Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (4): 543-558.DOI: 10.12211/2096-8280.2021-004
• Invited Review • Previous Articles Next Articles
From single-enzyme catalysis to multienzyme cascade: inspired from Professor Daniel I.C. Wang’s pioneer work in enzyme technology
WU Shuke1, ZHOU Yi1, WANG Wen2, ZHANG Wei3, GAO Pengfei4, LI Zhi5
- 1.College of Life Science and Technology,Huazhong Agricultural University,Wuhan 430070,Hubei,China
2.Nestlé R&D Center,Singapore 619625
3.Bioprocessing Technology Institute,Agency for Science,Technology and Research,Singapore 138668
4.GlaxoSmithKline (China) Investment Co. Ltd,Beijing 100025,China
5.Department of Chemical and Biomolecular Engineering,National University of Singapore,Singapore 117585
-
Received:2021-01-12Revised:2021-03-17Online:2021-09-10Published:2021-08-31 -
Contact:WU Shuke, LI Zhi
从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去
吴淑可1, 周颐1, 王文2, 张巍3, 高鹏飞4, 李智5
- 1.华中农业大学生命科学技术学院,湖北 武汉 430070
2.雀巢新加坡研发中心,新加坡 619625
3.新加坡科技研究局(A*STAR)生物过程技术研究所,新加坡 138668
4.葛兰素史克中国投资有限公司,北京 100025
5.新加坡国立大学化学与分子工程系,新加坡 117585
-
通讯作者:吴淑可,李智 -
作者简介:吴淑可 (1987—),男,博士,特聘教授,主要从事酶工程及微生物催化研究。E-mail:shukewu@mail.hzau.edu.cn李智 ,男,博士,新加坡国立大学化学与生物分子工程系副教授,主要从事酶催化与生物化工研究。E-mail:chelz@nus.edu.sg
CLC Number:
Cite this article
WU Shuke, ZHOU Yi, WANG Wen, ZHANG Wei, GAO Pengfei, LI Zhi. From single-enzyme catalysis to multienzyme cascade: inspired from Professor Daniel I.C. Wang’s pioneer work in enzyme technology[J]. Synthetic Biology Journal, 2021, 2(4): 543-558.
吴淑可, 周颐, 王文, 张巍, 高鹏飞, 李智. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-004
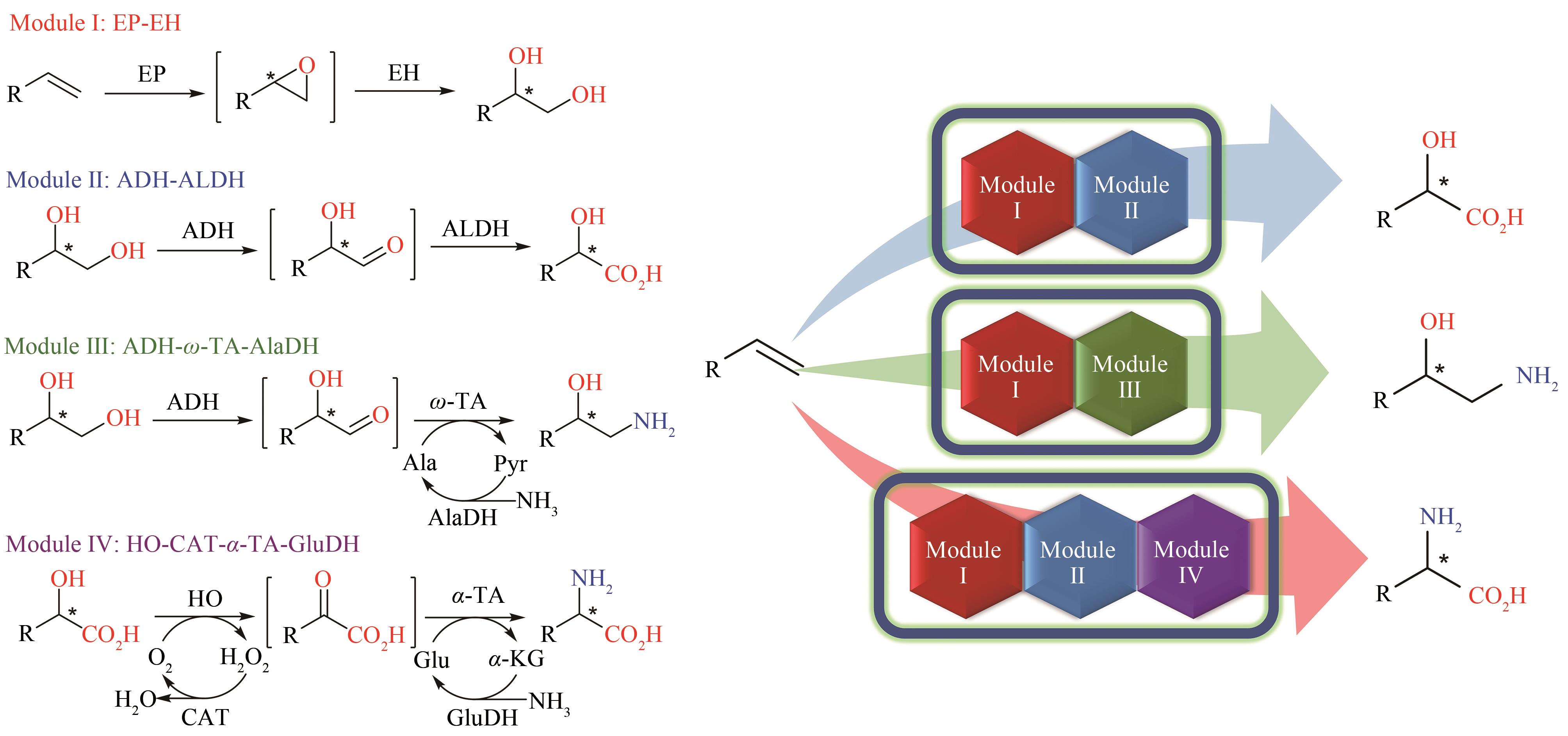
Fig. 3 Modular multi-enzyme cascade catalysis for the transformation of alkenes into three types of chiral molecules developed by Wu Shuke and co-supervised by Prof. Wang through the Singapore-MIT Alliance.EP—epoxidase; EH—epoxide hydrolase; ADH—alcohol dehydrogenase; ALDH—aldehyde dehydrogenase; ω-TA—ω-transaminase; AlaDH—alanine dehydrogenase; HO—hydroxy acid oxidase; α-TA—α-transaminase; CAT—catalase; GluDH—glutamate dehydrogenase
| 1 | AFEYAN N B, COONEY C L. Professor Daniel IC Wang: a legacy of education, innovation, publication, and leadership [J]. Biotechnology and Bioengineering, 2006, 95(2): 206-217. |
| 2 | 丁明珠, 李炳志, 王颖, 等. 合成生物学重要研究方向进展[J]. 合成生物学, 2020, 1(1) :7-28. |
| DING M, LI B, WANG Y, et al. Significant research progress in synthetic biology [J]. Synthetic Biology Journal, 2020, 1(1): 7-28. | |
| 3 | SCHMID A, DORDICK J S, HAUER B, et al. Industrial biocatalysis today and tomorrow [J]. Nature, 2001, 409(6817): 258-268. |
| 4 | BORNSCHEUER U T, HUISMAN G W, KAZLAUSKAS R J, et al. Engineering the third wave of biocatalysis [J]. Nature, 2012, 485(7397): 185-194. |
| 5 | KIRK O, BORCHERT T V, FUGLSANG C C. Industrial enzyme applications [J]. Current Opinion in Biotechnology, 2002, 13(4): 345-351. |
| 6 | REETZ M T. Biocatalysis in organic chemistry and biotechnology: past, present, and future [J]. Journal of the American Chemical Society, 2013, 135(34): 12480-12496. |
