Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (4): 559-576.DOI: 10.12211/2096-8280.2021-028
• Invited Review • Previous Articles Next Articles
Application of multi-enzyme catalytic system in the synthesis of pharmaceutical chemicals
TANG Heng, HAN Xin, ZOU Shuping, ZHENG Yuguo
- National and Local Joint Engineering Research Center for Chiral Biomanufacturing,College of Biotechnology and Bioengineering,Zhejiang University of Technology,Hangzhou 310014,Zhejiang,China
-
Received:2021-02-24Revised:2021-05-25Online:2021-09-10Published:2021-08-31 -
Contact:ZOU Shuping
多酶催化体系在医药化学品合成中的应用
汤恒, 韩鑫, 邹树平, 郑裕国
- 浙江工业大学生物工程学院手性生物制造国家地方联合工程研究中心,浙江 杭州 310014
-
通讯作者:邹树平 -
作者简介:汤恒 (1989—),男,博士,讲师。研究方向为酶工程。E-mail:tangheng@zjut.edu.cn邹树平 (1980—),男,博士,教授,博士生导师。研究方向为医药化学品生物催化合成。E-mail:zousp@zjut.edu.cn -
基金资助:国家重点研究计划(2019YFA0905000)
CLC Number:
Cite this article
TANG Heng, HAN Xin, ZOU Shuping, ZHENG Yuguo. Application of multi-enzyme catalytic system in the synthesis of pharmaceutical chemicals[J]. Synthetic Biology Journal, 2021, 2(4): 559-576.
汤恒, 韩鑫, 邹树平, 郑裕国. 多酶催化体系在医药化学品合成中的应用[J]. 合成生物学, 2021, 2(4): 559-576.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-028
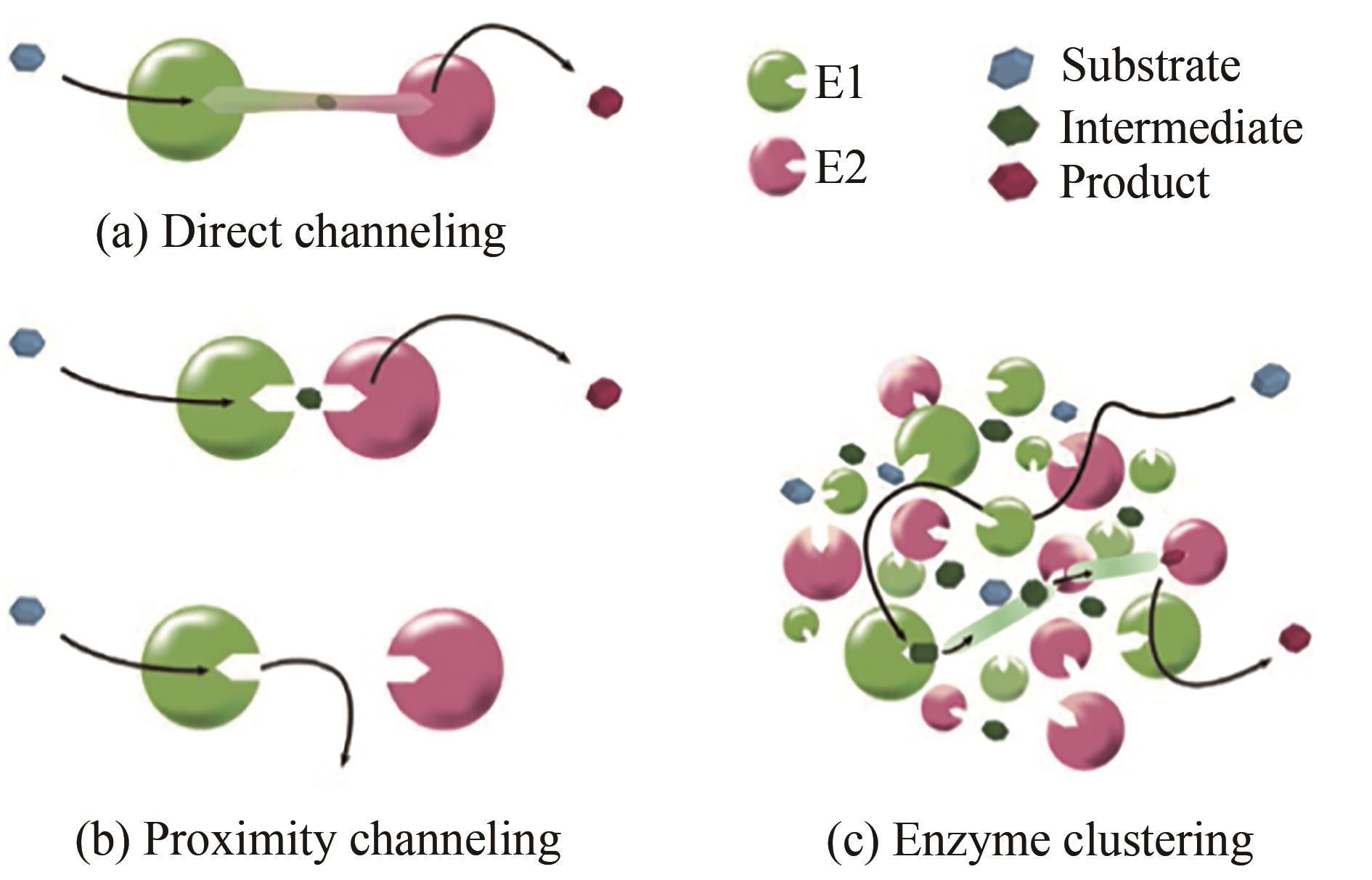
| 1 | CASTELLANA M, WILSON M Z, XU Y, et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling [J]. Nature Biotechnology, 2014, 32(10): 1011-1018. |
| 2 | WARNECKE T, GILL R T. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications [J]. Microbial Cell Factories, 2005, 4: 25. |
| 3 | GRAHAM J W A, WILLIAMS T C R, MORGAN M, et al. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling [J]. The Plant Cell, 2007, 19(11): 3723-3738. |
| 4 | HAGGIE P M, VERKMAN A S. Diffusion of tricarboxylic acid cycle enzymes in the mitochondrial matrix in vivo. Evidence for restricted mobility of a multienzyme complex [J]. The Journal of Biological Chemistry, 2002, 277(43): 40782-40788. |
| 5 | DING S Y, HIMMEL M E. The maize primary cell wall microfibril: a new model derived from direct visualization [J]. Journal of Agricultural and Food Chemistry, 2006, 54(3): 597-606. |
| 6 | GECK M K, KIRSCH J F. A novel, definitive test for substrate channeling illustrated with the aspartate aminotransferase/malate dehydrogenase system [J]. Biochemistry, 1999, 38(25): 8032-8037. |
| 7 | ZHANG Y H. Substrate channeling and enzyme complexes for biotechnological applications [J]. Biotechnology Advances, 2011, 29(6): 715-725. |
| 8 | WOODLEY J M. Microbial biocatalytic processes and their development [J]. Advances in Applied Microbiology, 2006, 60: 1-15. |
| 9 | 许可, 吕波, 李春. 无细胞的合成生物技术——多酶催化与生物合成[J]. 中国科学:化学, 2015, 45(5): 429-437. |
| XU K, LÜ B, LI C. Cell-free synthetic biotechnology — multi-enzyme catalysis and biosynthesis[J]. Scientia Sinica Chimica, 2015, 45(5): 429-437. | |
| 10 | SCHOFFELEN S, HEST J C M VAN. Multi-enzyme systems: bringing enzymes together in vitro [J]. Soft Matter, 2012, 8(6): 1736-1746. |
| 11 | FEIST A M, PALSSON B Ø. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli [J]. Nature Biotechnology, 2008, 26(6): 659-667. |
