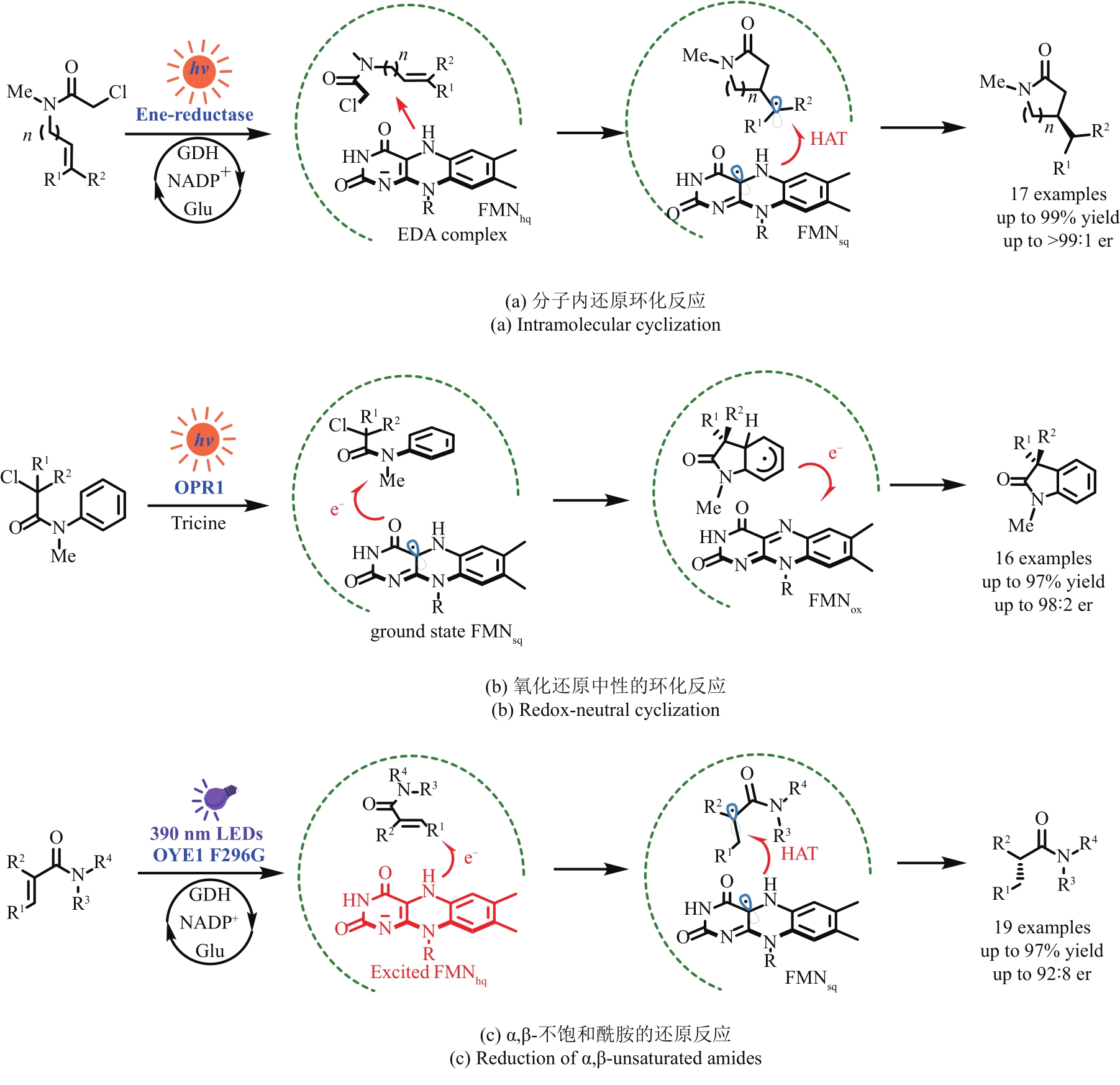
Fig. 5 Selected reductions of the enzyme activated substrates by photocatalysts to achieve unnatural transformationsRB—Rose Bengal; MorB—morphinone reductase from P. putida; RuⅡ—Ru(bpy)32+; NtDBR—Double bond reductase from Nicotiana tabacum; NostocER—Ene-Reductase from N. punctiforme; YqjM-S/R—Ene-Reductase from Bacillus subtili; OBzF5—perfluorobenzoyloxy
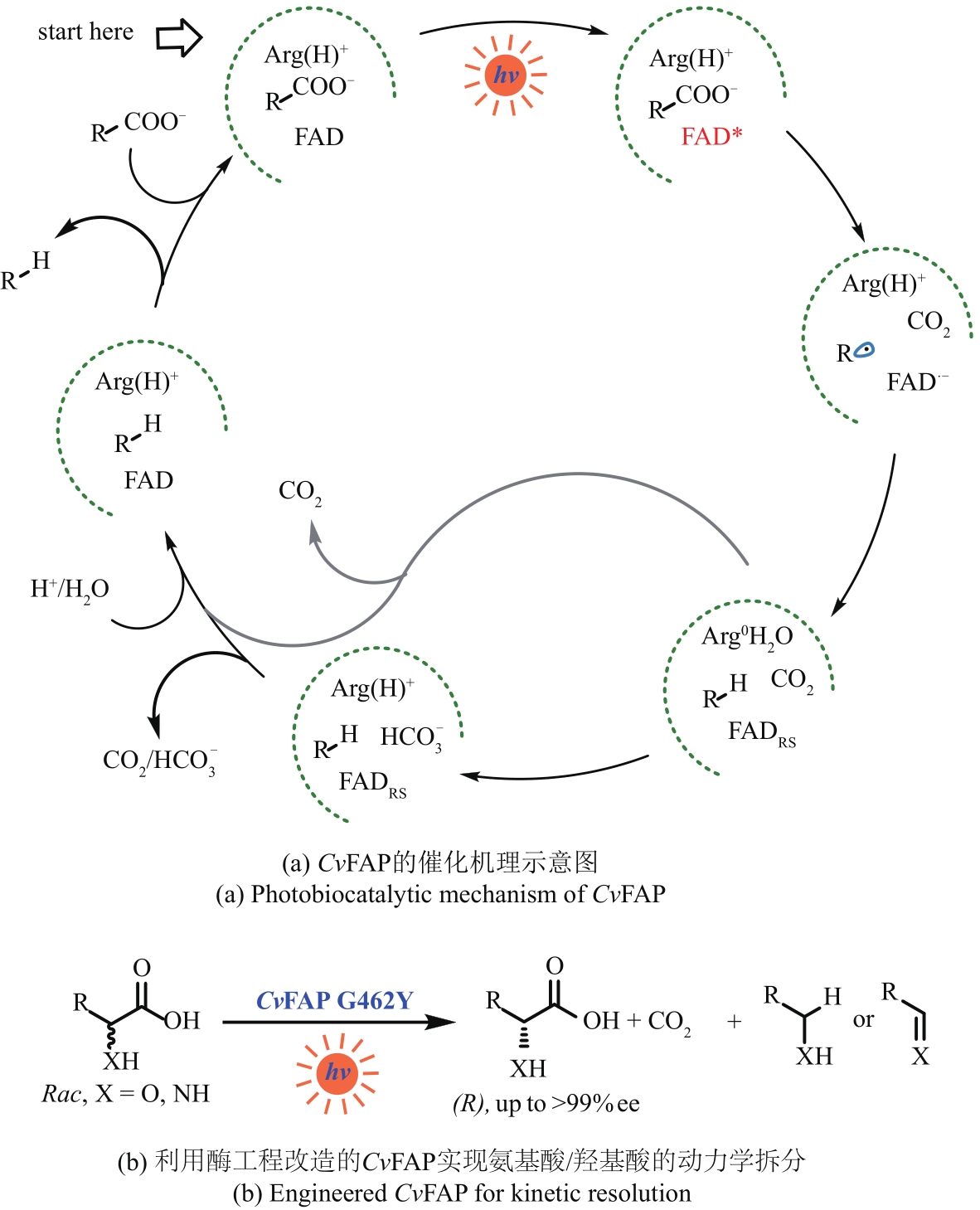
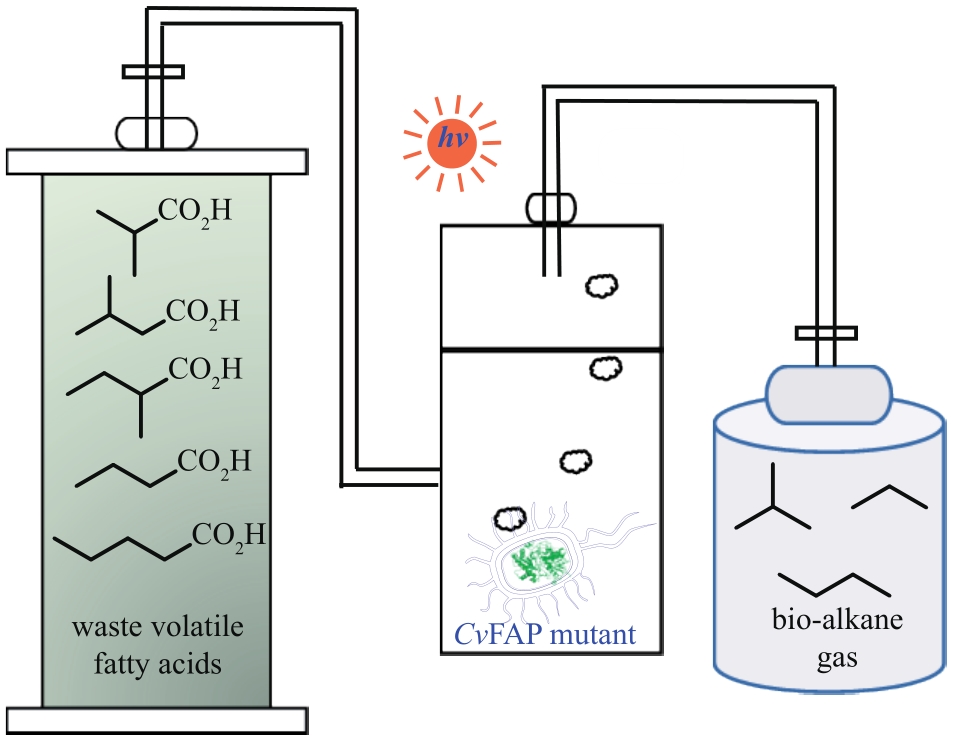
Fig. 7 Photobiocatalytic mechanism of CvFAP and the application exampleArg—Arginine; FAD—flavin adenine dinucleotide; FADRS—red-shifted oxidized flavin; Rac—racemic
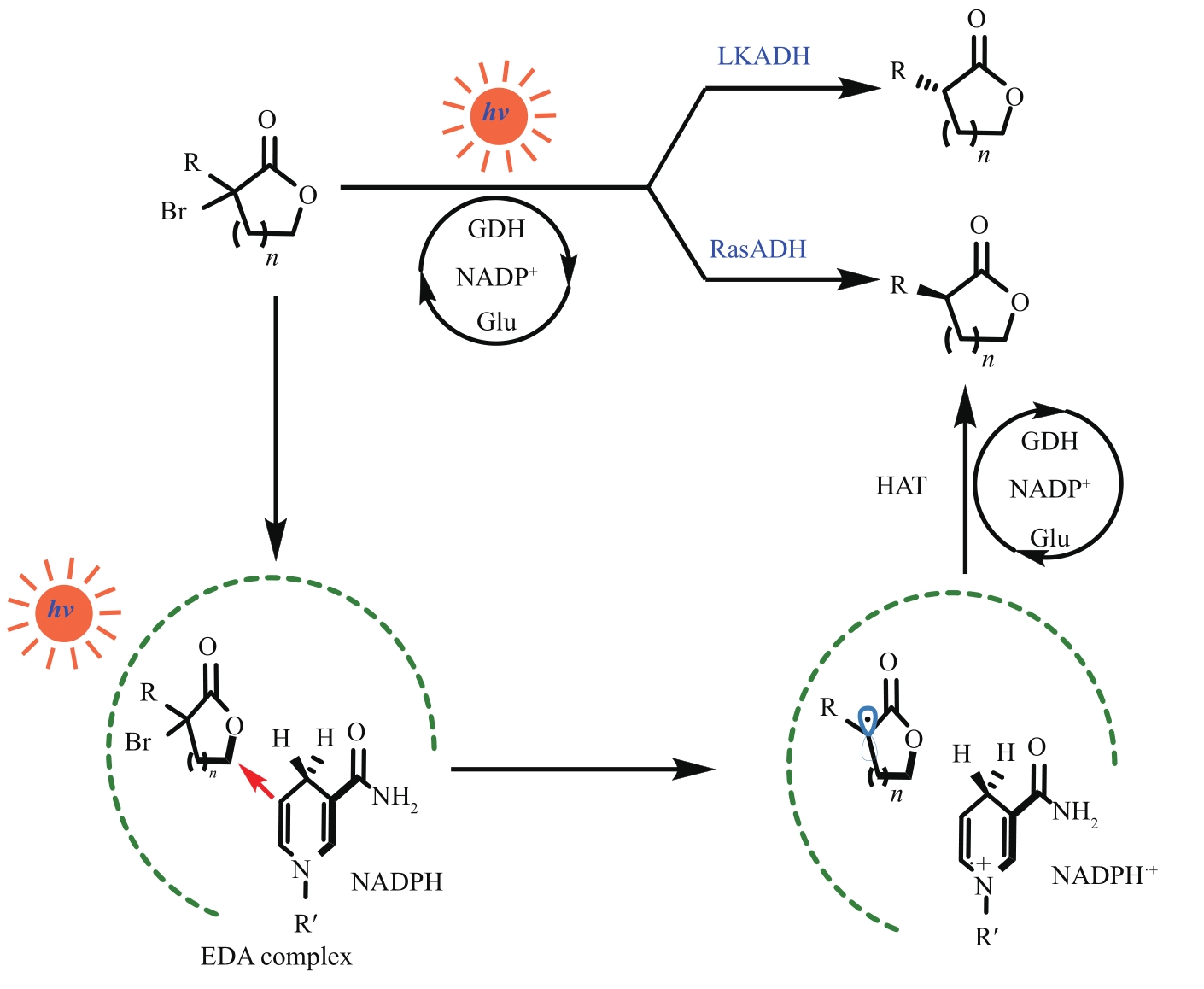
Fig. 9 Dehalogenation of halogenated lactones by light-induced alcohol dehydrogenaseLKADH—short-chain dehydrogenase from Lactobacillus kefiri; RasDH—short-chain dehydrogenase from Ralstonia species
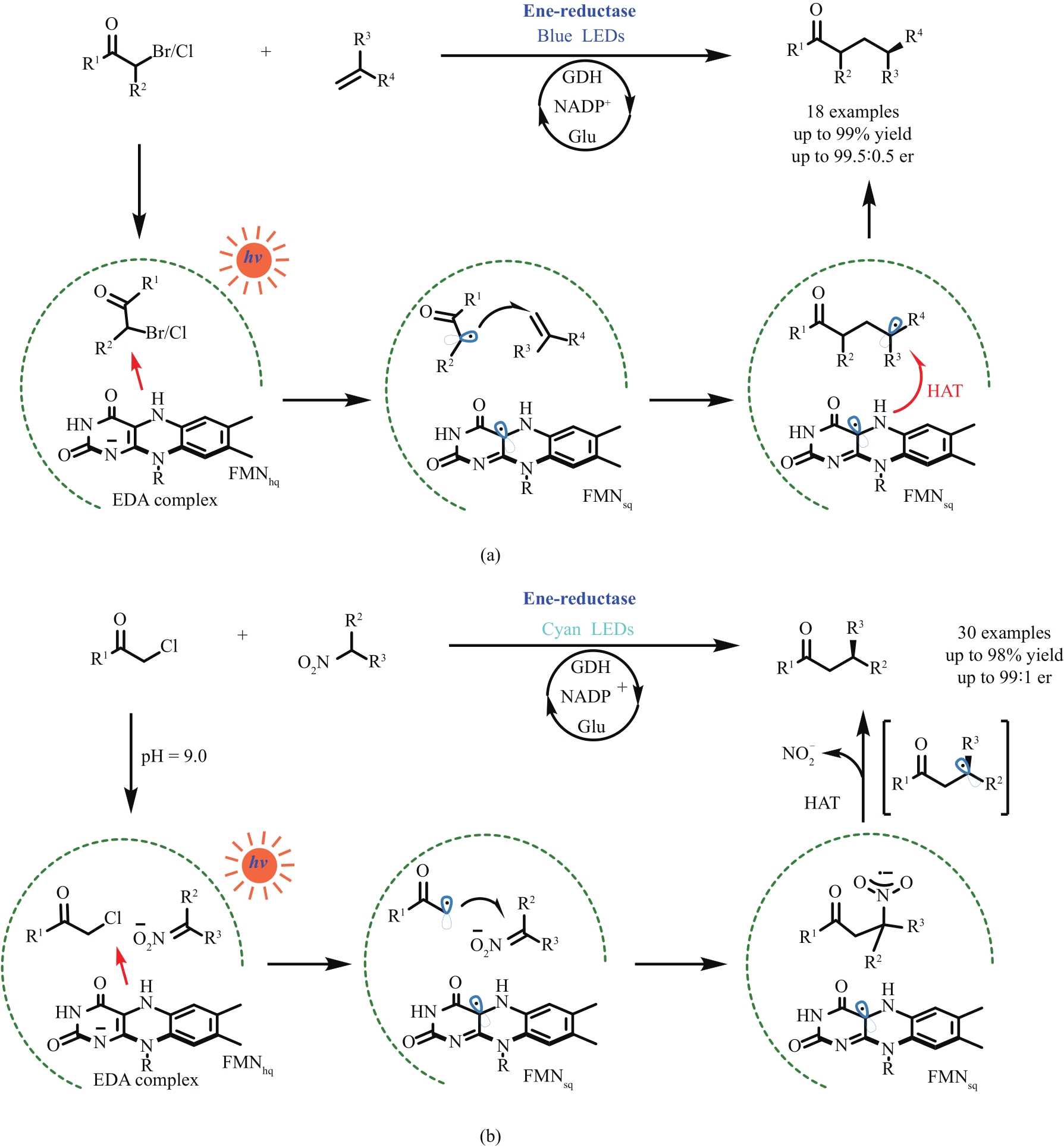
Fig. 12 Photoactivated ene-reductases enabled intermolecular reductive coupling couplings for alkene hydroalkylations (a) and Csp3—Csp3 bond formations (b)
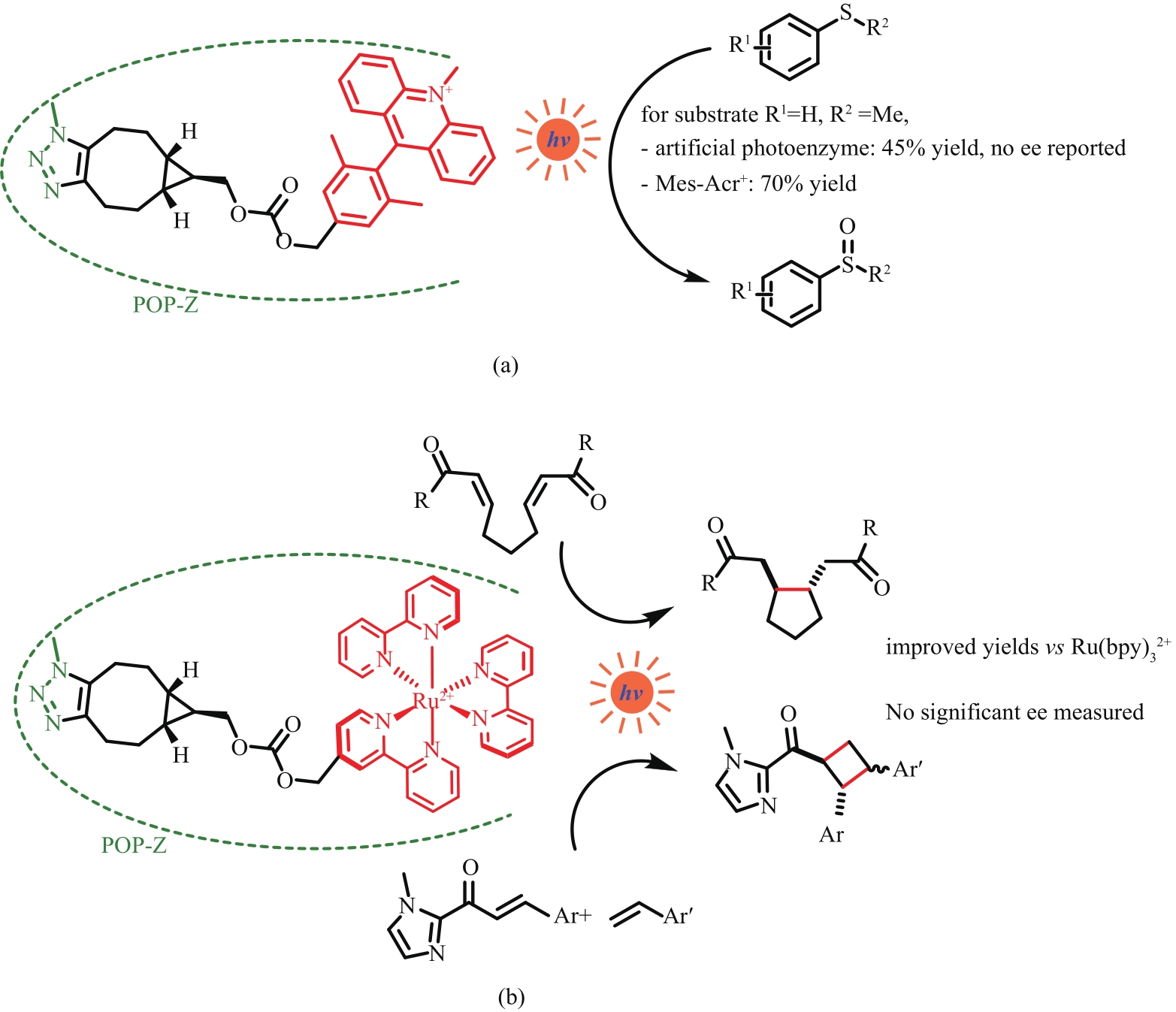
Fig. 13 Introduction of acridine photosensitizer (a) and tris(2,2′-bipyridyl) rutheniumⅡ (b) into protein by clicking chemistryPOP-Z—p-azido-L-phenylalanine (Z) incorporated prolyl oligopeptidase (POP)
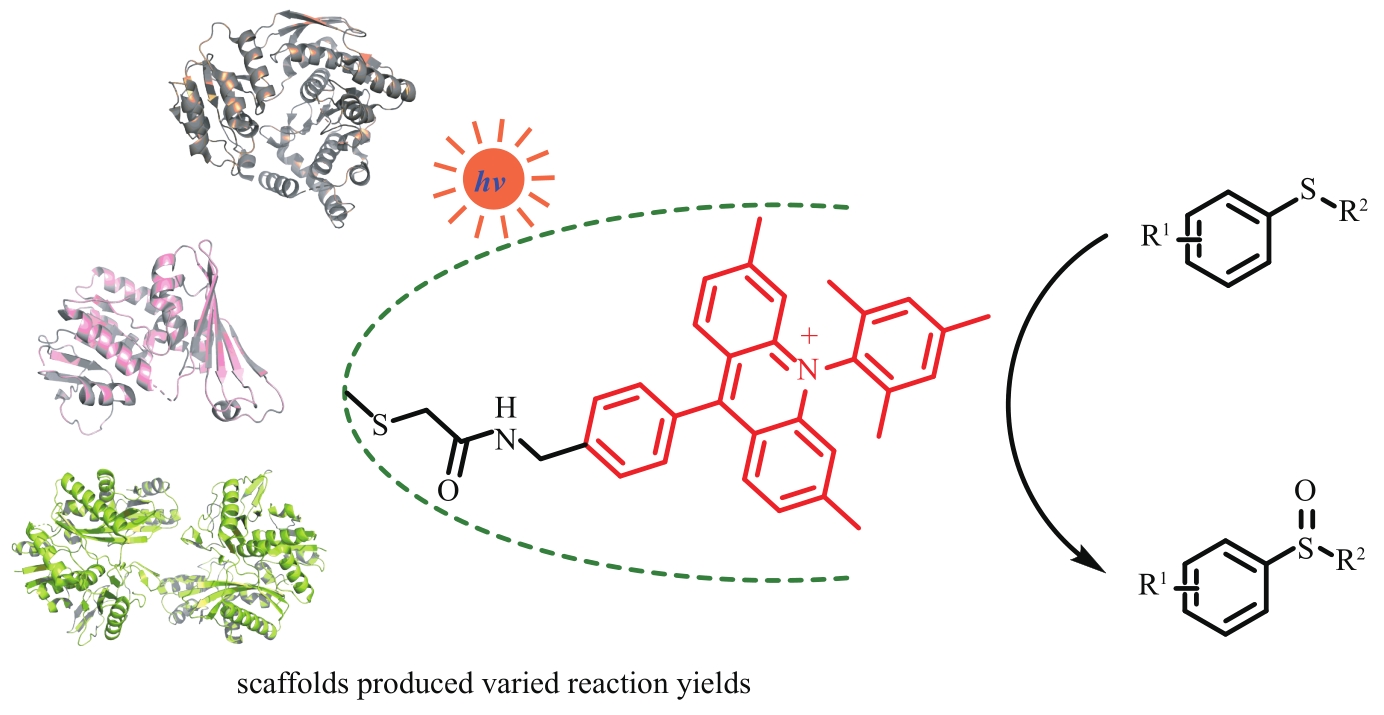
Fig. 14 Construction of different artificial photoenzymes by introducing photosensitizers through covalent cross-linking of cysteine residues with iodoacetamide derivatives
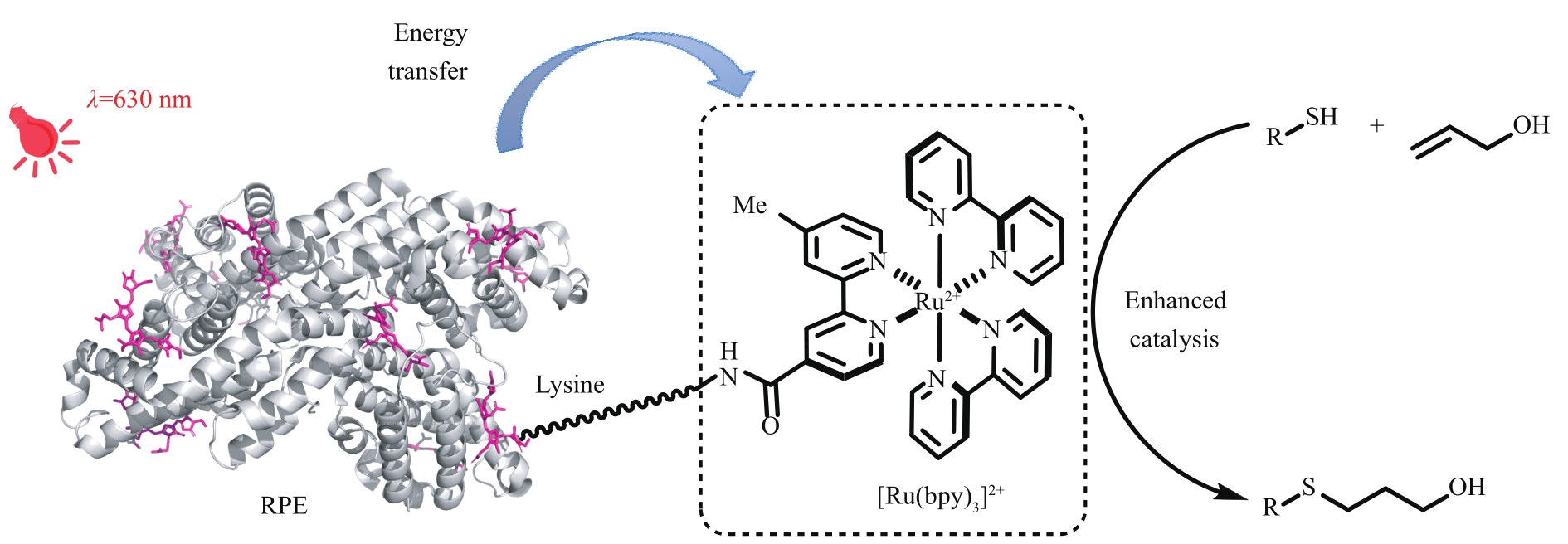
Fig. 15 Construction of low energy absorption photoenzyme via the combination of photosynthetic light-harvesting protein and photocatalystRPE—R-phycoerythrin, PDB 1EYX
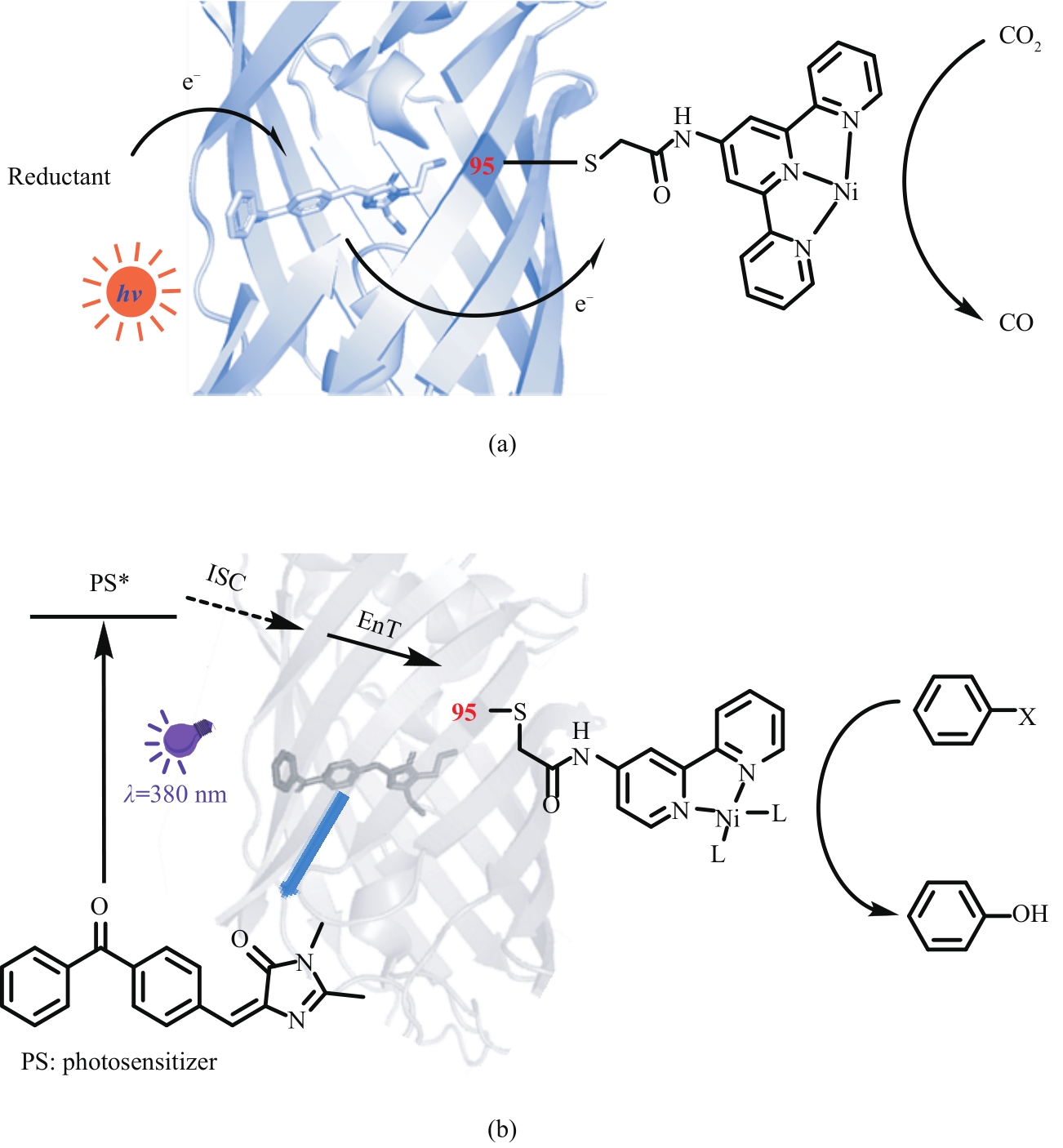
Fig. 16 Artificial photoenzymes with benzophenone photosensitive groups catalyze the reduction of carbon dioxide (a) and the dehalogenation and hydroxylation of aryl halides (b)
| 酶的种类 | 反应类型 | 参考文献 |
|---|---|---|
黄素依赖的 烯烃还原酶 | 利用光诱导能量转移促进烯烃异构化,酶优选底物发生对映选择性还原,实现立体汇聚式还原 | [ |
| 光催化剂-酶协同实现非天然底物的C=O、C=C双键的还原 | [ | |
| 光催化剂-酶协同实现不对称氢胺化反应 | [ | |
| 分子内自由基环化反应 | [ | |
| 氧化还原中性的不对称自由基环化反应 | [ | |
| 非天然底物α,β-不饱和酰胺的对映选择性还原 | [ | |
| α-卤代羰基化合物和烯烃的分子间氢烷基化反应 | [ | |
| 卤代烷烃和硝基烷烃的不对称Csp3—Csp3亲电交叉偶联反应 | [ | |
黄素依赖的 环己酮单加氧酶 | 光诱导酶催化的α-卤代-α-氟代酮的对映选择性还原脱卤反应 | [ |
烟酰胺依赖的 酮还原酶 | 结合光-小分子胺催化和酶催化,实现远程惰性C—H键的去消旋化 | [ |
| 卤代内酯的不对称自由基脱卤化反应 | [ | |
| 分子间自由基共轭加成反应 | [ | |
烟酰胺依赖的 双键还原酶 | 光催化剂-酶协同实现对映选择性脱乙酰氧基反应 | [ |
黄素依赖的 脂肪酸光脱羧酶 | 选择性催化S-构型底物的光脱羧反应,实现动力学拆分 | [ |
| 短链脂肪酸光脱羧、光脱羧氘化、反式脂肪酸的选择性光脱羧、生物燃料制造 | [ | |
| 人工光酶 | 催化硫茴香醚的氧化、二烯酮的分子内还原环化、[2+2]环加成反应以及硫醇与烯烃的偶联等 | [ |
| 二氧化碳还原、卤代芳烃脱卤羟化反应以及C—N键构建反应 | [ | |
| 紫外光激发插入的光敏非天然氨基酸,通过能量转移,实现对映选择性[2+2]环加成反应 | [ | |
| 过氧合酶 | 光催化剂-酶串联实现H2O2的原位生成及利用 | [ |
| 脂肪酶 | 通过光引发的电子转移促进底物消旋化,酶优选底物发生选择性酰胺化,实现动态动力学拆分 | [ |
| 光催化剂-酶串联实现2,2-二取代吲哚-3-酮的直接不对称合成 | [ |
Table 1 Catalogue of photoenzymes and photoenzymatic reactions
| 酶的种类 | 反应类型 | 参考文献 |
|---|---|---|
黄素依赖的 烯烃还原酶 | 利用光诱导能量转移促进烯烃异构化,酶优选底物发生对映选择性还原,实现立体汇聚式还原 | [ |