| 7 | WU S, SNAJDROVA R, MOORE J C, et al. Biocatalysis: enzymatic synthesis for industrial applications [J]. Angewandte Chemie International Edition, 2021, 60(1): 88-119. |
| 8 | ARCHER M C, RAGNARSSON J O, TANNENBAUM S R, et al. Enzymatic solubilization of an insoluble substrate, fish protein concentrate: process and kinetic considerations [J]. Biotechnology and Bioengineering, 1973, 15(1): 181-196. |
| 9 | ARCHER M C, STILLINGS B R, TANNENBAUM S R, et al. Reduction in mercury content of fish protein concentrate by enzymic digestion [J]. Journal of Agricultural and Food Chemistry, 1973, 21(6): 1116-1117. |
| 10 | BARATTI J, COUDERC R, COONEY C L, et al. Preparation and properties of immobilized methanol oxidase [J]. Biotechnology and Bioengineering, 1978, 20(3): 333-348. |
| 11 | HAMILTON B K, MONTGOMERY J P, WANG D I C. Enzyme reactions for preparative scale synthesis [M]. Enzyme Engineering Volume 2. Boston, MA: Springer, 1974, 153-159. |
| 12 | GARDNER C R, COLTON C K, LANGER R S, et al. Enzymatic regeneration of ATP from AMP and ADP part i. thermodynamics, kinetics, and process development [M]. Enzyme Engineering Volume 2. Boston, MA: Springer, 1974, 209-216. |
| 13 | TZENG C H, THRASHER K D, MONTGOMERY J P, et al. High productivity tank fermentation for gramicidin S synthetases [J]. Biotechnology and Bioengineering, 1975, 17(1): 143-152. |
| 14 | WANG D I C, HAMILTON B K. Kinetics of the enzymatic synthesis of peptide antibiotics [J]. Biotechnology and Bioengineering, 1977, 19(8): 1225-1232. |
| 15 | BOMMARIUS A S, HATTON T A, WANG D I C. Xanthine oxidase reactivity in reversed micellar systems: a contribution to the prediction of enzymic activity in organized media [J]. Journal of the American Chemical Society, 1995, 117(16): 4515-4523. |
| 16 | LASKO D R, WANG D I C. In situ fermentation monitoring with recombinant firefly luciferase [J]. Biotechnology and Bioengineering, 1993, 42(1): 30-36. |
| 17 | lASKO D R, WANG D I C. On-line monitoring of intracellular ATP concentration in Escherichia coli fermentations [J]. Biotechnology and Bioengineering, 1996, 52(3): 364-372. |
| 18 | FASAN R, JENNIFER KAN S B, ZHAO H. A continuing career in biocatalysis: Frances H. Arnold [J]. ACS Catalysis, 2019, 9(11): 9775-9788. |
| 19 | ACEVEDO-ROCHA C G, HOLLMANN F, SANCHIS J, et al. A pioneering career in catalysis: Manfred T. Reetz [J]. ACS Catalysis, 2020, 10(24): 15123-15139. |
| 20 | 刘延峰,周景文,刘龙, 等. 合成生物学与食品制造[J]. 合成生物学, 2020, 1(1): 84-91. |
| LIU Y F, ZHOU J W, LIU L, et al. Synthetic biology and food manufacturing [J]. Synthetic Biology Journal, 2020, 1(1): 84-91. | |
| 21 | 史硕博,孟琼宇,乔玮博,等. 塑造低碳经济的第三代固碳生物炼制[J]. 合成生物学, 2020, 1(1): 44-59. |
| SHI S B, MENG Q Y, QIAO W B, et al. Establishing carbon dioxide-based third-generation biorefinery for a sustainable low-carbon economy [J]. Synthetic Biology Journal, 2020, 1(1): 44-59. | |