| 12 | HAWKINS K M, SMOLKE C D. Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae [J]. Nature Chemical Biology, 2008, 4(9): 564-573. |
| 13 | FRANCE S P, HEPWORTH L J, TURNER N J, et al. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways [J]. ACS Catalysis, 2016, 7(1): 710-724. |
| 14 | BACHMANN B O. Biosynthesis: Is it time to go retro? [J]. Nature Chemical Biology, 2010, 6(6): 390-393. |
| 15 | DELÉPINE B, DUIGOU T, CARBONELL P, et al. RetroPath2.0: A retrosynthesis workflow for metabolic engineers [J]. Metabolic Engineering, 2018, 45: 158-170. |
| 16 | KUMAR A, WANG L, NG C Y, et al. Pathway design using de novo steps through uncharted biochemical spaces [J]. Nature Communications, 2018, 9(1): 184. |
| 17 | SHI J F, WU Y Z, ZHANG S H, et al. Bioinspired construction of multi-enzyme catalytic systems [J]. Chemical Society Reviews, 2018, 47(12): 4295-4313. |
| 18 | MUTTI F G, KNAUS T, SCRUTTON N S, et al. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades [J]. Science, 2015, 349(6255): 1525-1529. |
| 19 | KIM Y H, CAMPBELL E, YU J, et al. Complete oxidation of methanol in biobattery devices using a hydrogel created from three modified dehydrogenases[J]. Angewandte Chemie (International Ed in English), 2013, 52(5): 1437-1440. |
| 20 | ANDRE C, KIM S W, YU X H, et al. Fusing catalase to an alkane-producing enzyme maintains enzymatic activity by converting the inhibitory byproduct H2O2 to the cosubstrate O2 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(8): 3191-3196. |
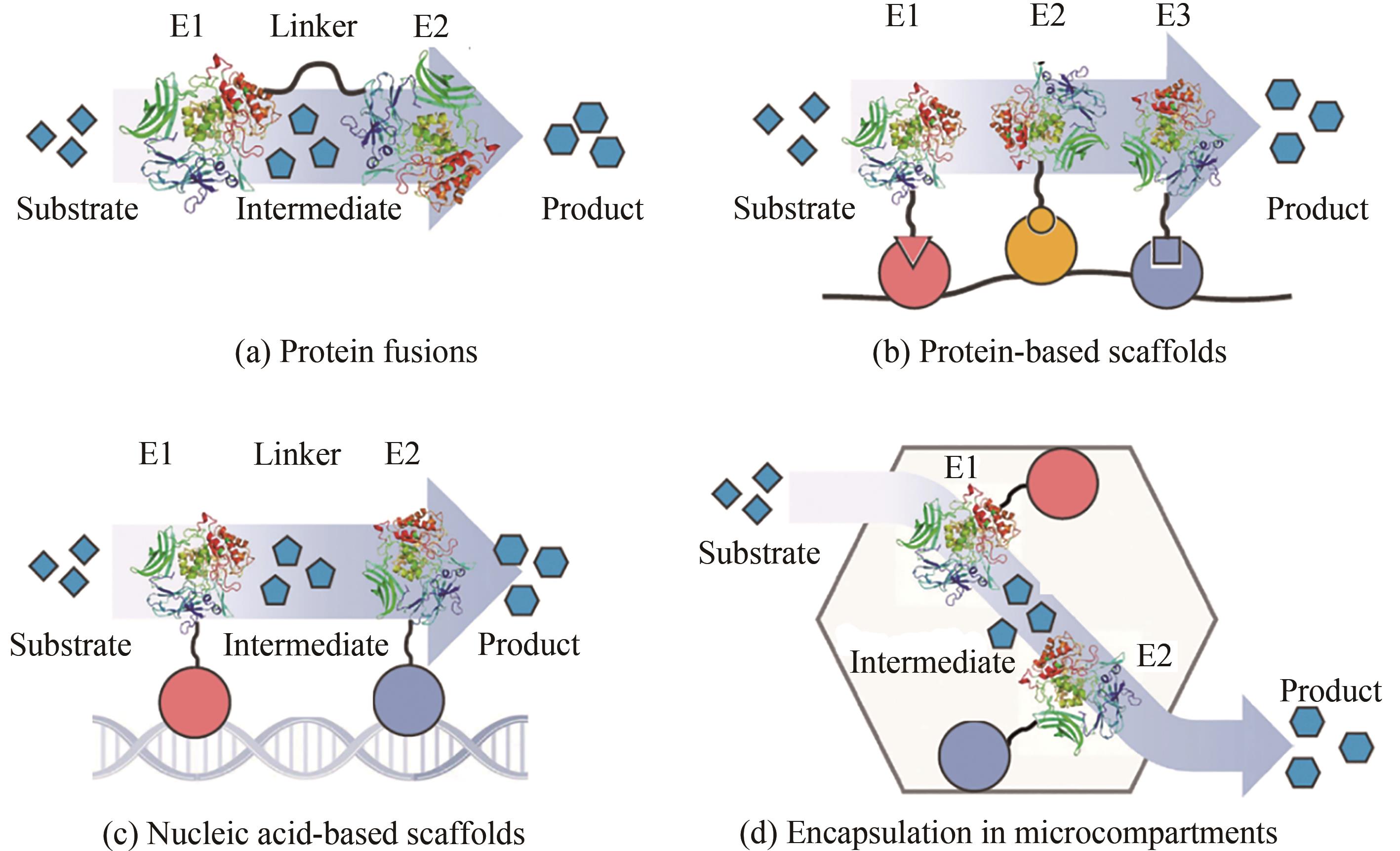
| 21 | QUIN M B, WALLIN K K, ZHANG G, et al. Spatial organization of multi-enzyme biocatalytic cascades [J]. Organic & Biomolecular Chemistry, 2017, 15(20): 4260-4271. |
| 22 | ZHANG G Q, QUIN M B, SCHMIDT-DANNERT C. Self-assembling protein scaffold system for easy in vitro coimmobilization of biocatalytic cascade enzymes [J]. ACS Catalysis, 2018, 8(6): 5611-5620. |
| 23 | DELEBECQUE C J, LINDNER A B, SILVER P A, et al. Organization of intracellular reactions with rationally designed RNA assemblies [J]. Science, 2011, 333(6041): 470-474. |
| 24 | MYHRVOLD C, POLKA J K, SILVER P A. Synthetic lipid-containing scaffolds enhance production by colocalizing enzymes[J]. ACS Synthetic Biology, 2016, 5(12): 1396-1403. |
| 25 | POLKA J K, HAYS S G, SILVER P A. Building spatial synthetic biology with compartments, scaffolds, and communities [J]. Cold Spring Harbor Perspectives in Biology, 2016, 8(8): a024018. |
| 26 | AGAPAKIS C M, BOYLE P M, SILVER P A. Natural strategies for the spatial optimization of metabolism in synthetic biology [J]. Nature Chemical Biology, 2012, 8(6): 527-535. |
| 27 | GIESSEN T W, SILVER P A. Encapsulation as a strategy for the design of biological compartmentalization[J]. Journal of Molecular Biology, 2016, 428(5pt b): 916-927. |
| 28 | LEE H, DELOACHE W C, DUEBER J E. Spatial organization of enzymes for metabolic engineering [J]. Metabolic Engineering, 2012, 14(3): 242-251. |
| 29 | YEATES T O, CROWLEY C S, TANAKA S. Bacterial microcompartment organelles: protein shell structure and evolution [J]. Annual Review of Biophysics, 2010, 39: 185-205. |