| 光催化剂-酶协同实现非天然底物的C=O、C=C双键的还原 | [ | |
| 光催化剂-酶协同实现不对称氢胺化反应 | [ | |
| 分子内自由基环化反应 | [ | |
| 氧化还原中性的不对称自由基环化反应 | [ | |
| 非天然底物α,β-不饱和酰胺的对映选择性还原 | [ | |
| α-卤代羰基化合物和烯烃的分子间氢烷基化反应 | [ | |
| 卤代烷烃和硝基烷烃的不对称Csp3—Csp3亲电交叉偶联反应 | [ | |
黄素依赖的 环己酮单加氧酶 | 光诱导酶催化的α-卤代-α-氟代酮的对映选择性还原脱卤反应 | [ |
烟酰胺依赖的 酮还原酶 | 结合光-小分子胺催化和酶催化,实现远程惰性C—H键的去消旋化 | [ |
| 卤代内酯的不对称自由基脱卤化反应 | [ | |
| 分子间自由基共轭加成反应 | [ | |
烟酰胺依赖的 双键还原酶 | 光催化剂-酶协同实现对映选择性脱乙酰氧基反应 | [ |
黄素依赖的 脂肪酸光脱羧酶 | 选择性催化S-构型底物的光脱羧反应,实现动力学拆分 | [ |
| 短链脂肪酸光脱羧、光脱羧氘化、反式脂肪酸的选择性光脱羧、生物燃料制造 | [ | |
| 人工光酶 | 催化硫茴香醚的氧化、二烯酮的分子内还原环化、[2+2]环加成反应以及硫醇与烯烃的偶联等 | [ |
| 二氧化碳还原、卤代芳烃脱卤羟化反应以及C—N键构建反应 | [ | |
| 紫外光激发插入的光敏非天然氨基酸,通过能量转移,实现对映选择性[2+2]环加成反应 | [ | |
| 过氧合酶 | 光催化剂-酶串联实现H2O2的原位生成及利用 | [ |
| 脂肪酶 | 通过光引发的电子转移促进底物消旋化,酶优选底物发生选择性酰胺化,实现动态动力学拆分 | [ |
| 光催化剂-酶串联实现2,2-二取代吲哚-3-酮的直接不对称合成 | [ |
| 1 | QU G, LI A T, ACEVEDO-ROCHA C G, et al. The crucial role of methodology development in directed evolution of selective enzymes[J]. Angewandte Chemie International Edition, 2020, 59(32): 13204-13231. |
| 2 | DEVINE P N, HOWARD R M, KUMAR R, et al. Extending the application of biocatalysis to meet the challenges of drug development[J]. Nature Reviews Chemistry, 2018, 2(12): 409-421. |
| 3 | SCHMID A, DORDICK J S, HAUER B, et al. Industrial biocatalysis today and tomorrow[J]. Nature, 2001, 409(6817): 258-268. |
| 4 | YI D, BAYER T, BADENHORST C P S, et al. Recent trends in biocatalysis[J]. Chemical Society Reviews, 2021, 50(14): 8003-8049. |
| 5 | PYSER J B, CHAKRABARTY S, ROMERO E O, et al. State-of-the-art biocatalysis[J]. ACS Central Science, 2021, 7(7): 1105-1116. |
| 6 | SAJEEV SURAJ C, SUMANT O. Enzymes market: opportunities and forecast[R/OL]. 2022, 2021-2031. (2022-07-17)[2022-11-01]. . |
| 7 | HANEFELD U, HOLLMANN F, PAUL C E. Biocatalysis making waves in organic chemistry[J]. Chemical Society Reviews, 2022, 51(2): 594-627. |
| 8 | PENG Y Z, CHEN Z C, XU J, et al. Recent advances in photobiocatalysis for selective organic synthesis[J]. Organic Process Research & Development, 2022, 26(7): 1900-1913. |
| 9 | CHEN K, ARNOLD F H. Engineering new catalytic activities in enzymes[J]. Nature Catalysis, 2020, 3(3): 203-213. |
| 10 | YOON T P, ISCHAY M A, DU J A. Visible light photocatalysis as a greener approach to photochemical synthesis[J]. Nature Chemistry, 2010, 2(7): 527-532. |
| 11 | CIAMICIAN G. The photochemistry of the future[J]. Science, 1912, 36(926): 385-394. |
| 12 | XU C P, RAVI ANUSUYADEVI P, AYMONIER C, et al. Nanostructured materials for photocatalysis[J]. Chemical Society Reviews, 2019, 48(14): 3868-3902. |
| 13 | HUANG X Q, MEGGERS E. Asymmetric photocatalysis with bis-cyclometalated rhodium complexes[J]. Accounts of Chemical Research, 2019, 52(3): 833-847. |
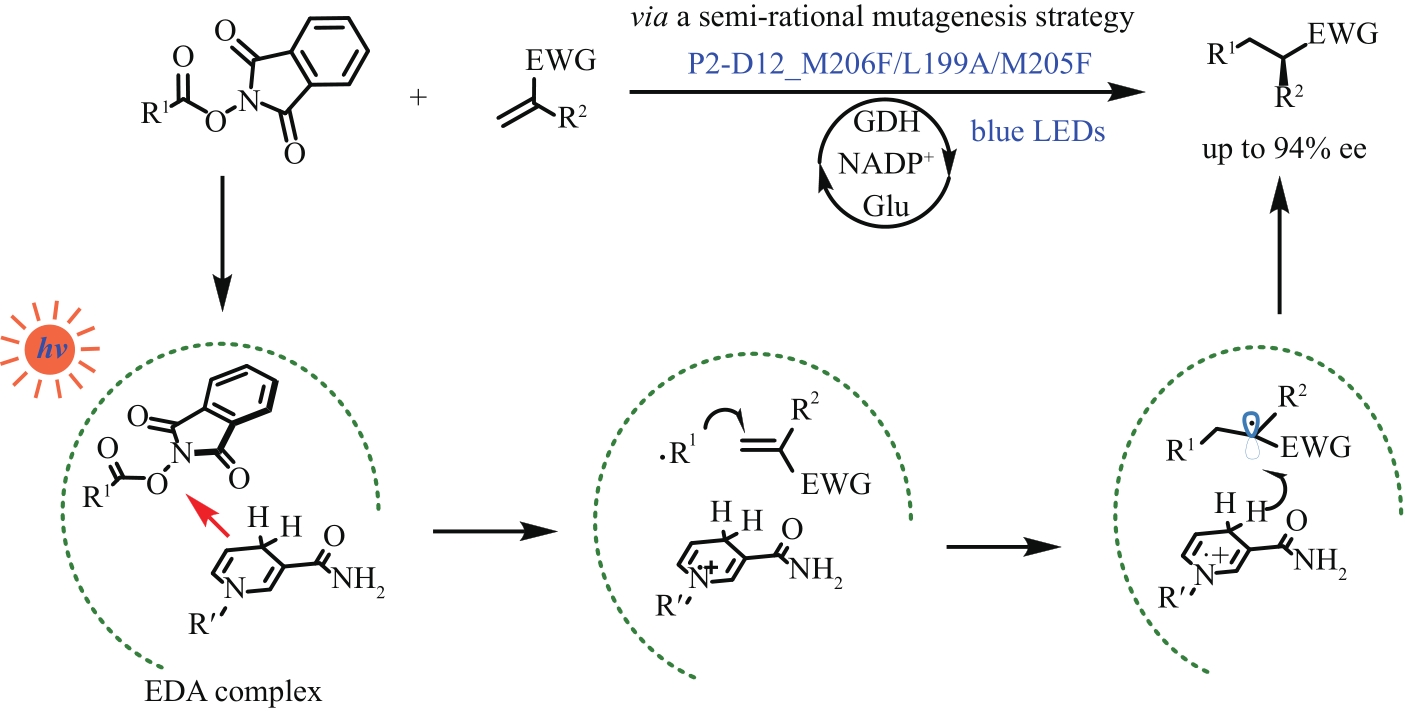
| 14 | BUZZETTI L, CRISENZA G E M, MELCHIORRE P. Mechanistic studies in photocatalysis[J]. Angewandte Chemie International Edition, 2019, 58(12): 3730-3747. |
| 15 | ZHANG J N, HU W P, CAO S, et al. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting[J]. Nano Research, 2020, 13(9): 2313-2322. |