| 22 | 王凯, 刘子鹤, 陈必强, 等. 微生物利用二氧化碳合成燃料及化学品——第三代生物炼制[J]. 合成生物学, 2020, 1(1): 60-70. |
| WANG K, LIU Z H, CHEN B Q, et al. Microbial utilization of carbon dioxide to synthesize fuels and chemicals——third-generation biorefineries [J]. Synthetic Biology Journal, 2020, 1(1): 60-70. | |
| 23 | 高教琪, 周雍进. 甲醇生物转化的机遇与挑战[J]. 合成生物学, 2020, 1(2): 158-173. |
| GAO J Q, ZHOU Y J. Advances in methanol bio-transformation [J]. Synthetic Biology Journal, 2020, 1(2): 158-173. | |
| 24 | 贺俊斌, 孟松, 潘海学, 等. 多酶催化串联策略在复杂天然产物合成中的应用[J]. 合成生物学, 2020, 1(2): 226-246. |
| HE J B, MENG S, PAN H X, et al. Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products [J]. Synthetic Biology Journal, 2020, 1(2): 226-246. | |
| 25 | WANG W, YAO L, CHENG C Y, et al. Harnessing the hygroscopic and biofluorescent behaviors of genetically tractable microbial cells to design biohybrid wearables [J]. Science Advances, 2017, 3(5): e1601984. |
| 26 | MATEO C, PALOMO J M, FERNANDEZ-LORENTE G, et al. Improvement of enzyme activity, stability and selectivity via immobilization techniques [J]. Enzyme and Microbial Technology, 2007, 40(6): 1451-1463. |
| 27 | SHELDON R A. Enzyme immobilization: the quest for optimum performance [J]. Advanced Synthesis & Catalysis, 2007, 349(8-9): 1289-1307. |
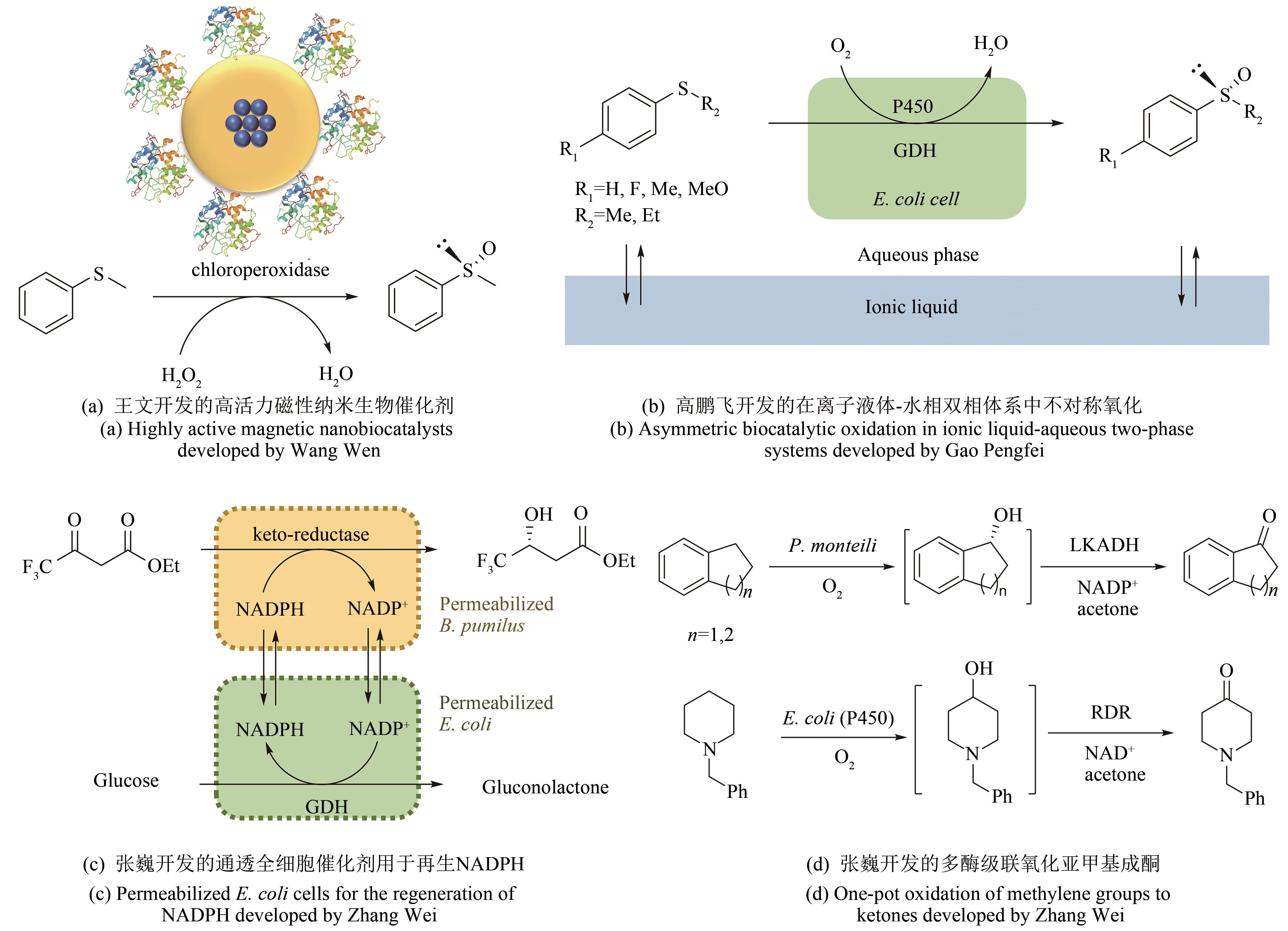
| 28 | WANG W, XU Y, WANG D I C, et al. Recyclable nanobiocatalyst for enantioselective sulfoxidation: facile fabrication and high performance of chloroperoxidase-coated magnetic nanoparticles with iron oxide core and polymer shell [J]. Journal of the American Chemical Society, 2009, 131(36): 12892-12893. |
| 29 | WANG W, WANG D I C, LI Z. Facile fabrication of recyclable and active nanobiocatalyst: purification and immobilization of enzyme in one pot with Ni-NTA functionalized magnetic nanoparticle [J]. Chemical Communications, 2011; 47(28): 8115-8117. |
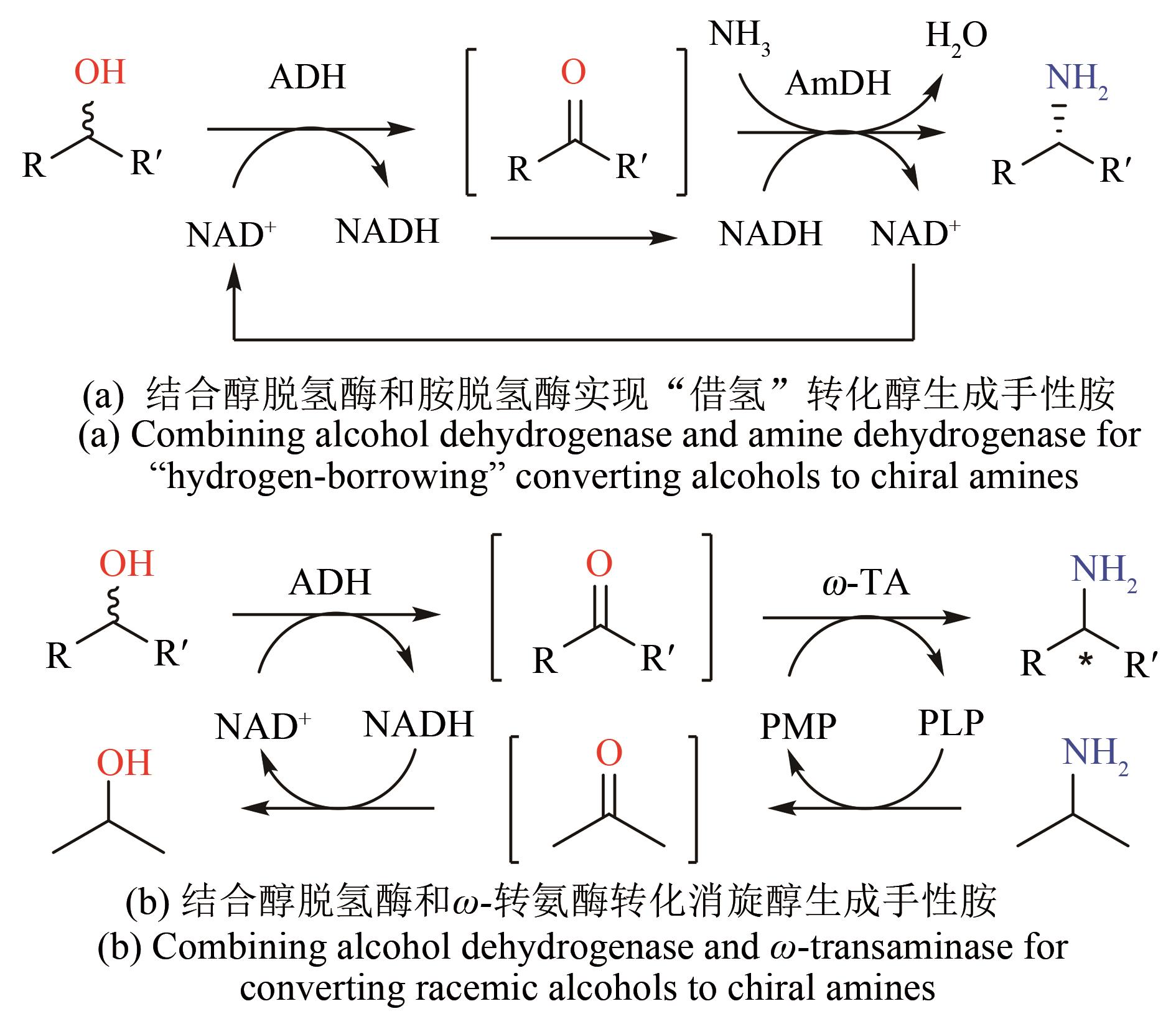
| 30 | NGO T P, ZHANG W, WANG W, et al. Reversible clustering of magnetic nanobiocatalysts for high-performance biocatalysis and easy catalyst recycling [J]. Chemical Communications, 2012, 48(38): 4585-4587. |
| 31 | VAHIDI A K, YANG Y, NGO T P, et al. Simple and efficient immobilization of extracellular his-tagged enzyme directly from cell culture supernatant as active and recyclable nanobiocatalyst: high-performance production of biodiesel from waste grease [J]. ACS Catalysis, 2015, 5(6): 3157-3161. |
| 32 | VAHIDI A K, WANG Z, LI Z. Facile synthesis of S-substituted L-cysteines with nano-sized immobilized O-acetylserine sulfhydrylase [J]. ChemCatChem, 2018, 10(17): 3671-3674. |
| 33 | RANTWIJK F VAN, SHELDON R A. Biocatalysis in ionic liquids [J]. Chemical Reviews, 2007,107(6): 2757-2785. |
| 34 | XU P, ZHENG G W, ZONG M H, et al. Recent progress on deep eutectic solvents in biocatalysis [J]. Bioresources and Bioprocessing, 2017, 4(1): 34. |
| 35 | GAO P, LI A, LEE H H, et al. Enhancing enantioselectivity and productivity of P450-catalyzed asymmetric sulfoxidation with an aqueous/ionic liquid biphasic system [J]. ACS Catalysis, 2014, 4(10): 3763-3771. |