| 30 | BOBIK T A, LEHMAN B P, YEATES T O. Bacterial microcompartments: widespread prokaryotic organelles for isolation and optimization of metabolic pathways [J]. Molecular Microbiology, 2015, 98(2): 193-207. |
| 31 | LAWRENCE A D, FRANK S, NEWNHAM S, et al. Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor [J]. ACS Synthetic Biology, 2014, 3(7): 454-465. |
| 32 | LI C, ZHANG R, WANG J, et al. Protein engineering for improving and diversifying natural product biosynthesis [J]. Trends in Biotechnology, 2020, 38(7): 729-744. |
| 33 | XUE R, WOODLEY J M. Process technology for multi-enzymatic reaction systems [J]. Bioresource Technology, 2012, 115: 183-195. |
| 34 | WAHL C, HIRTZ D, ELLING L. Multiplexed capillary electrophoresis as analytical tool for fast optimization of multi-enzyme cascade reactions - synthesis of nucleotide sugars: Dedicated to Prof. Dr. Vladimir Křen on the occasion of his 60th birthday [J]. Biotechnology Journal, 2016, 11(10): 1298-1308. |
| 35 | ARANAZ I, ACOSTA N, FéRNANDEZ-VALLE M E, et al. Optimization of D-amino acid production catalyzed by immobilized multi-enzyme system in polyelectrolyte complex gel capsules [J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 121: 45-52. |
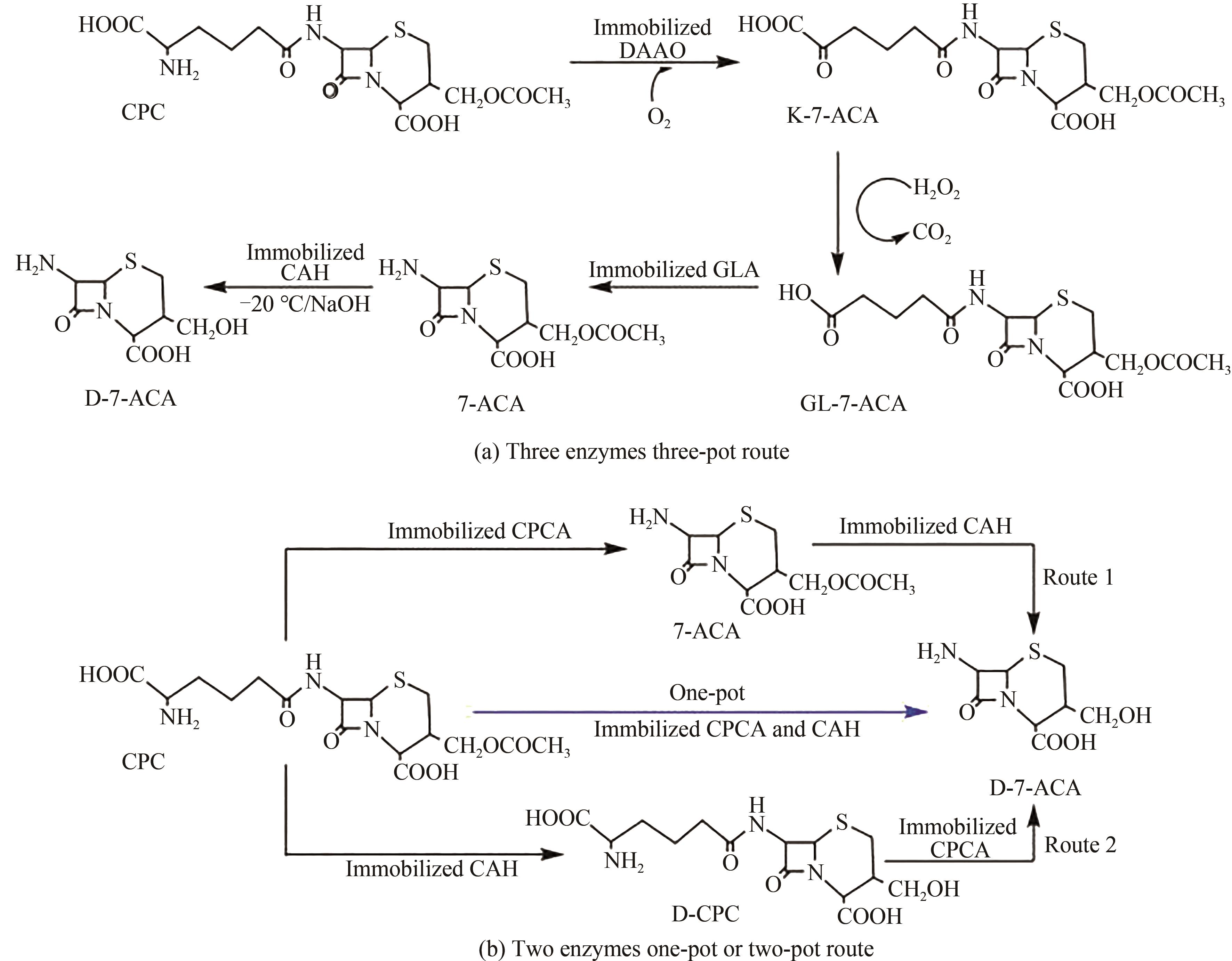
| 36 | KIM Y, YOON K, KHANG Y, et al. The 2.0 Å crystal structure of cephalosporin acylase [J]. Structure, 2000, 8(10): 1059-1068. |
| 37 | HAMAD B. The antibiotics market [J]. Nature Reviews Drug Discovery, 2010, 9(9): 675-676. |
| 38 | DING J M, ZHOU Y, ZHU H J, et al. Characterization of EstZY: a new acetylesterase with 7-aminocephalosporanic acid deacetylase activity from Alicyclobacillus tengchongensis [J]. International Journal of Biological Macromolecules, 2020, 148: 333-341. |
| 39 | TAKIMOTO A, TAKAKURA T, TANI H, et al. Batch production of deacetyl 7-aminocephalosporanic acid by immobilized cephalosporin-C deacetylase [J]. Applied Microbiology and Biotechnology, 2004, 65(3): 263-267. |
| 40 | YAMANAKA H, CHIBA T, KAWABATA K, et al. Studies on β-lactam antibiotics IX. Synthesis and biological activity of a new orally active cephalosporin, cefixime (FK027) [J]. The Journal of Antibiotics, 1985, 38(12): 1738-1751. |
| 41 | GONZÁLEZ M, RODRÍGUEZ Z, TOLÓN B, et al. An alternative procedure for preparation of cefdinir [J]. Farmaco, 2003, 58(6): 409-418. |
| 42 | MA X Q, DENG S W, SU E Z, et al. One-pot enzymatic production of deacetyl-7-aminocephalosporanic acid from cephalosporin C via immobilized cephalosporin C acylase and deacetylase [J]. Biochemical Engineering Journal, 2015, 95: 1-8. |
| 43 | JIANG J J, CHEN X, ZHANG D L, et al. Characterization of (R)-selective amine transaminases identified by in silico motif sequence blast [J]. Applied Microbiology and Biotechnology, 2015, 99(6): 2613-2621. |
| 44 | LEY S V, PRIOUR A. Total synthesis of the cyclic peptide Argyrin B [J]. European Journal of Organic Chemistry, 2002(23): 3995-4004. |
| 45 | SHAGINIAN A, ROSEN M C, BINKOWSKI B F, et al. Solid-phase synthesis of dihydrovirginiamycin S1, a streptogramin B antibiotic [J]. Chemistry, 2004, 10(17): 4334-4340. |