| 16 | FU J W, JIANG K X, QIU X Q, et al. Product selectivity of photocatalytic CO2 reduction reactions[J]. Materials Today, 2020, 32: 222-243. |
| 17 | CHEN D J, CHENG Y L, ZHOU N, et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: a review[J]. Journal of Cleaner Production, 2020, 268: 121725. |
| 18 | SILVESTRI S, FAJARDO A R, IGLESIAS B A. Supported porphyrins for the photocatalytic degradation of organic contaminants in water: a review[J]. Environmental Chemistry Letters, 2022, 20(1): 731-771. |
| 19 | XUAN J, HE X K, XIAO W J. Visible light-promoted ring-opening functionalization of three-membered carbo- and heterocycles[J]. Chemical Society Reviews, 2020, 49(9): 2546-2556. |
| 20 | YIN Y L, ZHAO X W, QIAO B K, et al. Cooperative photoredox and chiral hydrogen-bonding catalysis[J]. Organic Chemistry Frontiers, 2020, 7(10): 1283-1296. |
| 21 | CHAN A Y, PERRY I B, BISSONNETTE N B, et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis[J]. Chemical Reviews, 2022, 122(2): 1485-1542. |
| 22 | BRIMIOULLE R, LENHART D, MATURI M M, et al. Enantioselective catalysis of photochemical reactions[J]. Angewandte Chemie International Edition, 2015, 54(13): 3872-3890. |
| 23 | WANG C F, LU Z. Catalytic enantioselective organic transformations via visible light photocatalysis[J]. Organic Chemistry Frontiers, 2015, 2(2): 179-190. |
| 24 | MEGGERS E. Asymmetric catalysis activated by visible light[J]. Chemical Communications, 2015, 51(16): 3290-3301. |
| 25 | HARRISON W, HUANG X Q, ZHAO H M. Photobiocatalysis for abiological transformations[J]. Accounts of Chemical Research, 2022, 55(8): 1087-1096. |
| 26 | GUO X W, OKAMOTO Y, SCHREIER M R, et al. Enantioselective synthesis of amines by combining photoredox and enzymatic catalysis in a cyclic reaction network[J]. Chemical Science, 2018, 9(22): 5052-5056. |
| 27 | ZHANG W Y, BUREK B O, FERNÁNDEZ-FUEYO E, et al. Selective activation of C—H bonds in a cascade process combining photochemistry and biocatalysis[J]. Angewandte Chemie International Edition, 2017, 56(48): 15451-15455. |
| 28 | ÖZGEN F F, RUNDA M E, SCHMIDT S. Photo-biocatalytic cascades: combining chemical and enzymatic transformations fueled by light[J]. Chembiochem, 2021, 22(5): 790-806. |
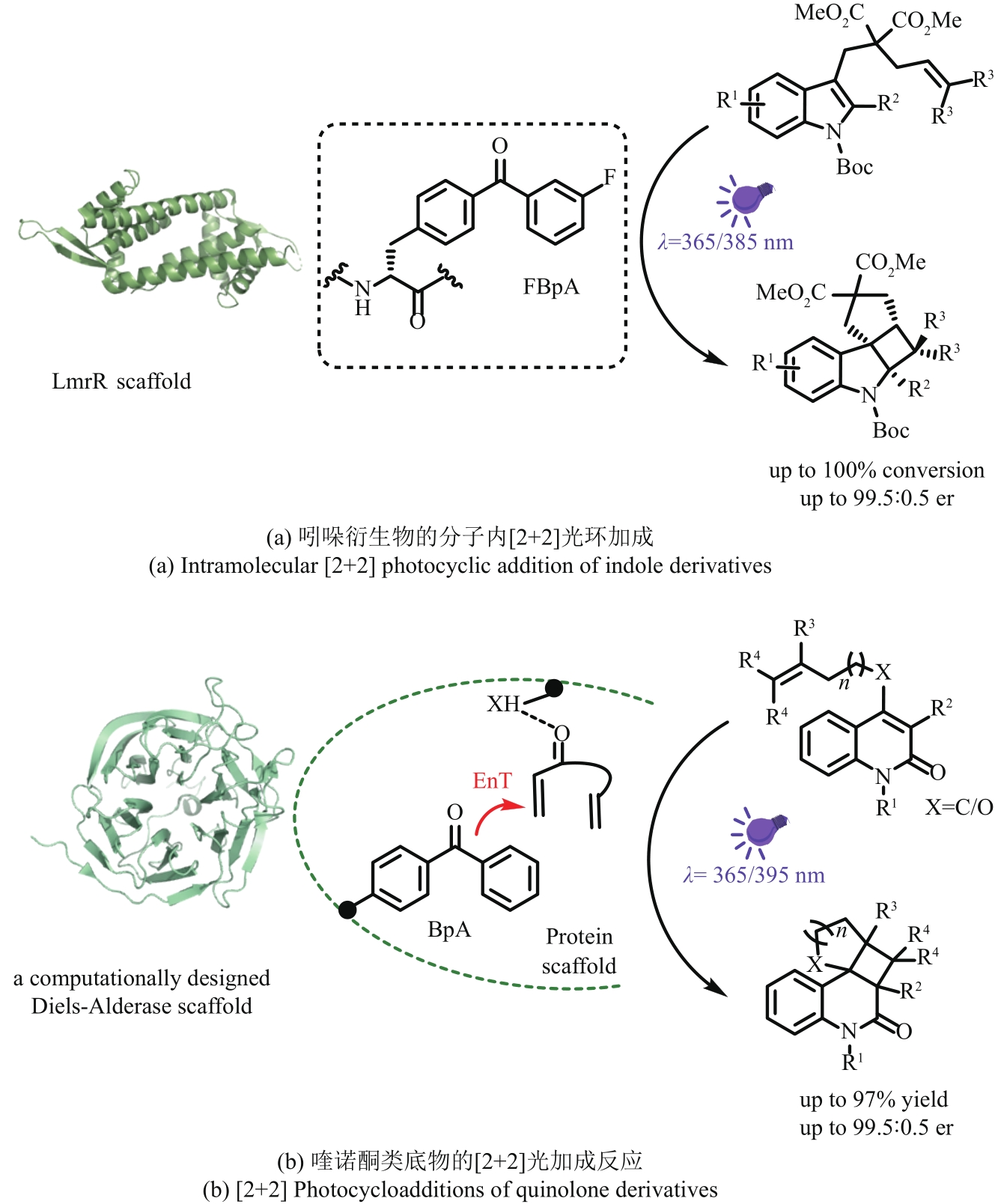
| 29 | 蒋迎迎, 曲戈, 孙周通. 机器学习助力酶定向进化[J]. 生物学杂志, 2020, 37(4): 1-11. |
| JIANG Y Y, QU G, SUN Z T. Machine learning-assisted enzyme directed evolution[J]. Journal of Biology, 2020, 37(4): 1-11. | |
| 30 | WANG Y J, XUE P, CAO M F, et al. Directed evolution: methodologies and applications[J]. Chemical Reviews, 2021, 121(20): 12384-12444. |
| 31 | CHITNIS P R. PHOTOSYSTEM I: Function and physiology[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 2001, 52: 593-626. |
| 32 | GAO J L, WANG H, YUAN Q P, et al. Structure and function of the photosystem supercomplexes[J]. Frontiers in Plant Science, 2018, 9: 357. |
| 33 | KAVAKLI I H, BARIS I, TARDU M, et al. The photolyase/cryptochrome family of proteins as DNA repair enzymes and transcriptional repressors[J]. Photochemistry and Photobiology, 2017, 93(1): 93-103. |