| 36 | REETZ M T. Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions [J]. Angewandte Chemie International Edition, 2011, 50(1): 138-174. |
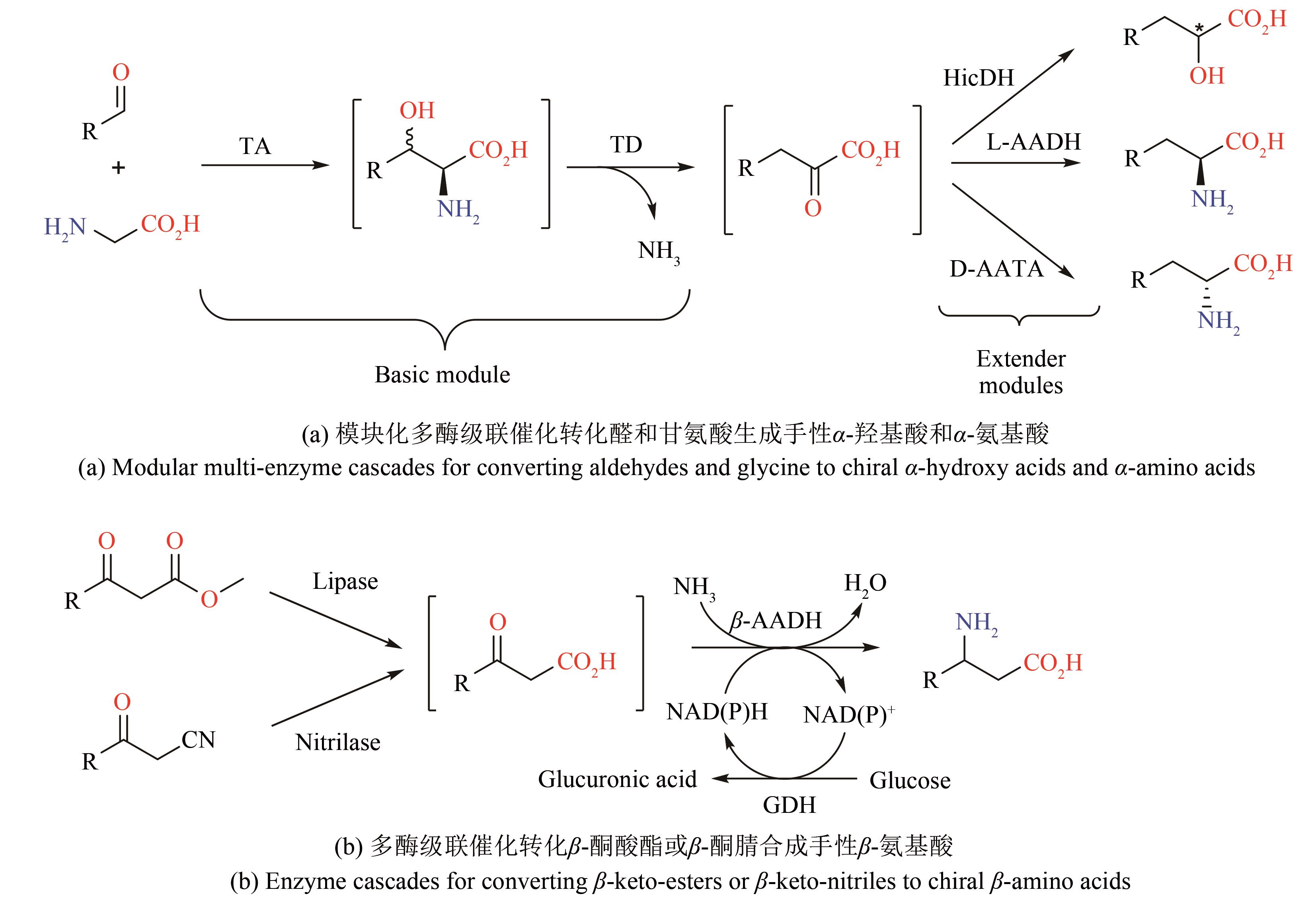
| 37 | TANG W L, LI Z, ZHAO H. Inverting the enantioselectivity of P450pyr monooxygenase by directed evolution [J]. Chemical Communications, 2010, 46(30): 5461-5463. |
| 38 | PHAM S Q, POMPIDOR G, LIU J, et al. Evolving P450pyr hydroxylase for highly enantioselective hydroxylation at non-activated carbon atom [J]. Chemical Communications, 2012, 48(38): 4618-4620. |
| 39 | YANG Y, LIU J, LI Z. Engineering of P450pyr hydroxylase for the highly regio- and enantioselective subterminal hydroxylation of alkanes [J]. Angewandte Chemie International Edition, 2014, 53(12): 3120-3124. |
| 40 | LIU W, WANG P. Cofactor regeneration for sustainable enzymatic biosynthesis [J]. Biotechnology Advances, 2007, 25(4): 369-384. |
| 41 | ZHANG J, DUETZ W A, WITHOLT B, et al. Rapid identification of new bacterial alcohol dehydrogenases for (R)-and (S)-enantioselective reduction of β-ketoesters [J]. Chemical Communications, 2004(18): 2120-2121. |
| 42 | ZHANG W, O'CONNOR K, WANG D I C, et al. Bioreduction with efficient recycling of NADPH by coupled permeabilized microorganisms[J]. Applied and Environmental Microbiology, 2009, 75(3): 687-694. |
| 43 | SCHRITTWIESER J H, VELIKOGNE S, HALL M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules [J]. Chemical Reviews, 2018, 118(1): 270-348. |
| 44 | FRANCE S P, HEPWORTH L J, TURNER N J, et al. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways [J]. ACS Catalysis, 2017, 7(1): 710-724. |
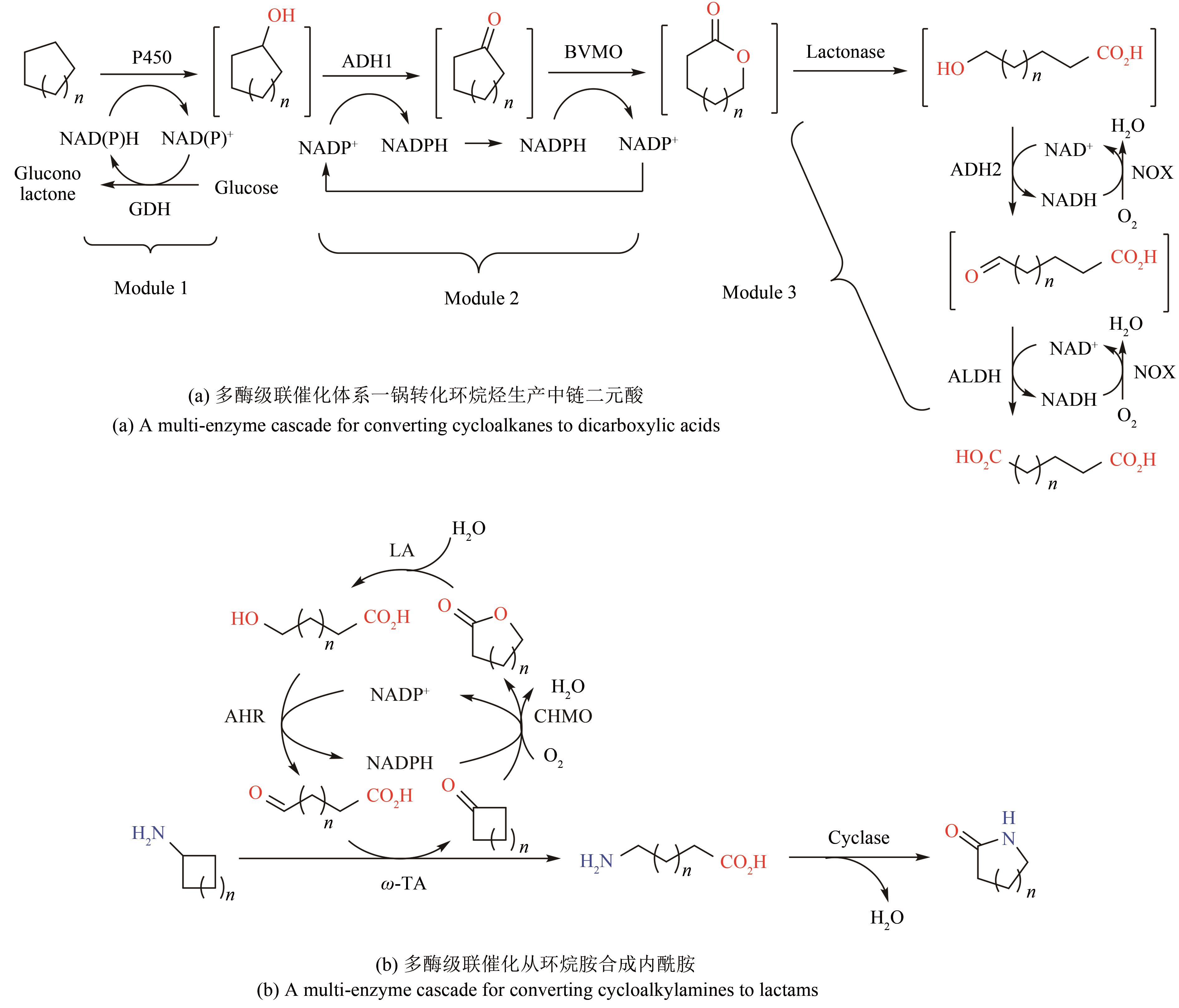
| 45 | WU S K, LI Z. Whole-cell cascade biotransformations for one-pot multistep organic synthesis [J]. ChemCatChem, 2018, 10(10): 2164-2178. |
| 46 | KUSKA J, O'REILLY E. Engineered biosynthetic pathways and biocatalytic cascades for sustainable synthesis [J]. Current Opinion in Chemical Biology, 2020, 58: 146-54. |
| 47 | LI R J, ZHANG Z, ACEVEDO-ROCHA C G, et al. Biosynthesis of organic molecules via artificial cascade reactions based on cytochrome P450 monooxygenases [J]. Green Synthesis and Catalysis, 2020, 1(1): 52-59. |
| 48 | VÁZQUEZ-GONZÁLEZ M, WANG C, WILLNER I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments [J]. Nature Catalysis, 2020, 3(3): 256-273. |
| 49 | WANG Z, SEKAR B S, LI Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals [J]. Bioresource Technology, 2021, 323: 124551. |
| 50 | ZHANG W, TANG W L, WANG D I C, et al. Concurrent oxidations with tandem biocatalysts in one pot: green, selective and clean oxidations of methylene groups to ketones [J]. Chemical Communications, 2011, 47(11): 3284-3286. |
| 51 | WU S K, CHEN Y, XU Y, et al. Enantioselective trans-dihydroxylation of aryl olefins by cascade biocatalysis with recombinant Escherichia coli coexpressing monooxygenase and epoxide hydrolase [J]. ACS Catalysis, 2014, 4(2): 409-420. |