| 46 | DEATON D N, GRAHAM K P, GROSS J W, et al. Thiol-based angiotensin-converting enzyme 2 inhibitors: P1' modifications for the exploration of the S1' subsite [J]. Bioorganic & Medicinal Chemistry Letters, 2008, 18(5): 1681-1687. |
| 47 | AUBELE D L, HOM R K, ADLER M, et al. Selective and brain-permeable polo-like kinase-2 (Plk-2) inhibitors that reduce α-synuclein phosphorylation in rat brain [J]. ChemMedChem, 2013, 8(8): 1295-1313. |
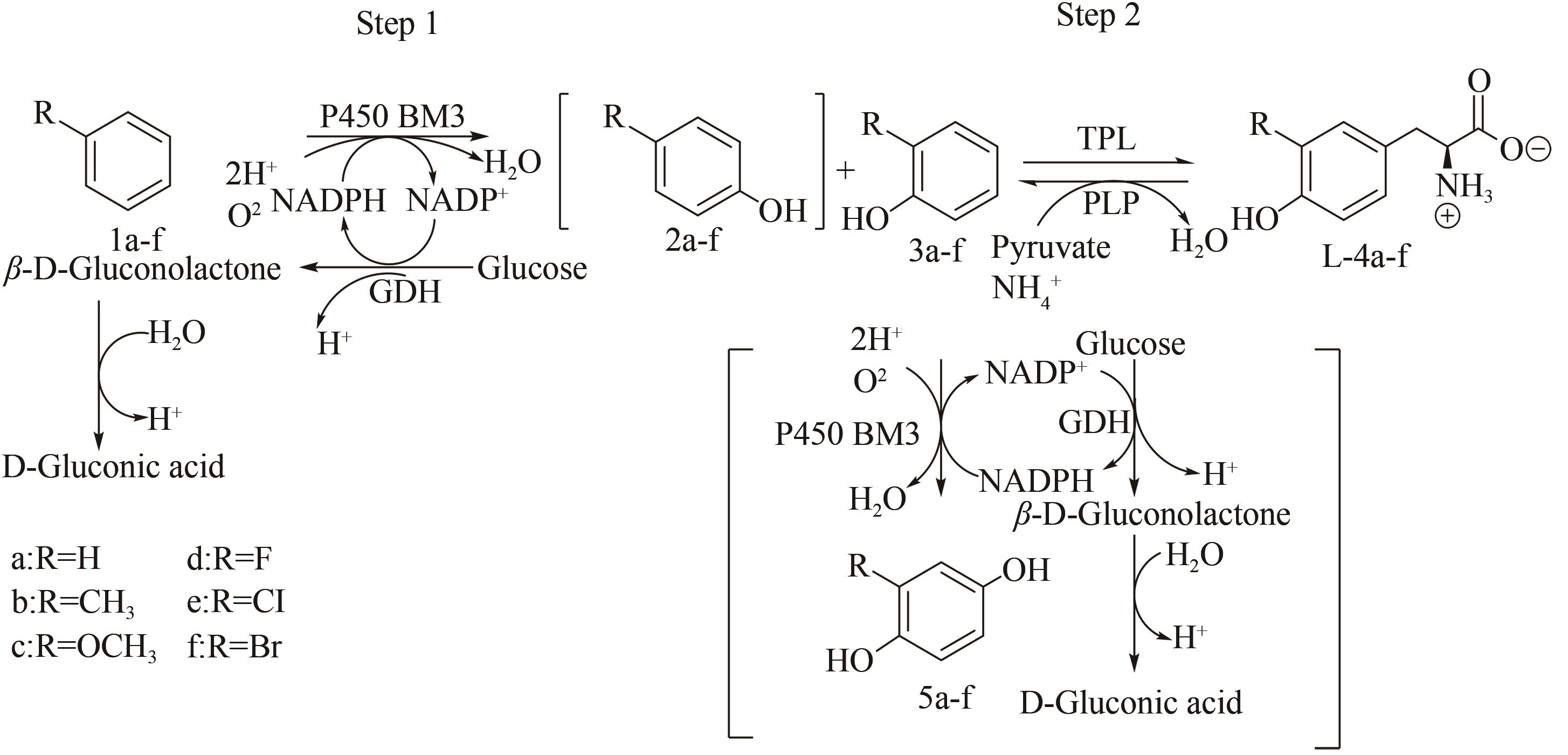
| 48 | SCHAROW A, KNAPPE D, REINDL W, et al. Development of bifunctional inhibitors of polo-like Kinase 1 with low-nanomolar activities against the polo-box domain [J]. ChemBioChem, 2016, 17(8): 759-767. |
| 49 | BEHRENDS M, WAGNER S, KOPKA K, et al. New matrix metalloproteinase inhibitors based on γ-fluorinated α-aminocarboxylic and α-aminohydroxamic acids [J]. Bioorganic & Medicinal Chemistry, 2015, 23(13): 3809-3818. |
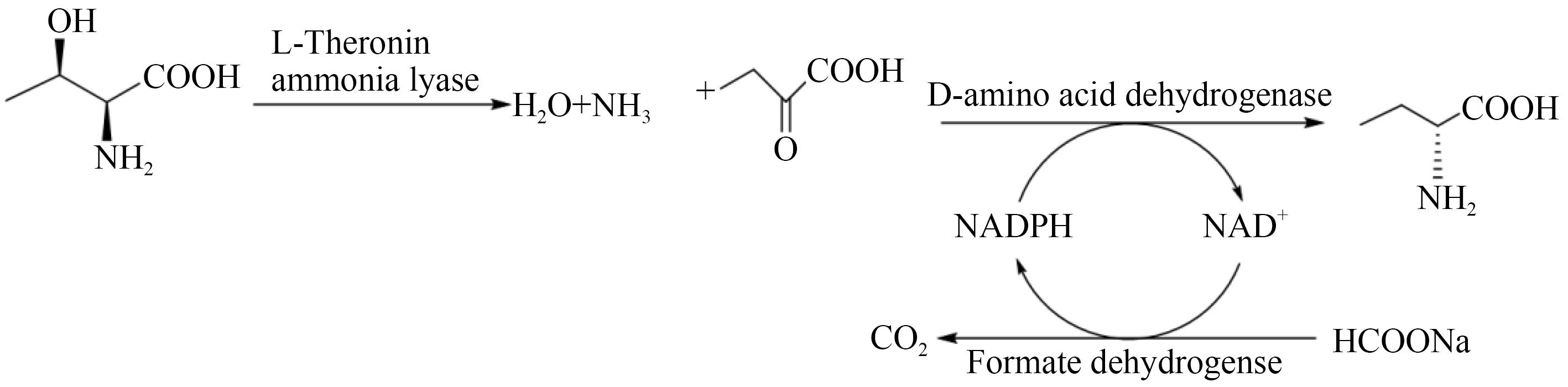
| 50 | CHEN X, CUI Y F, CHENG X K, et al. Highly atom economic synthesis of d-2-aminobutyric acid through an in vitro tri-enzymatic catalytic system [J]. ChemistryOpen, 2017, 6(4): 534-540. |
| 51 | SHINDE P, BANERJEE P, MANDHARE A. Marine natural products as source of new drugs: a patent review (2015—2018) [J]. Expert Opinion on Therapeutic Patents, 2019, 29(4): 283-309. |
| 52 | LIU J, HU K F, QU J P, et al. Organopromoted selectivity-switchable synthesis of polyketones [J]. Organic Letters, 2017, 19(20): 5593-5596. |
| 53 | BRAÏEK O BEN, SMAOUI S, SMAOUI S. Enterococci: between emerging pathogens and potential probiotics [J]. BioMed Research International, 2019, 2019: 5938210. |
| 54 | CHENG Q, XIANG L K, IZUMIKAWA M, et al. Enzymatic total synthesis of enterocin polyketides [J]. Nature Chemical Biology, 2007, 3(9): 557-558. |
| 55 | LEE G E, JOSHI B V, CHEN W, et al. Synthesis and structure-activity relationship studies of tyrosine-based antagonists at the human P2X7 receptor [J]. Bioorganic & Medicinal Chemistry Letters, 2008, 18(2): 571-575. |
| 56 | CHEN P W, LEE N C, CHIEN Y H, et al. Diagnosis of aromatic L-amino acid decarboxylase deficiency by measuring 3-O-methyldopa concentrations in dried blood spots [J]. Clinica Chimica Acta, International Journal of Clinical Chemistry, 2014, 431: 19-22. |
| 57 | DONG W F, LIU W, LIAO X W, et al. Asymmetric total synthesis of (-)-saframycin A from L-tyrosine [J]. The Journal of Organic Chemistry, 2011, 76(13): 5363-5368. |
| 58 | OHTAKE K, YAMAGUCHI A, MUKAI T, et al. Protein stabilization utilizing a redefined codon [J]. Scientific Reports, 2015, 5: 9762. |
| 59 | MCCUBBIN J A, MADDESS M L, LAUTENS M. Total synthesis of cryptophycin analogues via a scaffold approach[J]. Organic Letters, 2006, 8(14): 2993-2996. |
| 60 | CHEN X C, ZHU J. Total synthesis of the marine natural product (-)‐Cribrostatin 4 (Renieramycin H) [J]. Angewandte Chemie International Edition, 2007, 46(21): 3962-3965. |