| 34 | SANCAR A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors[J]. Chemical Reviews, 2003, 103(6): 2203-2237. |
| 35 | SORIGUÉ D, LÉGERET B, CUINÉ S, et al. An algal photoenzyme converts fatty acids to hydrocarbons[J]. Science, 2017, 357(6354): 903-907. |
| 36 | SORIGUÉ D, HADJIDEMETRIOU K, BLANGY S, et al. Mechanism and dynamics of fatty acid photodecarboxylase[J]. Science, 2021, 372(6538): eabd5687. |
| 37 | SCHMERMUND L, JURKAŠ V, ÖZGEN F F, et al. Photo-biocatalysis: biotransformations in the presence of light[J]. ACS Catalysis, 2019, 9(5): 4115-4144. |
| 38 | SEEL C J, GULDER T. Biocatalysis fueled by light: on the versatile combination of photocatalysis and enzymes[J]. Chembiochem, 2019, 20(15): 1871-1897. |
| 39 | LEE S H, CHOI D S, KUK S K, et al. Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons[J]. Angewandte Chemie International Edition, 2018, 57(27): 7958-7985. |
| 40 | 张武元, 袁波, 曲戈, 等. 光促酶催化反应设计及生物合成应用[J]. 生物学杂志, 2021, 38(5): 1-11. |
| ZHANG W Y, YUAN B, QU G, et al. Photobiocatalytic reaction design and its biosynthetic applications[J]. Journal of Biology, 2021, 38(5): 1-11. | |
| 41 | YUN C H, KIM J, HOLLMANN F, et al. Light-driven biocatalytic oxidation[J]. Chemical Science, 2022, 13(42): 12260-12279. |
| 42 | KIM J, LEE S H, TIEVES F, et al. Biocatalytic C=C bond reduction through carbon nanodot-sensitized regeneration of NADH analogues[J]. Angewandte Chemie International Edition, 2018, 57(42): 13825-13828. |
| 43 | SCHROEDER L, FRESE M, MÜLLER C, et al. Photochemically driven biocatalysis of halogenases for the green production of chlorinated compounds[J]. ChemCatChem, 2018, 10(15): 3336-3341. |
| 44 | CHENG Y Q, SHI J F, WU Y Z, et al. Intensifying electron utilization by surface-anchored Rh complex for enhanced nicotinamide cofactor regeneration and photoenzymatic CO2 reduction[J]. Research, 2021, 2021: 8175709. |
| 45 | ZHANG S H, ZHANG Y S, CHEN Y, et al. Metal hydride-embedded titania coating to coordinate electron transfer and enzyme protection in photo-enzymatic catalysis[J]. ACS Catalysis, 2021, 11(1): 476-483. |
| 46 | JIA J S, HUO Q, YANG D, et al. Granum-inspired photoenzyme-coupled catalytic system via stacked polymeric carbon nitride[J]. ACS Catalysis, 2021, 11(15): 9210-9220. |
| 47 | SUN Y Y, SHI J F, WANG Z, et al. Thylakoid membrane-inspired capsules with fortified cofactor shuttling for enzyme-photocoupled catalysis[J]. Journal of the American Chemical Society, 2022, 144(9): 4168-4177. |
| 48 | STRIETH-KALTHOFF F, JAMES M J, TEDERS M, et al. Energy transfer catalysis mediated by visible light: principles, applications, directions[J]. Chemical Society Reviews, 2018, 47(19): 7190-7202. |
| 49 | ZHANG Y, LIU H Z, SHI Q S, et al. Enantiocomplementary synthesis of vicinal fluoro alcohols through photo-bio cascade reactions[J]. Green Chemistry, 2022, 24(20): 7889-7893. |
| 50 | SUN Y Y, LI W P, WANG Z, et al. General framework for enzyme-photo-coupled catalytic system toward carbon dioxide conversion[J]. Current Opinion in Biotechnology, 2022, 73: 67-73. |