| 52 | WU S K, ZHOU Y, WANG T, et al. Highly regio- and enantioselective multiple oxy-and amino-functionalizations of alkenes by modular cascade biocatalysis [J]. Nature Communications, 2016, 7: 11917. |
| 53 | ZHOU Y, WU S K, LI Z. One-pot enantioselective synthesis of d-phenylglycines from racemic mandelic acids, styrenes, or biobased l-phenylalanine via cascade biocatalysis [J]. Advanced Synthesis & Catalysis, 2017, 359(24): 4305-4316. |
| 54 | WU S K, LIU J, LI Z. Biocatalytic formal anti-Markovnikov hydroamination and hydration of aryl alkenes [J]. ACS Catalysis, 2017, 7(8): 5225-5233. |
| 55 | WU S K, ZHOU Y, SEET D, et al. Regio-and stereoselective oxidation of styrene derivatives to arylalkanoic acids via one-pot cascade biotransformations [J]. Advanced Synthesis & Catalysis, 2017, 359(12): 2132-2141. |
| 56 | ZHOU Y, WU S K, MAO J, et al. Bioproduction of benzylamine from renewable feedstocks via a nine-step artificial enzyme cascade and engineered metabolic pathways [J]. ChemSusChem, 2018, 11(13): 2221-2228. |
| 57 | ZHOU Y, SEKAR B S, WU S K, et al. Benzoic acid production via cascade biotransformation and coupled fermentation-biotransformation [J]. Biotechnology and Bioengineering, 2020, 117(8): 2340-2350. |
| 58 | SEKAR B S, MAO J, LUKITO B R, et al. Bioproduction of enantiopure (R)- and (S)-2-phenylglycinols from styrenes and renewable feedstocks [J]. Advanced Synthesis & Catalysis, 2021, 363(7): 1892-1903. |
| 59 | ZHANG J, YANG X, DONG R, et al. Cascade biocatalysis for regio- and stereoselective aminohydroxylation of styrenyl olefins to enantiopure arylglycinols [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(49): 18277-18285. |
| 60 | ZHOU Y, WU S K, LI Z. Cascade biocatalysis for sustainable asymmetric synthesis: from biobased l-phenylalanine to high-value chiral chemicals [J]. Angewandte Chemie International Edition, 2016, 55(38): 11647-11650. |
| 61 | SEKAR B S, LUKITO B R, LI Z. Production of natural 2-phenylethanol from glucose or glycerol with coupled Escherichia coli strains expressing l-phenylalanine biosynthesis pathway and artificial biocascades [J]. ACS Sustainable Chemistry & Engineering. 2019, 7(14): 12231-12239. |
| 62 | NUGENT T C, EL-SHAZLY M. Chiral amine synthesis-recent developments and trends for enamide reduction, reductive amination, and imine reduction [J]. Advanced Synthesis & Catalysis, 2010, 352(5): 753-819. |
| 63 | SAVILE C K, JANEY J M, MUNDORFF E C, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture [J]. Science, 2010, 329(5989): 305-309. |
| 64 | ABRAHAMSON M J, VAZQUEZ-FIGUEROA E, WOODALL N B, et al. Development of an amine dehydrogenase for synthesis of chiral amines [J]. Angewandte Chemie International Edition, 2012, 51(16): 3969-3972. |
| 65 | MUTTI F G, KNAUS T, SCRUTTON N S, et al. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades [J]. Science, 2015, 349(6255): 1525-1529. |