| 61 | SEYEDSAYAMDOST M R, REECE S Y, NOCERA D G, et al. Mono-, di-, tri-, and tetra-substituted fluorotyrosines: new probes for enzymes that use tyrosyl radicals in catalysis [J]. Journal of the American Chemical Society, 2006, 128(5): 1569-1579. |
| 62 | NATARAJAN A, SCHWANS J P, HERSCHLAG D. Using unnatural amino acids to probe the energetics of oxyanion hole hydrogen bonds in the ketosteroid isomerase active site [J]. Journal of the American Chemical Society, 2014, 136(21): 7643-7654. |
| 63 | LI F H, SHI P, LI J S, et al. A genetically encoded 19F NMR probe for tyrosine phosphorylation [J]. Angewandte Chemie (International Ed in English), 2013, 52(14): 3958-3962. |
| 64 | DI STEFANO A, SOZIO P, CERASA L S. Antiparkinson prodrugs [J]. Molecules, 2008, 13(1): 46-68. |
| 65 | SWOBODA K J, SAUL J P, MCKENNA C E, et al. Aromatic L‐amino acid decarboxylase deficiency: overview of clinical features and outcomes [J]. Annals of Neurology, 2003, 54(S6): S49-S55. |
| 66 | DENNIG A, BUSTO E, KROUTIL W, et al. Biocatalytic one-pot synthesis of L-tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia [J]. ACS Catalysis, 2015, 5(12): 7503-7506. |
| 67 | DAMARAJU V L, DAMARAJU S, YOUNG J D, et al. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy [J]. Oncogene, 2003, 22(47): 7524-7536. |
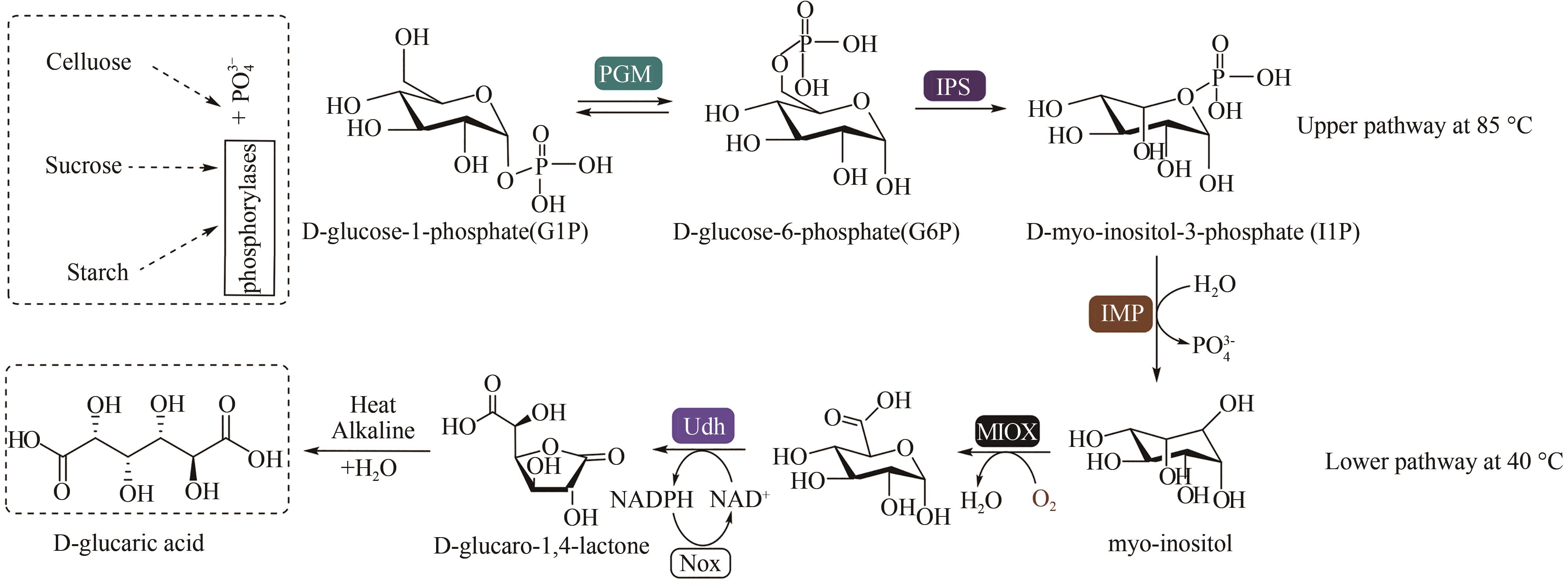
| 68 | ROBAK T, LECH-MARANDA E, KORYCKA A, et al. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity [J]. Current Medicinal Chemistry, 2006, 13(26): 3165-3189. |
| 69 | MESAROS C, ARORA J S, WHOLER A, et al. 8-Oxo-2'-deoxyguanosine as a biomarker of tobacco-smoking-induced oxidative stress [J]. Free Radical Biology & Medicine, 2012, 53(3): 610-617. |
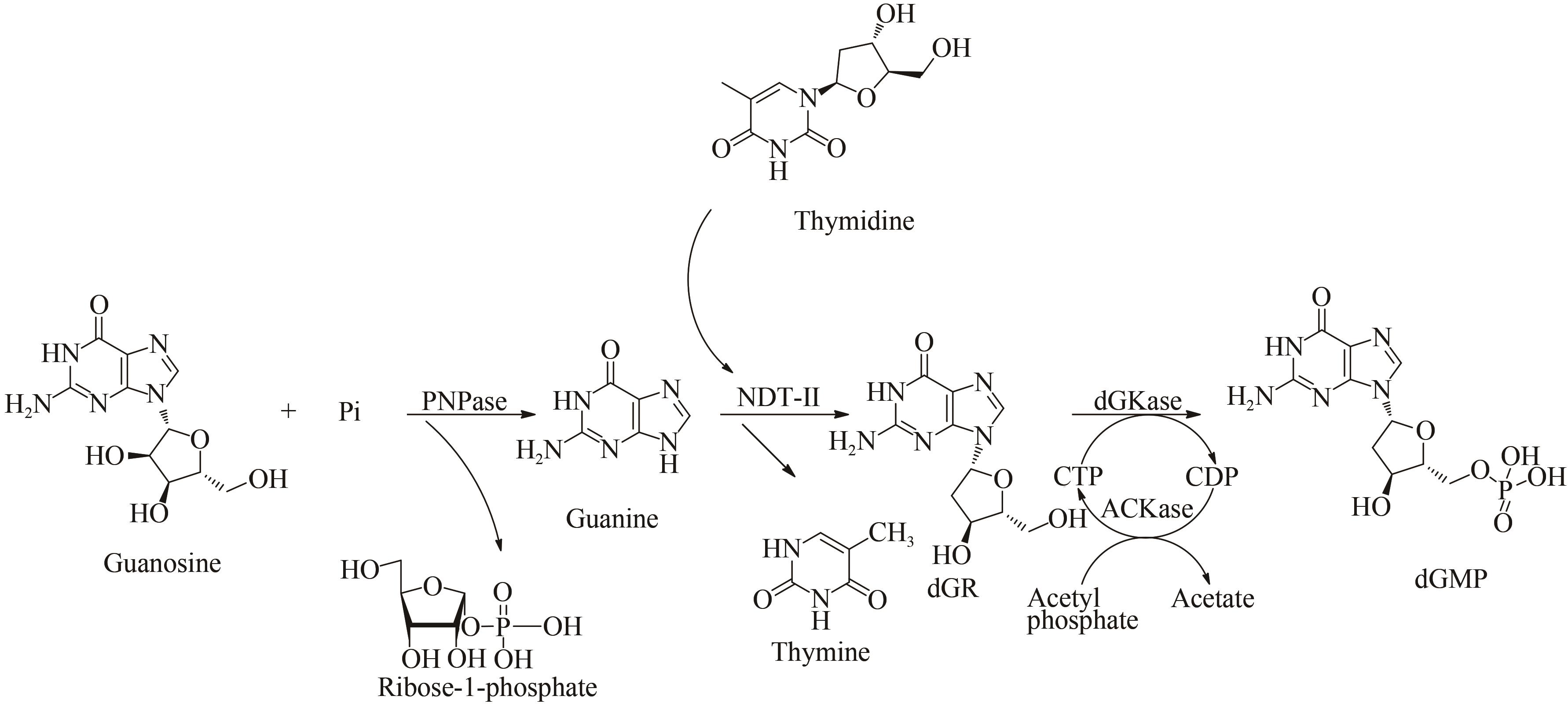
| 70 | LI Y Y, DING Q B, OU L, et al. One-pot process of 2'-deoxyguanylic acid catalyzed by a multi-enzyme system [J]. Biotechnology and Bioprocess Engineering, 2015, 20(1): 37-43. |
| 71 | ENDO A. The origin of the statins [J]. International Congress Series, 2004, 1262: 3-8. |
| 72 | PATEL R N. Biocatalysis for synthesis of pharmaceuticals [J]. Bioorganic & Medicinal Chemistry, 2018, 26(7): 1252-1274. |
| 73 | SIERRA S, RAMOS M C, MOLINA P, et al. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death [J]. Journal of Alzheimer's Disease, 2011, 23(2): 307-318. |
| 74 | HOYOS P, PACE V, ALCÁNTARA A R. Biocatalyzed synthesis of statins: a sustainable strategy for the preparation of valuable drugs [J]. Catalysts, 2019, 9(3): 260. |
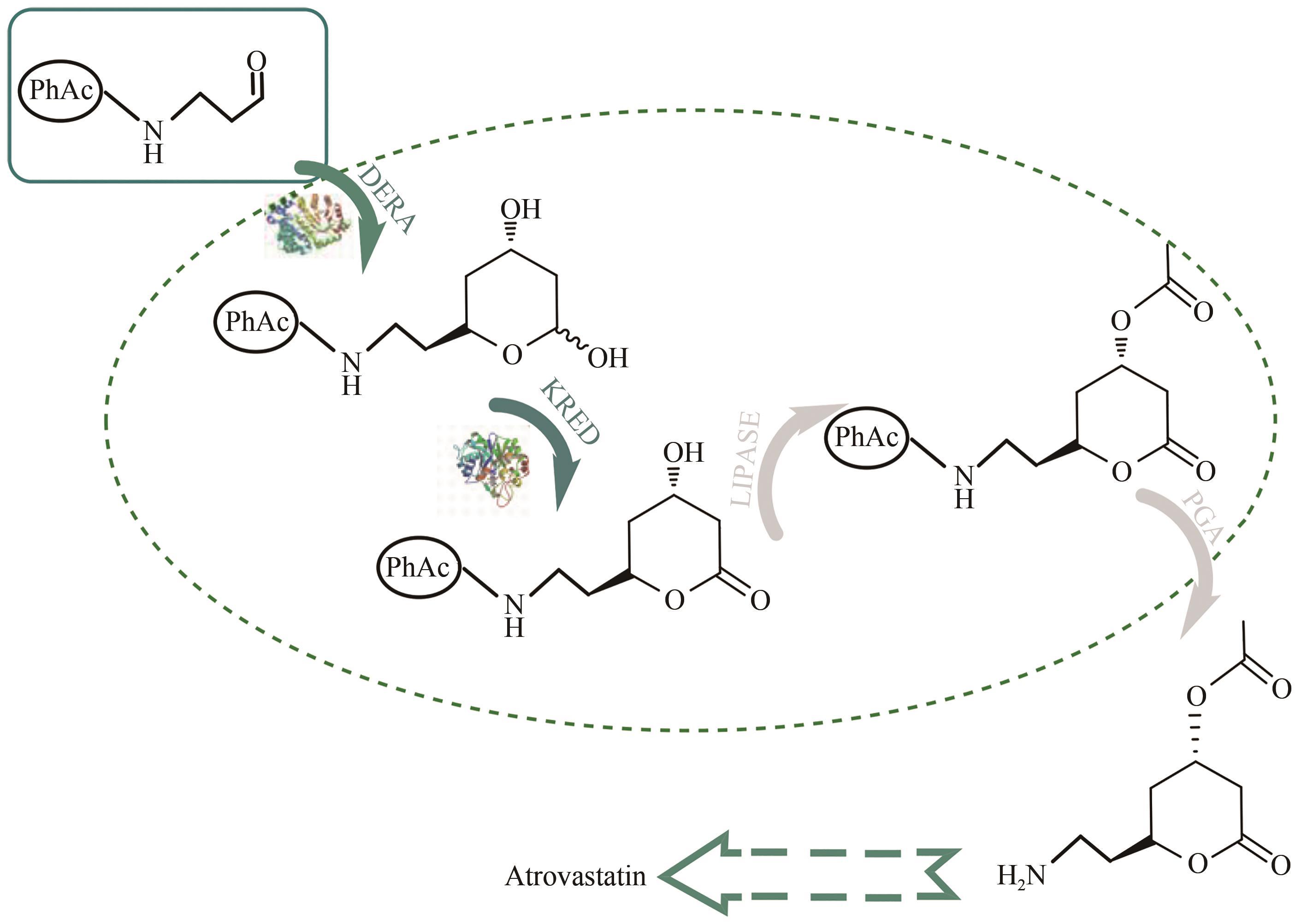
| 75 | ŠVARC A, FEKETE M, HERNANDEZ K, et al. An innovative route for the production of atorvastatin side-chain precursor by DERA-catalysed double aldol addition [J]. Chemical Engineering Science, 2021, 231: 116312. |
| 76 | LINDOR K D, KOWDLEY K V, HEATHCOTE E J, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial[J]. Hepatology, 2004, 39(3): 770-778. |
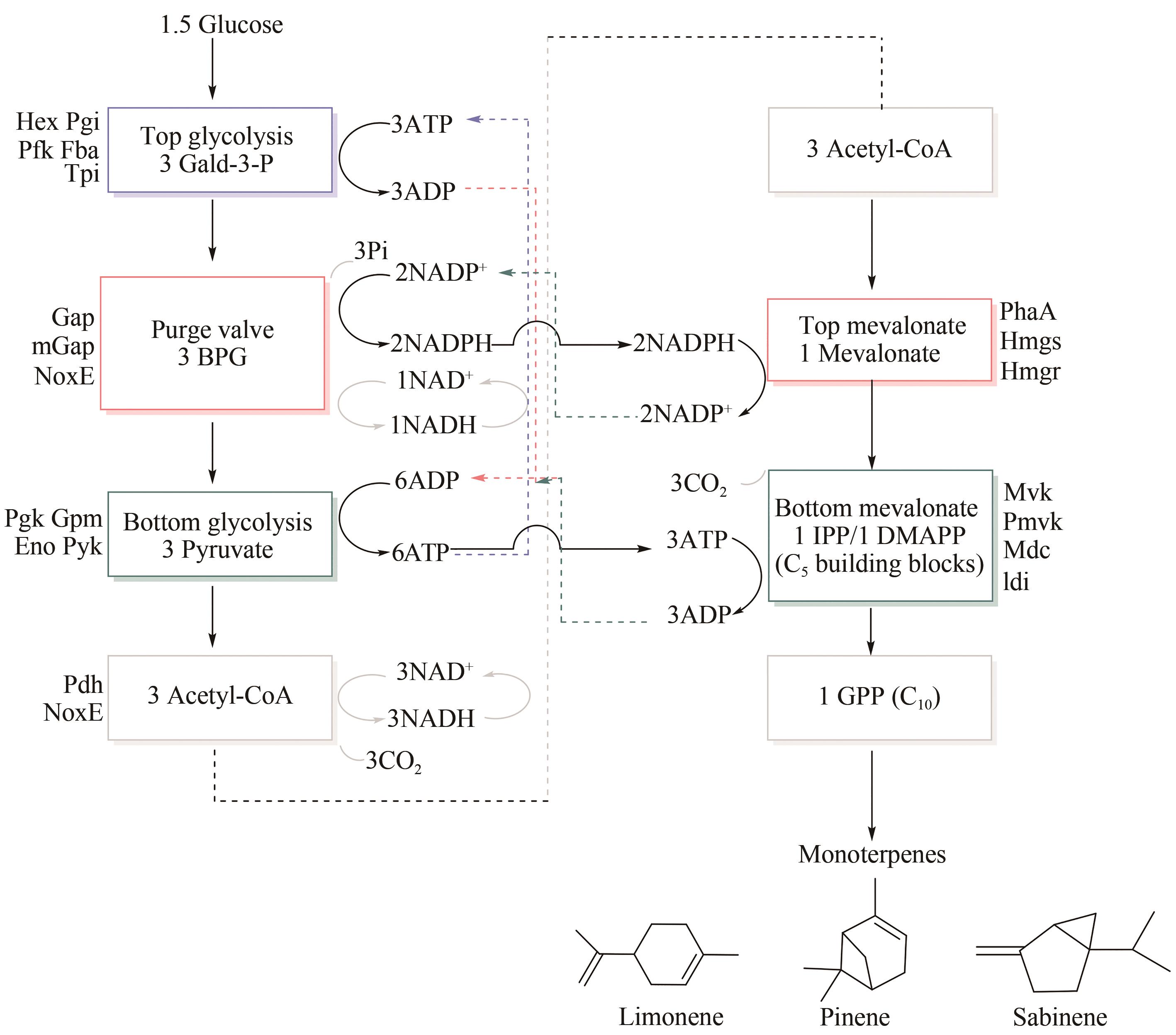
| 77 | ZHANG Y J, ZHENG X J, HUANG F J, et al. Ursodeoxycholic acid alters bile acid and fatty acid profiles in a mouse model of diet-induced obesity [J]. Frontiers in Pharmacology, 2019, 10: 842. |
| 78 | HANAFI N I, MOHAMED A S, SHEIKH A K S H, et al. Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart [J]. Biomolecules, 2018, 8(4): 159. |