| 51 | LITMAN Z C, WANG Y J, ZHAO H M, et al. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis[J]. Nature, 2018, 560(7718): 355-359. |
| 52 | WANG Y J, HUANG X Q, HUI J S, et al. Stereoconvergent reduction of activated alkenes by a nicotinamide free synergistic photobiocatalytic system[J]. ACS Catalysis, 2020, 10(16): 9431-9437. |
| 53 | VERHO O, BÄCKVALL J E. Chemoenzymatic dynamic kinetic resolution: a powerful tool for the preparation of enantiomerically pure alcohols and amines[J]. Journal of the American Chemical Society, 2015, 137(12): 3996-4009. |
| 54 | BHAT V, WELIN E R, GUO X L, et al. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations[J]. Chemical Reviews, 2017, 117(5): 4528-4561. |
| 55 | YANG Q, ZHAO F Q, ZHANG N, et al. Mild dynamic kinetic resolution of amines by coupled visible-light photoredox and enzyme catalysis[J]. Chemical Communications, 2018, 54(100): 14065-14068. |
| 56 | DEHOVITZ J S, LOH Y Y, KAUTZKY J A, et al. Static to inducibly dynamic stereocontrol: the convergent use of racemic β-substituted ketones[J]. Science, 2020, 369(6507): 1113-1118. |
| 57 | PIRNOT M T, RANKIC D A, MARTIN D B C, et al. Photoredox activation for the direct β-arylation of ketones and aldehydes[J]. Science, 2013, 339(6127): 1593-1596. |
| 58 | BIEGASIEWICZ K F, COOPER S J, EMMANUEL M A, et al. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases[J]. Nature Chemistry, 2018, 10(7): 770-775. |
| 59 | SANDOVAL B A, KURTOIC S I, CHUNG M M, et al. Photoenzymatic catalysis enables radical-mediated ketone reduction in ene-reductases[J]. Angewandte Chemie International Edition, 2019, 58(26): 8714-8718. |
| 60 | NAKANO Y, BLACK M J, MEICHAN A J, et al. Photoenzymatic hydrogenation of heteroaromatic olefins using 'ene'-reductases with photoredox catalysts[J]. Angewandte Chemie International Edition, 2020, 59(26): 10484-10488. |
| 61 | YE Y X, CAO J Z, OBLINSKY D G, et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination[J]. Nature Chemistry, 2023, 15(2): 206-212. |
| 62 | LAUDER K, TOSCANI A, QI Y Y, et al. Photo-biocatalytic one-pot cascades for the enantioselective synthesis of 1, 3-mercaptoalkanol volatile sulfur compounds[J]. Angewandte Chemie International Edition, 2018, 57(20): 5803-5807. |
| 63 | YU Y, LIN R D, YAO Y, et al. Development of a metal- and oxidant-free enzyme-photocatalyst hybrid system for highly efficient C-3 acylation reactions of indoles with aldehydes[J]. ACS Catalysis, 2022, 12(20): 12543-12554. |
| 64 | DING X, DONG C L, GUAN Z, et al. Concurrent asymmetric reactions combining photocatalysis and enzyme catalysis: direct enantioselective synthesis of 2,2-disubstituted indol-3-ones from 2-arylindoles[J]. Angewandte Chemie International Edition, 2019, 58(1): 118-124. |
| 65 | BUREK B O, BORMANN S, HOLLMANN F, et al. Hydrogen peroxide driven biocatalysis[J]. Green Chemistry, 2019, 21(12): 3232-3249. |