| 66 | CHEN F F, LIU Y Y, ZHENG G W, et al. Asymmetric amination of secondary alcohols by using a redox-neutral two-enzyme cascade [J]. ChemCatChem, 2015, 7(23): 3838-3841. |
| 67 | YU H L, LI T, CHEN F F, et al. Bioamination of alkane with ammonium by an artificially designed multienzyme cascade [J]. Metabolic Engineering, 2018, 47: 184-189. |
| 68 | TAUBER K, FUCHS M, SATTLER J H, et al. Artificial multi-enzyme networks for the asymmetric amination of sec-alcohols [J]. Chemistry-A European Journal, 2013, 19(12): 4030-4035. |
| 69 | TIAN K, LI Z. A simple biosystem for the high-yielding cascade conversion of racemic alcohols to enantiopure amines [J]. Angewandte Chemie International Edition, 2020, 59(48): 21745-21751. |
| 70 | ALEKU G A, FRANCE S P, MAN H, et al. A reductive aminase from Aspergillus oryzae [J]. Nature Chemistry, 2017, 9(10): 961-969. |
| 71 | RAMSDEN J I, HEATH R S, DERRINGTON S R, et al. Biocatalytic N-alkylation of amines using either primary alcohols or carboxylic acids via reductive aminase cascades [J]. Journal of the American Chemical Society, 2019, 141(3): 1201-1206. |
| 72 | XUE Y P, CAO C H, ZHENG Y G. Enzymatic asymmetric synthesis of chiral amino acids [J]. Chemical Society Reviews, 2018, 47(4): 1516-1561. |
| 73 | ALMHJELL P J, BOVILLE C E, ARNOLD F H. Engineering enzymes for noncanonical amino acid synthesis [J]. Chemical Society Reviews, 2018, 47(24): 8980-8997. |
| 74 | ALTENBUCHNER J, SIEMANN-HERZBERG M, SYLDATK C. Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids [J]. Current Opinion in Biotechnology, 2001, 12(6): 559-563. |
| 75 | LIU F, ZHOU J, XU M, et al. One-pot biocatalytic preparation of enantiopure unusual α-amino acids from α-hydroxy acids via a hydrogen-borrowing dual-enzyme cascade [J]. Catalysts, 2020, 10(12): 1470. |
| 76 | SONG W, WANG J H, WU J, et al. Asymmetric assembly of high-value α-functionalized organic acids using a biocatalytic chiral-group-resetting process [J]. Nature Communications, 2018, 9: 3818. |
| 77 | LI R, WIJMA H J, SONG L, et al. Computational redesign of enzymes for regio- and enantioselective hydroamination [J]. Nature Chemical Biology, 2018, 14(7): 664-670. |
| 78 | WANG J, SONG W, WU J, et al. Efficient production of phenylpropionic acids by an amino-group-transformation biocatalytic cascade [J]. Biotechnology and Bioengineering, 2020, 117(3): 614-625. |
| 79 | ZHANG D, CHEN X, ZHANG R, et al. Development of β-amino acid dehydrogenase for the synthesis of β-amino acids via reductive amination of β-keto acids [J]. ACS Catalysis, 2015, 5(4): 2220-2224. |
| 80 | AHMED S T, LEFERINK N G, SCRUTTON N S. Chemo-enzymatic routes towards the synthesis of bio-based monomers and polymers [J]. Molecular Catalysis, 2019, 467: 95-110. |
| 81 | GE J, YANG X, YU H, et al. High-yield whole cell biosynthesis of Nylon 12 monomer with self-sufficient supply of multiple cofactors [J]. Metabolic Engineering, 2020, 62: 172-185. |