| 79 | HIRSCHFIELD G M, MASON A, LUKETIC V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid [J]. Gastroenterology, 2015, 148(4): 751-61.e8. |
| 80 | HE H W, MENNONE A, BOYER J L, et al. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells [J]. Hepatology, 2011, 53(2): 548-557. |
| 81 | TONIN F, ARENDS I W. Latest development in the synthesis of ursodeoxycholic acid (UDCA): a critical review [J]. Beilstein Journal of Organic Chemistry, 2018, 14(1): 470-483. |
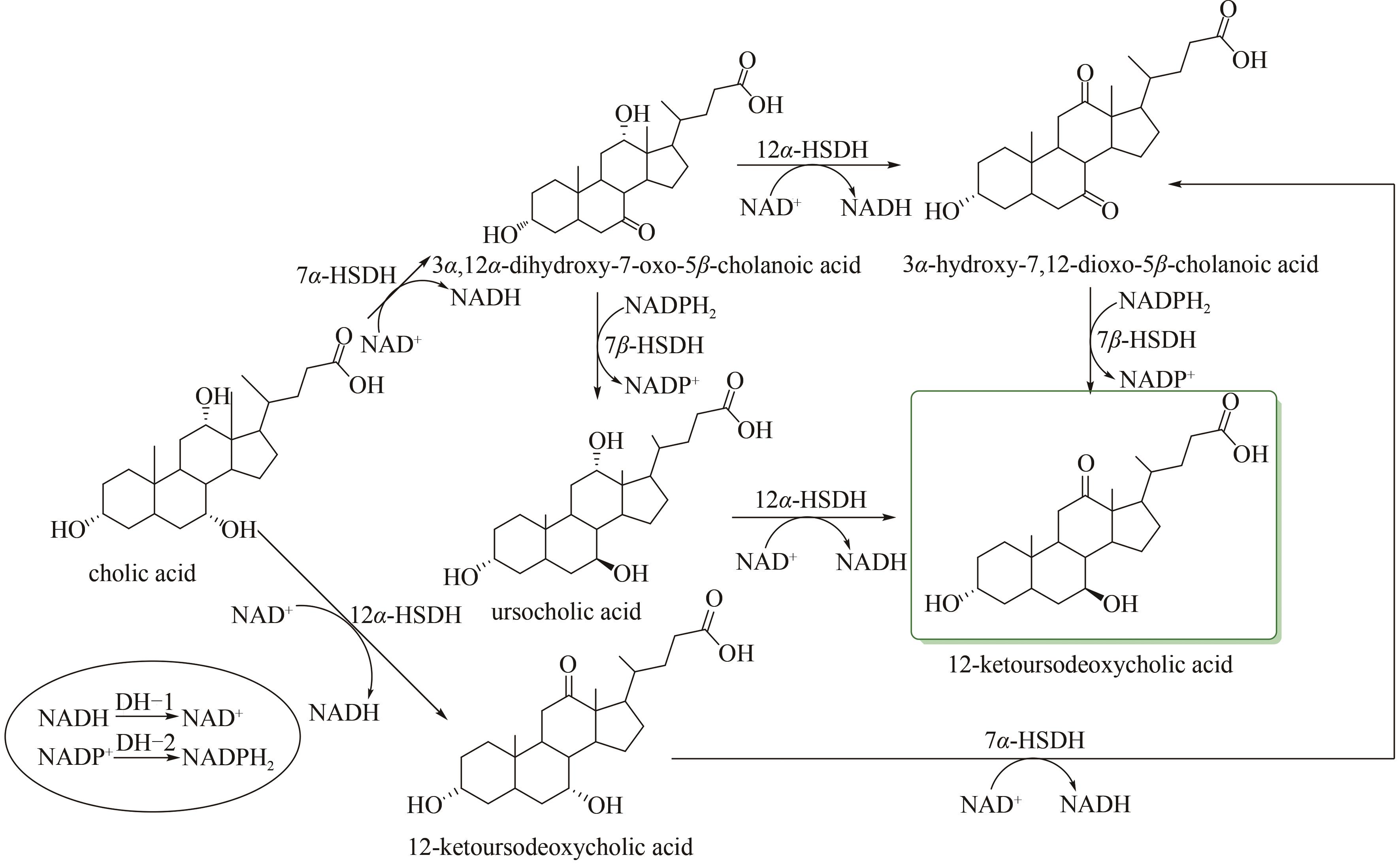
| 82 | POLYKETIDES N T S O E, MONTI D, FERRANDI E E, et al. One-Pot multienzymatic synthesis of 12-ketoursodeoxycholic acid: subtle cofactor specificities rule the reaction equilibria of five biocatalysts working in a row [J]. Advanced Synthesis & Catalysis, 2009, 351(9): 1303-1311. |
| 83 | PANDEY R K, FERNANDES R A, KUMAR P. An asymmetric dihydroxylation route to enantiomerically pure norfluoxetine and fluoxetine [J]. Tetrahedron Letters, 2002, 43(25): 4425-4426. |
| 84 | KUMAR P, UPADHYAY R K, PANDEY R K. Asymmetric dihydroxylation route to (R)-isoprenaline,(R)-norfluoxetine and (R)-fluoxetine [J]. Tetrahedron: Asymmetry, 2004, 15(24): 3955-3959. |
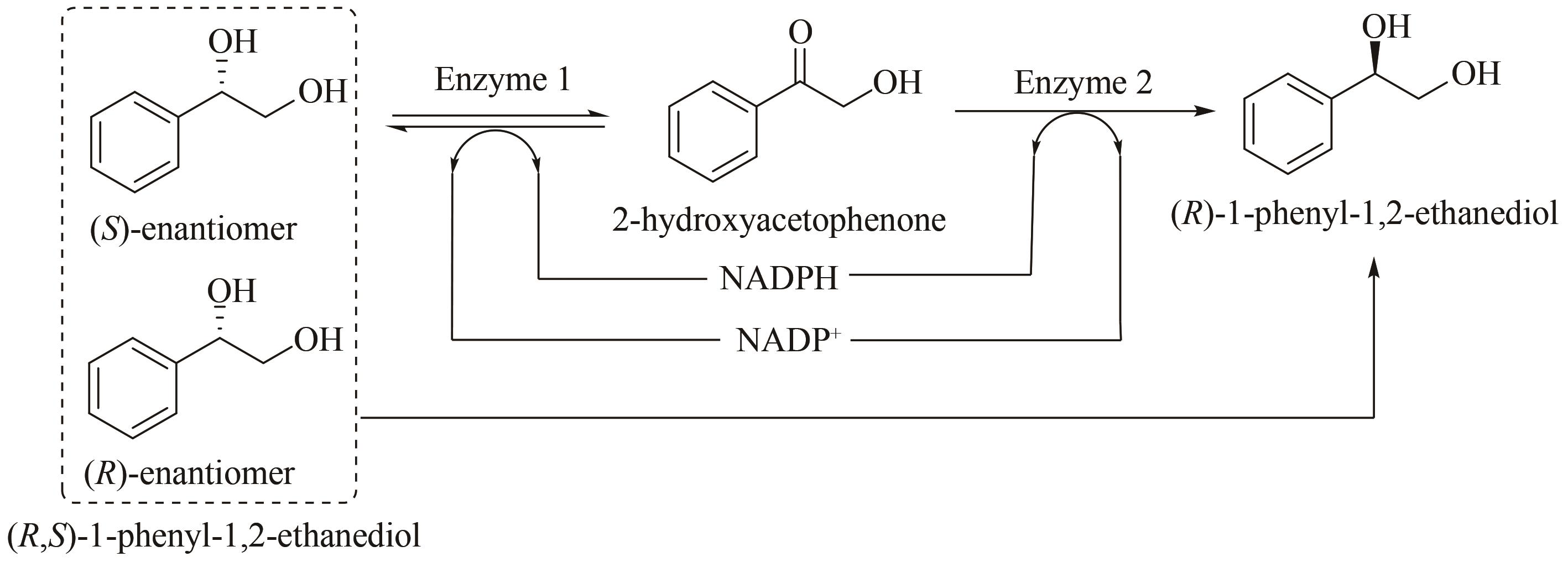
| 85 | CAO L, LEE J, CHEN W, et al. Enantioconvergent production of (R)-1-phenyl-1,2-ethanediol from styrene oxide by combining the Solanum tuberosum and an evolved Agrobacterium radiobacter AD1 epoxide hydrolases [J]. Biotechnology and Bioengineering, 2006, 94(3): 522-529. |
| 86 | HU Q S, XU Y, NIE Y. Highly enantioselective reduction of 2-hydroxy-1-phenylethanone to enantiopure (R)-phenyl-1,2-ethanediol using Saccharomyces cerevisiae of remarkable reaction stability [J]. Bioresource Technology, 2010, 101(22): 8502-8508. |
| 87 | LI B, NIE Y, MU X Q, et al. De novo construction of multi-enzyme system for one-pot deracemization of (R,S)-1-phenyl-1,2-ethanediol by stereoinversion of (S)-enantiomer to the corresponding counterpart [J]. Journal of Molecular Catalysis B: Enzymatic, 2016, 129: 21-28. |
| 88 | RAMACHANDRAN S, FONTANILLE P, PANDEY A, et al. Gluconic acid: properties, applications and microbial production [J]. Food Technology & Biotechnology, 2006, 44(2): 185-195. |
| 89 | ANASTASSIADIS S, MORGUNOV I G. Gluconic acid production [J]. Recent Patents on Biotechnology, 2007, 1(2): 167-180. |
| 90 | SU H H, GUO Z W, WU X L, et al. Efficient bioconversion of sucrose to high-value-added glucaric acid by in vitro metabolic engineering [J]. ChemSusChem, 2019, 12(10): 2278-2285. |
| 91 | ZHAO F H, LI H, JIANG Y J, et al. Co-immobilization of multi-enzyme on control-reduced graphene oxide by non-covalent bonds: an artificial biocatalytic system for the one-pot production of gluconic acid from starch [J]. Green Chemistry, 2014, 16(5): 2558-2565. |
| 92 | PETROLL K, CARE A, BERGQUIST P L, et al. A novel framework for the cell-free enzymatic production of glucaric acid [J]. Metabolic Engineering, 2020, 57: 162-173. |