| 66 | LÜTZ S, STECKHAN E, LIESE A. First asymmetric electroenzymatic oxidation catalyzed by a peroxidase[J]. Electrochemistry Communications, 2004, 6(6): 583-587. |
| 67 | ZHANG W Y, FERNÁNDEZ-FUEYO E, NI Y, et al. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalizations[J]. Nature Catalysis, 2018, 1(1): 55-62. |
| 68 | YUAN B, MAHOR D, FEI Q, et al. Water-soluble anthraquinone photocatalysts enable methanol-driven enzymatic halogenation and hydroxylation reactions[J]. ACS Catalysis, 2020, 10(15): 8277-8284. |
| 69 | ZHANG W Y, MA M, HUIJBERS M M E, et al. Hydrocarbon synthesis via photoenzymatic decarboxylation of carboxylic acids[J]. Journal of the American Chemical Society, 2019, 141(7): 3116-3120. |
| 70 | XU J, HU Y J, FAN J J, et al. Light-driven kinetic resolution of α-functionalized carboxylic acids enabled by an engineered fatty acid photodecarboxylase[J]. Angewandte Chemie International Edition, 2019, 58(25): 8474-8478. |
| 71 | LI D Y, WU Q, REETZ M T. Focused rational iterative site-specific mutagenesis (FRISM)[J]. Methods in Enzymology, 2020, 643: 225-242. |
| 72 | XU J, FAN J J, LOU Y J, et al. Light-driven decarboxylative deuteration enabled by a divergently engineered photodecarboxylase[J]. Nature Communications, 2021, 12: 3983. |
| 73 | LI D Y, HAN T, XUE J D, et al. Engineering fatty acid photodecarboxylase to enable highly selective decarboxylation of trans fatty acids[J]. Angewandte Chemie International Edtion, 2021, 60(38): 20695-20699. |
| 74 | GE R, ZHANG P P, DONG X T, et al. Photobiocatalytic decarboxylation for the synthesis of fatty epoxides from renewable fatty acids[J]. ChemSusChem, 2022, 15(20): e202201275. |
| 75 | AMER M, WOJCIK E Z, SUN C H, et al. Low carbon strategies for sustainable bio-alkane gas production and renewable energy[J]. Energy & Environmental Science, 2020, 13(6): 1818-1831. |
| 76 | BRUDER S, MOLDENHAUER E J, LEMKE R D, et al. Drop-in biofuel production using fatty acid photodecarboxylase from Chlorella variabilis in the oleaginous yeast Yarrowia lipolytica [J]. Biotechnology for Biofuels, 2019, 12: 202. |
| 77 | XU W H, MOU K H, ZHOU H N, et al. Transformation of triolein to biogasoline by photo-chemo-biocatalysis[J]. Green Chemistry, 2022, 24(17): 6589-6598. |
| 78 | SELLÉS VIDAL L, KELLY C L, MORDAKA P M, et al. Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application[J]. Biochimica et Biophysica Acta-Proteins and Proteomics, 2018, 1866(2): 327-347. |
| 79 | TOOGOOD H S, SCRUTTON N S. Discovery, characterisation, engineering and applications of ene reductases for industrial biocatalysis[J]. ACS Catalysis, 2019, 8(4): 3532-3549. |
| 80 | MULLIKEN R S. Molecular compounds and their spectra. Ⅱ[J]. Journal of the American Chemical Society, 1952, 74(3): 811-824. |
| 81 | CRISENZA G E M, MAZZARELLA D, MELCHIORRE P. Synthetic methods driven by the photoactivity of electron donor-acceptor complexes[J]. Journal of the American Chemical Society, 2020, 142(12): 5461-5476. |
| 82 | EMMANUEL M A, GREENBERG N R, OBLINSKY D G, et al. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light[J]. Nature, 2016, 540(7633): 414-417. |