| 82 | WANG F, ZHAO J, LI Q, et al. One-pot biocatalytic route from cycloalkanes to α-ω-dicarboxylic acids by designed Escherichia coli consortia [J]. Nature Communications, 2020, 11: 5035. |
| 83 | ZHANG Z, LI Q, WANG F, et al. One-pot biosynthesis of 1,6-hexanediol from cyclohexane by de novo designed cascade biocatalysis [J]. Green Chemistry, 2020, 22(21): 7476-7483. |
| 84 | SARAK S, SUNG S, JEON H, et al. An integrated cofactor/co-product recycling cascade for the biosynthesis of nylon monomers from cycloalkylamines [J]. Angewandte Chemie International Edition, 2021, 60(7): 3481-3486. |
| 85 | 张建志, 付立豪, 唐婷, 等. 酶蛋白元件的规模化挖掘[J]. 合成生物学, 2020,1 (3): 267-284. |
| ZHANG J Z, FU L H, TANG T, et al. Scalable enzyme mining via synthetic biology [J]. Synthetic Biology Journal, 2020, 1(3): 267-284. | |
| 86 | 曲戈,朱彤,蒋迎迎, 等. 蛋白质工程:从定向进化到计算设计[J]. 生物工程学报, 2019, 35(10): 1843-1856. |
| QU G, ZHU T, JIANG Y Y, et al. Protein engineering: from directed evolution to computational design [J]. Chinese Journal of Biotechnology, 2019, 35(10): 1843-1856. | |
| 87 | 于勇, 朱欣娜, 张学礼. 大宗化学品细胞工厂的构建与应用[J]. 合成生物学,2020,1(6):674-684. |
| YU Y, ZHU X N, ZHANG X L. Construction and application of microbial cell factories for production of bulk chemicals [J]. Synthetic Biology Journal, 2020, 1(6): 674-684. | |
| 88 | WALSH C T, MOORE B S. Enzymatic cascade reactions in biosynthesis [J]. Angewandte Chemie International Edition, 2019, 58(21): 6846-6879. |
| 89 | HUFFMAN M A, FRYSZKOWSKA A, ALVIZO O, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir [J]. Science, 2019, 366(6470): 1255-1259. |
| 90 | RUDROFF F, MIHOVILOVIC M D, GROEGER H, et al. Opportunities and challenges for combining chemo- and biocatalysis [J]. Nature Catalysis, 2018, 1(1): 12-22. |
| 91 | HUANG X, CAO M, ZHAO H. Integrating biocatalysis with chemocatalysis for selective transformations [J]. Current Opinion in Chemical Biology, 2020, 55: 161-170. |
| 92 | LITMAN ZC, WANG Y, ZHAO H, et al. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis [J]. Nature, 2018, 560(7718): 355-359. |
| 93 | WU S K, ZHOU Y, GERNGROSS D, et al. Chemo-enzymatic cascades to produce cycloalkenes from bio-based resources [J]. Nature Communications, 2019, 10: 5060. |
| 94 | ZHANG W, LEE J H, YOUNES S H, et al. Photobiocatalytic synthesis of chiral secondary fatty alcohols from renewable unsaturated fatty acids [J]. Nature Communications, 2020, 11: 2258. |
| 95 | WEI X, HAN P, YOU C. Facilitation of cascade biocatalysis by artificial multi-enzyme complexes—a review [J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2799-2809. |
| 96 | KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux [J]. Nature Communications, 2019, 10: 4248. |
| 97 | NI J, GAO Y Y, TAO F, et al. Temperature-directed biocatalysis for the sustainable production of aromatic aldehydes or alcohols [J]. Angewandte Chemie International Edition, 2018, 57(5): 1214-1217. |
| 98 | NI J, WU Y T, TAO F, et al. A coenzyme-free biocatalyst for the value-added utilization of lignin-derived aromatics [J]. Journal of the American Chemical Society, 2018, 140(47): 16001-16005. |