| 93 | PETROLL K, KOPP D, CARE A, et al. Tools and strategies for constructing cell-free enzyme pathways [J]. Biotechnology Advances, 2019, 37(1): 91-108. |
| 94 | OLDFIELD E, LIN F Y. Terpene biosynthesis: modularity rules [J]. Angewandte Chemie International Edition, 2012, 51(5): 1124-1137. |
| 95 | DIXON R A. Plant natural products: the molecular genetic basis of biosynthetic diversity [J]. Current Opinion in Biotechnology, 1999, 10(2): 192-197. |
| 96 | WITHERS S T, KEASLING J D. Biosynthesis and engineering of isoprenoid small molecules [J]. Applied Microbiology and Biotechnology, 2007, 73(5): 980-990. |
| 97 | KRINGS U, BERGER R G. Biotechnological production of flavours and fragrances [J]. Applied Microbiology and Biotechnology, 1998, 49(1): 1-8. |
| 98 | AJIKUMAR P K, TYO K, CARLSEN S, et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms [J]. Molecular Pharmaceutics, 2008, 5(2): 167-190. |
| 99 | ALONSO-GUTIERREZ J, CHAN R, BATTH T S, et al. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production [J]. Metabolic Engineering, 2013, 19: 33-41. |
| 100 | YANG J M, NIE Q J, REN M, et al. Metabolic engineering of Escherichia coli for the biosynthesis of α-pinene [J]. Biotechnology for Biofuels, 2013, 6(1): 60. |
| 101 | ZHANG H, LIU Q, CAO Y, et al. Microbial production of sabinene—a new terpene-based precursor of advanced biofuel [J]. Microbial Cell Factories, 2014, 13: 20. |
| 102 | KORMAN T P, OPGENORTH P H, BOWIE J U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose [J]. Nature Communications, 2017, 8: 15526. |
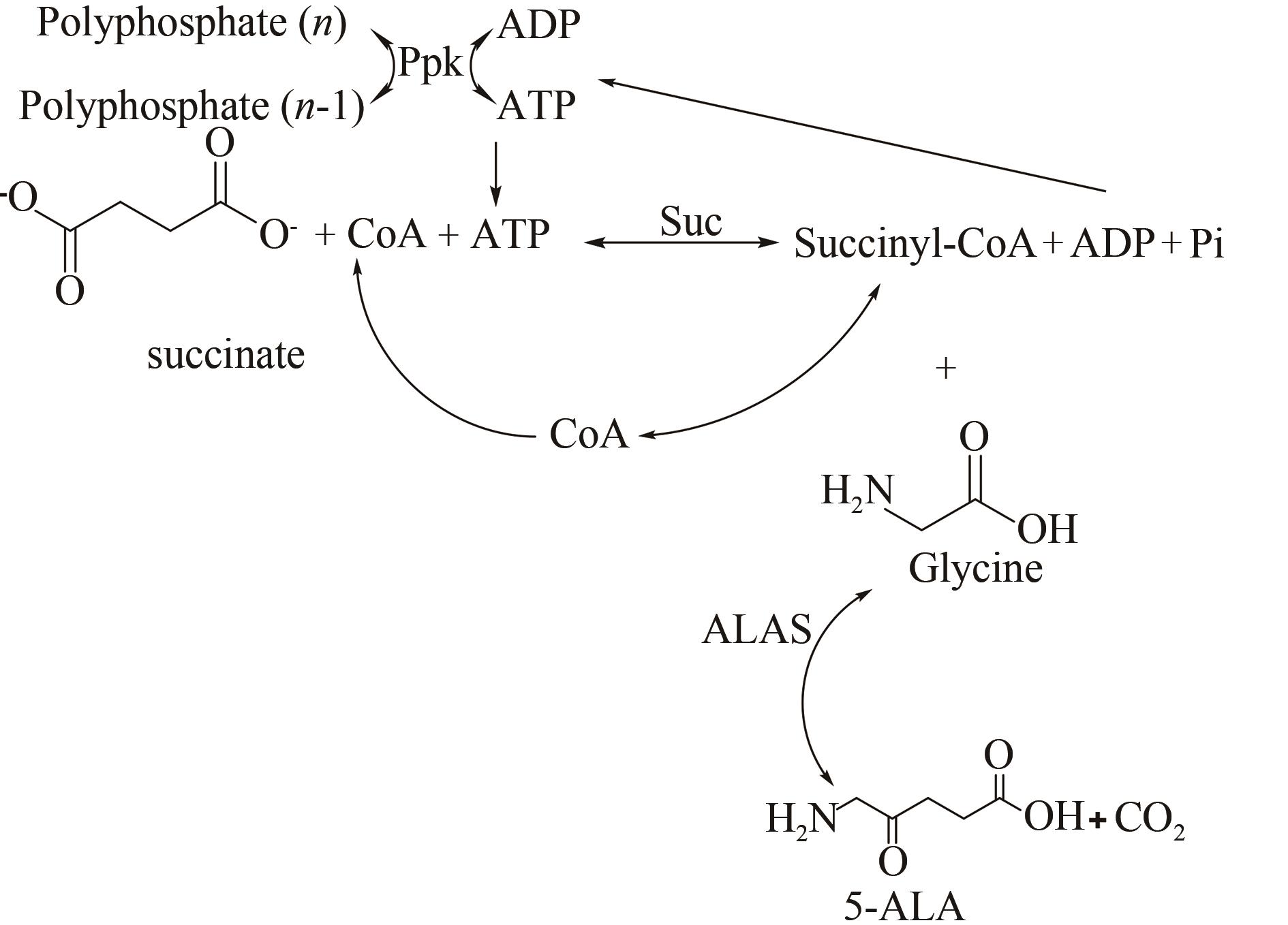
| 103 | YANG M-L, KUN Y, GUO Y-P, et al. A photosensitivity insecticide, 5-aminolevulinic acid, exerts effectivetoxicity to Oxya chinensis (Orthoptera: Acridoidea) [J]. Agricultural Sciences in China, 2011, 10(7): 1056-1063. |
| 104 | ZHANG J L, KANG Z, CHEN J, et al. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli [J]. Scientific Reports, 2015, 5: 8584. |
| 105 | SASAKI K, WATANABE M, TANAKA T, et al. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid [J]. Applied Microbiology and Biotechnology, 2002, 58(1): 23-29. |
| 106 | LI T, GUO Y Y, QIAO G Q, et al. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate [J]. ACS Synthetic Biology, 2016, 5(11): 1264-1274. |
| 107 | MENG Q L, ZHANG Y F, JU X Z, et al. Production of 5-aminolevulinic acid by cell free multi-enzyme catalysis [J]. Journal of Biotechnology, 2016, 226: 8-13. |
| 108 | WANG S Z, ZHANG Y H, REN H, et al. Strategies and perspectives of assembling multi-enzyme systems [J]. Critical Reviews in Biotechnology, 2017, 37(8): 1024-1037. |
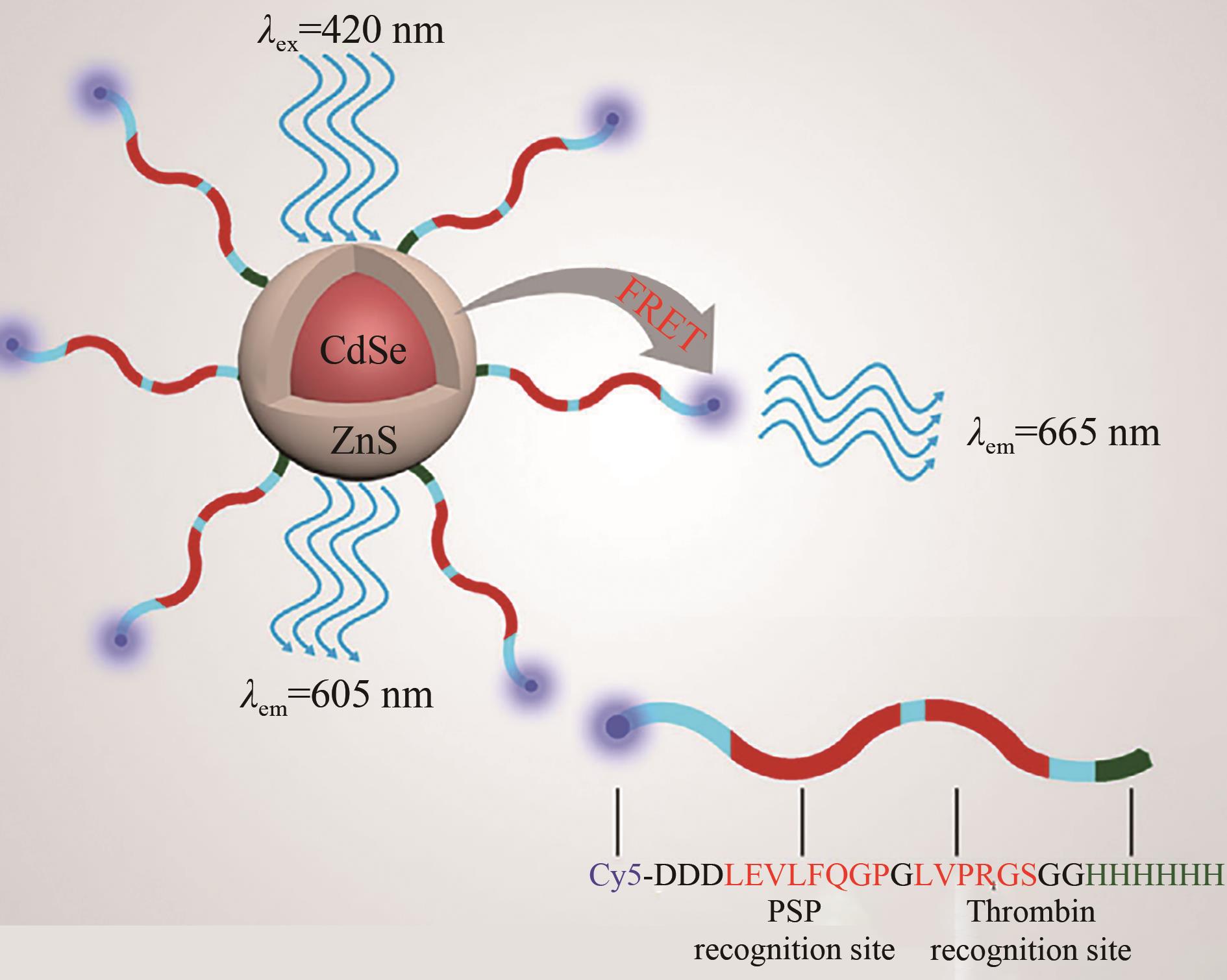
| 109 | QIU L, CUI P F, ZHU Z L, et al. Multienzyme detection and in-situ monitoring of enzyme activity by bending CE using quantum dots-based polypeptide substrate [J]. Electrophoresis. 2020, 41(12): 1103-1108. |
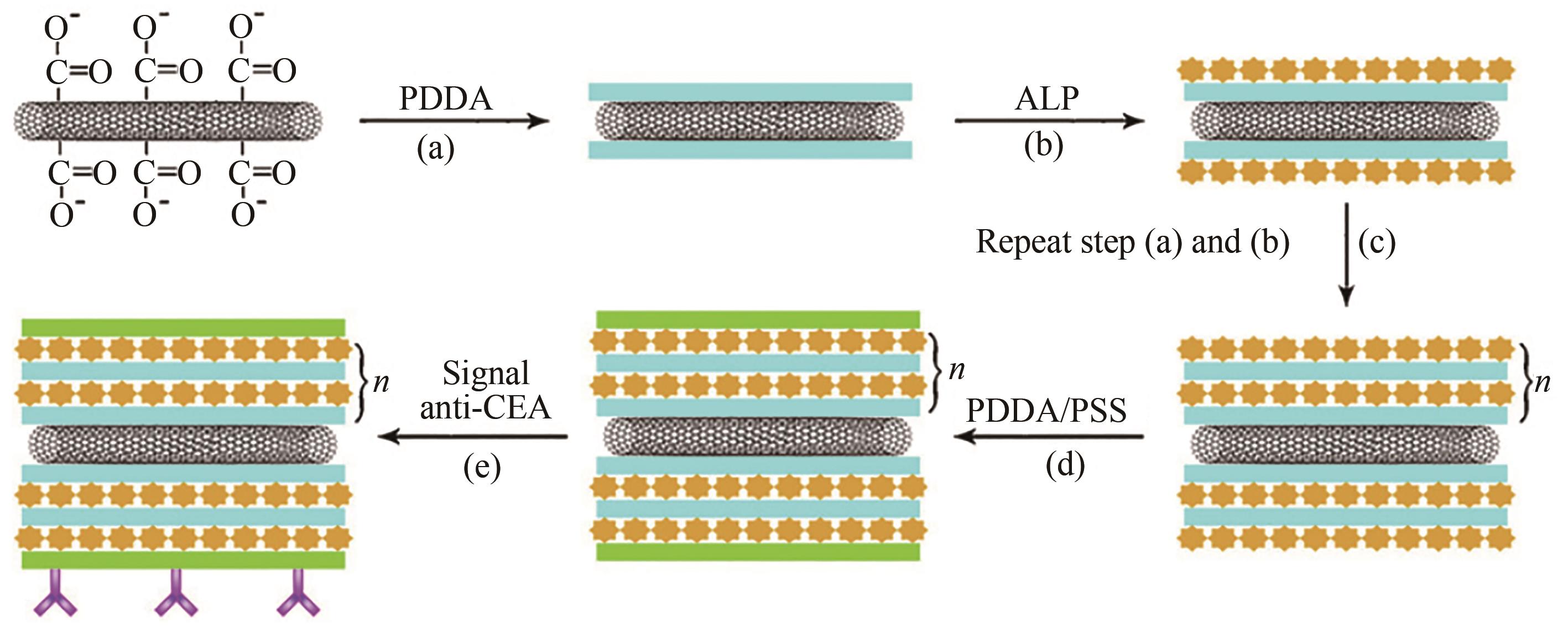
| 110 | XIANG Y, ZHANG Y Y, JIANG B Y, et al. Multi-enzyme layer-by-layer assembly for dual amplified ultrasensitive electronic detection of cancer biomarkers [J]. Sensors and Actuators B: Chemical, 2011, 155(1): 317-322. |
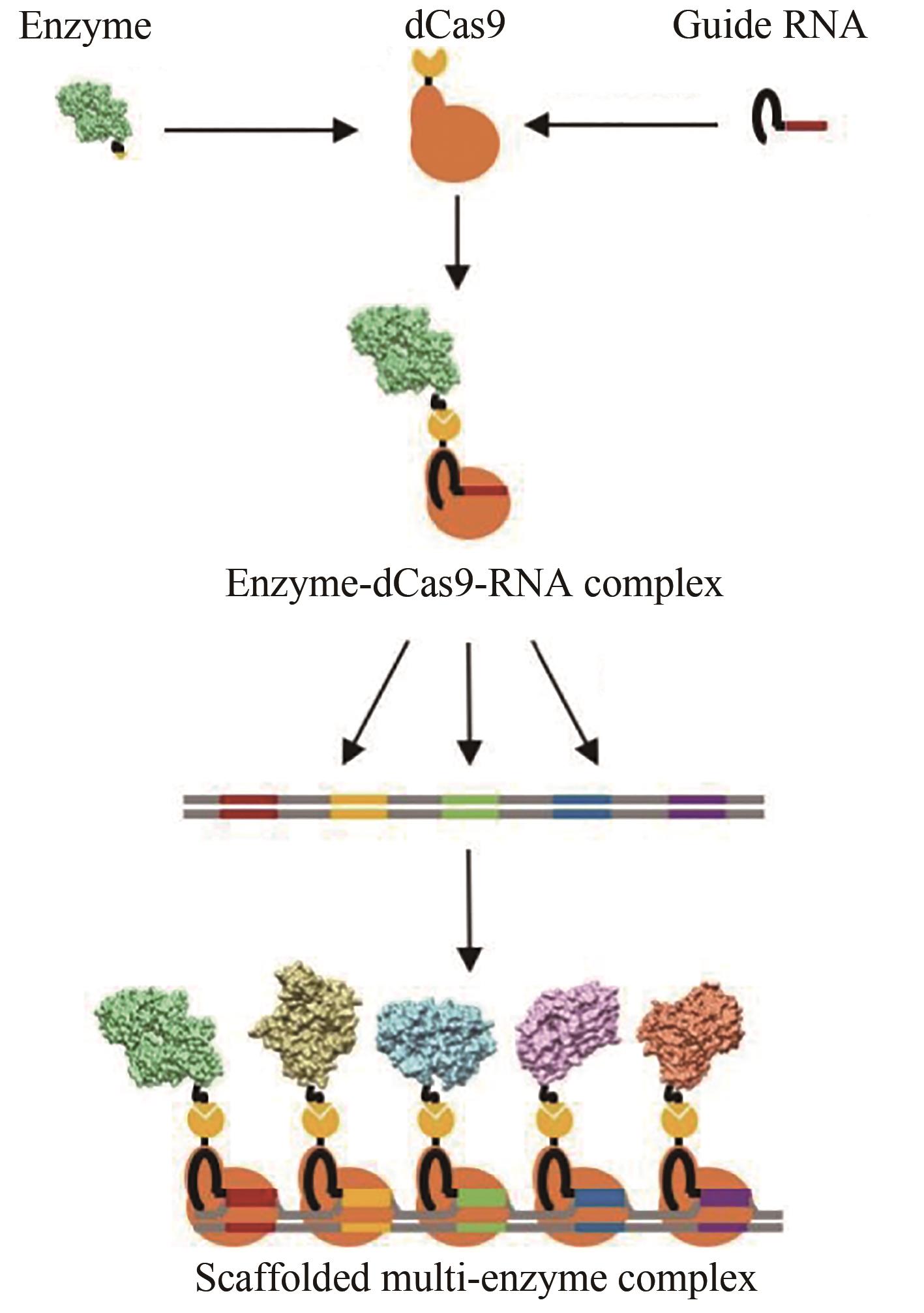