| 83 | PENG Y Z, WANG Z G, CHEN Y, et al. Photoinduced promiscuity of cyclohexanone monooxygenase for the enantioselective synthesis of α-fluoroketones[J]. Angewandte Chemie International Edtion, 2022, 61(50): e202211199. |
| 84 | HUANG X Q, FENG J Q, CUI J W, et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition[J]. Nature Catalysis, 2022, 5(7): 586-593. |
| 85 | XU J, CEN Y X, SINGH W, et al. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters[J]. Journal of the American Chemical Society, 2019, 141(19): 7934-7945. |
| 86 | BIEGASIEWICZ K F, COOPER S J, GAO X, et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization[J]. Science, 2019, 364(6446): 1166-1169. |
| 87 | LAGUERRE N, RIEHL P S, OBLINSKY D G, et al. Radical termination via β-scission enables photoenzymatic allylic alkylation using "ene"-reductases[J]. ACS Catalysis, 2022, 12(15): 9801-9805. |
| 88 | BLACK M J, BIEGASIEWICZ K F, MEICHAN A J, et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent 'ene'-reductases[J]. Nature Chemistry, 2020, 12(1): 71-75. |
| 89 | SANDOVAL B A, CLAYMAN P D, OBLINSKY D G, et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent 'ene'-reductases[J]. Journal of the American Chemical Society, 2021, 143(4): 1735-1739. |
| 90 | HUANG X Q, WANG B J, WANG Y J, et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation[J]. Nature, 2020, 584(7819): 69-74. |
| 91 | PAGE C G, COOPER S J, DEHOVITZ J S, et al. Quaternary charge-transfer complex enables photoenzymatic intermolecular hydroalkylation of olefins[J]. Journal of the American Chemical Society, 2021, 143(1): 97-102. |
| 92 | FU H G, LAM H, EMMANUEL M A, et al. Ground-state electron transfer as an initiation mechanism for biocatalytic C—C bond forming reactions[J]. Journal of the American Chemical Society, 2021, 143(25): 9622-9629. |
| 93 | FU H G, CAO J Z, QIAO T Z, et al. An asymmetric sp3-sp3 cross-electrophile coupling using 'ene'-reductases[J]. Nature, 2022, 610(7931): 302-307. |
| 94 | GU Y F, ELLIS-GUARDIOLA K, SRIVASTAVA P, et al. Preparation, characterization, and oxygenase activity of a photocatalytic artificial enzyme[J]. Chembiochem, 2015, 16(13): 1880-1883. |
| 95 | ZUBI Y S, LIU B Q, GU Y F, et al. Controlling the optical and catalytic properties of artificial metalloenzyme photocatalysts using chemogenetic engineering[J]. Chemical Science, 2022, 13(5): 1459-1468. |
| 96 | SOSA V, MELKIE M, SULCA C, et al. Selective light-driven chemoenzymatic trifluoromethylation/hydroxylation of substituted arenes[J]. ACS Catalysis, 2018, 8(3): 2225-2229. |
| 97 | SCHWOCHERT T D, CRUZ C L, WATTERS J W, et al. Design and evaluation of artificial hybrid photoredox biocatalysts[J]. Chembiochem, 2020, 21(21): 3146-3150. |
| 98 | CESANA P T, LI B X, SHEPARD S G, et al. A biohybrid strategy for enabling photoredox catalysis with low-energy light[J]. Chem, 2022, 8(1): 174-185. |