| 99 | SCHWANDER T, BORZYSKOWSKI L S VON, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| 100 | LU X, LIU Y, YANG Y, et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design [J]. Nature Communications, 2019, 10: 1378. |
| [1] | WEN Yanhua, LIU Hedong, CAO Chunlai, WU Ruibo. Applications of protein engineering in pharmaceutical industry [J]. Synthetic Biology Journal, 2025, 6(1): 65-86. |
| [2] | CHENG Feng, ZOU Shuping, XU Jianmiao, TANG Heng, XUE Yaping, ZHENG Yuguo. BioHPP®: a benchmark of biomanufacturing for high optically pure L-phosphinothricin [J]. Synthetic Biology Journal, 2024, 5(6): 1404-1418. |
| [3] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [4] | FU Yu, ZHONG Fangrui. Recent advances in chemically driven enantioselective photobiocatalysis [J]. Synthetic Biology Journal, 2024, 5(5): 1021-1049. |
| [5] | MING Yang, CHEN Bin, HUANG Xiaoqiang. Recent advances in photoenzymatic synthesis [J]. Synthetic Biology Journal, 2023, 4(4): 651-675. |
| [6] | QI Yanping, ZHU Jin, ZHANG Kai, LIU Tong, WANG Yajie. Recent development of directed evolution in protein engineering [J]. Synthetic Biology Journal, 2022, 3(6): 1081-1108. |
| [7] | WANG Huibin, CHE Changli, YOU Song. Recent advances of enzymatic synthesis of organohalogens catalyzed by Fe/αKG-dependent halogenases [J]. Synthetic Biology Journal, 2022, 3(3): 545-566. |
| [8] | LOU Yujiao, XU Jian, WU Qi. Progress of biocatalytic deuteration of inert carbon-hydrogen bonds [J]. Synthetic Biology Journal, 2022, 3(3): 530-544. |
| [9] | YANG Lu, QU Xudong. Application of imine reductase in the synthesis of chiral amines [J]. Synthetic Biology Journal, 2022, 3(3): 516-529. |
| [10] | XIONG Liangbin, SONG Lu, ZHAO Yunqiu, LIU Kun, LIU Yongjun, WANG Fengqing, WEI Dongzhi. Green biomanufacturing of steroids: from biotransformation to de novo synthesis by microorganisms [J]. Synthetic Biology Journal, 2021, 2(6): 942-963. |
| [11] | ZHANG Faguang, QU Ge, SUN Zhoutong, MA Jun′an. From chemical synthesis to biosynthesis: trends toward total synthesis of natural products [J]. Synthetic Biology Journal, 2021, 2(5): 674-696. |
| [12] | TANG Heng, HAN Xin, ZOU Shuping, ZHENG Yuguo. Application of multi-enzyme catalytic system in the synthesis of pharmaceutical chemicals [J]. Synthetic Biology Journal, 2021, 2(4): 559-576. |
| [13] | ZHANG Yi-Heng. Remembering Professor Daniel I.C. Wang’s contribution to biorefining and my perspective on the progress [J]. Synthetic Biology Journal, 2021, 2(4): 497-508. |
| [14] | WANG Junting, GUO Xiaojia, LI Qing, WAN Li, ZHAO Zongbao. Creation of non-natural cofactor-dependent methanol dehydrogenase [J]. Synthetic Biology Journal, 2021, 2(4): 651-661. |
| [15] | SU Zhiguo. Great impact of Professor Daniel I.C. Wang and BPEC on development of biochemical engineering [J]. Synthetic Biology Journal, 2021, 2(4): 470-481. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||