| 111 | LIM S, KIM J, KIM Y, et al. CRISPR/Cas-directed programmable assembly of multi-enzyme complexes [J]. Chemical Communications, 2020, 56(36): 4950-4953. |
| 112 | DUDLEY Q M, KARIM A S, JEWETT M C. Cell-free metabolic engineering: biomanufacturing beyond the cell [J]. Biotechnology Journal, 2015, 10(1): 69-82. |
| [1] | WEN Yanhua, LIU Hedong, CAO Chunlai, WU Ruibo. Applications of protein engineering in pharmaceutical industry [J]. Synthetic Biology Journal, 2025, 6(1): 65-86. |
| [2] | LEI Hangbin, HE Ning, LI Feixuan, DONG Lingling, WANG Shizhen. Advance in the immobilization of hydrogenases [J]. Synthetic Biology Journal, 2024, 5(6): 1485-1497. |
| [3] | CHENG Feng, ZOU Shuping, XU Jianmiao, TANG Heng, XUE Yaping, ZHENG Yuguo. BioHPP®: a benchmark of biomanufacturing for high optically pure L-phosphinothricin [J]. Synthetic Biology Journal, 2024, 5(6): 1404-1418. |
| [4] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [5] | FU Yu, ZHONG Fangrui. Recent advances in chemically driven enantioselective photobiocatalysis [J]. Synthetic Biology Journal, 2024, 5(5): 1021-1049. |
| [6] | LIU Wanqiu, JI Xiangyang, XU Huiling, LU Yicong, LI Jian. Cell-free protein synthesis system enables rapid and efficient biosynthesis of restriction endonucleases [J]. Synthetic Biology Journal, 2023, 4(4): 840-851. |
| [7] | TANG Shiming, HU Jiyuan, ZHENG Suiping, HAN Shuangyan, LIN Ying. Designing, building and rapid prototyping of biosynthesis module based on cell-free system [J]. Synthetic Biology Journal, 2022, 3(6): 1250-1261. |
| [8] | QI Yanping, ZHU Jin, ZHANG Kai, LIU Tong, WANG Yajie. Recent development of directed evolution in protein engineering [J]. Synthetic Biology Journal, 2022, 3(6): 1081-1108. |
| [9] | LIU Jianming, ZENG Anping. Cell-free multi-enzyme machines for CO2 capture, utilization and its associated challenges [J]. Synthetic Biology Journal, 2022, 3(5): 825-832. |
| [10] | JI Botao, QIAN Zhigang, XIA Xiaoxia. Application of cell-free synthesis strategy in biomaterial research [J]. Synthetic Biology Journal, 2022, 3(4): 658-675. |
| [11] | WANG Huibin, CHE Changli, YOU Song. Recent advances of enzymatic synthesis of organohalogens catalyzed by Fe/αKG-dependent halogenases [J]. Synthetic Biology Journal, 2022, 3(3): 545-566. |
| [12] | LOU Yujiao, XU Jian, WU Qi. Progress of biocatalytic deuteration of inert carbon-hydrogen bonds [J]. Synthetic Biology Journal, 2022, 3(3): 530-544. |
| [13] | HOU Jiaqi, JIANG Nan, MA Lianju, LU Yuan. Cell-free protein synthesis: from basic research to engineering applications [J]. Synthetic Biology Journal, 2022, 3(3): 465-486. |
| [14] | YANG Lu, QU Xudong. Application of imine reductase in the synthesis of chiral amines [J]. Synthetic Biology Journal, 2022, 3(3): 516-529. |
| [15] | XIONG Liangbin, SONG Lu, ZHAO Yunqiu, LIU Kun, LIU Yongjun, WANG Fengqing, WEI Dongzhi. Green biomanufacturing of steroids: from biotransformation to de novo synthesis by microorganisms [J]. Synthetic Biology Journal, 2021, 2(6): 942-963. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||