| 99 | CESANA P T, PAGE C G, HARRIS D, et al. Photoenzymatic catalysis in a new light: Gluconobacter "ene"-reductase conjugates possessing high-energy reactivity with tunable low-energy excitation[J]. Journal of the American Chemical Society, 2022, 144(38): 17516-17521. |
| 100 | YU Y, LIU X H, WANG J Y. Expansion of redox chemistry in designer metalloenzymes[J]. Accounts of Chemical Research, 2019, 52(3): 557-565. |
| 101 | LIU X H, LIU P C, LI H J, et al. Excited-state intermediates in a designer protein encoding a phototrigger caught by an X-ray free-electron laser[J]. Nature Chemistry, 2022, 14(9): 1054-1060. |
| 102 | ZHENG D D, TAO M, YU L J, et al. Ultrafast photoinduced electron transfer in a photosensitizer protein[J]. CCS Chemistry, 2022, 4(4): 1217-1223. |
| 103 | LIU X H, KANG F Y, HU C, et al. A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2-reducing enzyme[J]. Nature Chemistry, 2018, 10(12): 1201-1206. |
| 104 | FU Y, HUANG J, WU Y Z, et al. Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase[J]. Journal of the American Chemical Society, 2021, 143(2): 617-622. |
| 105 | SUN N N, HUANG J J, QIAN J Y, et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes[J]. Nature, 2022, 611(7937): 715-720. |
| 106 | ROELFES G. LmrR: A privileged scaffold for artificial metalloenzymes[J]. Accounts of Chemical Research, 2019, 52(3): 545-556. |
| 107 | TRIMBLE J S, CRAWSHAW R, HARDY F J, et al. A designed photoenzyme for enantioselective [2+2]cycloadditions[J]. Nature, 2022, 611(7937): 709-714. |
| 108 | SIEGEL J B, ZANGHELLINI A, LOVICK H M, et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction[J]. Science, 2010, 329(5989): 309-313. |
| [1] | FU Yu, ZHONG Fangrui. Recent advances in chemically driven enantioselective photobiocatalysis [J]. Synthetic Biology Journal, 2024, 5(5): 1021-1049. |
| [2] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [3] | SUN Mengchu, LU Liangyu, SHEN Xiaolin, SUN Xinxiao, WANG Jia, YUAN Qipeng. Fluorescence detection-based high-throughput screening systems and devices facilitate cell factories construction [J]. Synthetic Biology Journal, 2023, 4(5): 947-965. |
| [4] | Liqi KANG, Pan TAN, Liang HONG. Enzyme engineering in the age of artificial intelligence [J]. Synthetic Biology Journal, 2023, 4(3): 524-534. |
| [5] | Qingyun RUAN, Xin HUANG, Zijun MENG, Shu QUAN. Computational design and directed evolution strategies for optimizing protein stability [J]. Synthetic Biology Journal, 2023, 4(1): 5-29. |
| [6] | Yanping QI, Jin ZHU, Kai ZHANG, Tong LIU, Yajie WANG. Recent development of directed evolution in protein engineering [J]. Synthetic Biology Journal, 2022, 3(6): 1081-1108. |
| [7] | Yuqi TANG, Songtao YE, Jia LIU, Xin ZHANG. Molecular chaperones promote protein stability and evolution [J]. Synthetic Biology Journal, 2022, 3(3): 445-464. |
| [8] | Shuke WU, Yi ZHOU, Wen WANG, Wei ZHANG, Pengfei GAO, Zhi LI. From single-enzyme catalysis to multienzyme cascade: inspired from Professor Daniel I.C. Wang’s pioneer work in enzyme technology [J]. Synthetic Biology Journal, 2021, 2(4): 543-558. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||