Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 1050-1071.DOI: 10.12211/2096-8280.2024-006
• Invited Review • Previous Articles Next Articles
Recent research progress in non-canonical biosynthesis of terpenoids
CHENG Xiaolei, LIU Tiangang, TAO Hui
- Key Laboratory of Combinatorial Biosynthesis and New Drug Discovery,Ministry of Education and School of Pharmacy,Wuhan University,Wuhan 430071,Hubei,China
-
Received:2024-01-10Revised:2024-04-09Online:2024-11-20Published:2024-10-31 -
Contact:LIU Tiangang, TAO Hui
萜类化合物的非常规生物合成研究进展
程晓雷, 刘天罡, 陶慧
- 武汉大学药学院,组合生物合成与新药发现教育部重点实验室,湖北 武汉 430071
-
通讯作者:刘天罡,陶慧 -
作者简介:程晓雷 (1999—),女,硕士研究生。研究方向为真菌来源萜类合酶的功能。 E-mail:2021206500005@whu.edu.cn刘天罡 (1979—),男,教授,博士生导师。研究方向为萜类等天然产物的高效合成与创新发现;基于底盘细胞和自动化平台的天然产物基因组挖掘;微生物与人体的代谢互作。 E-mail:liutg@whu.edu.cn陶慧 (1990—),女,教授,博士生导师。研究方向为复杂微生物来源天然产物的生物合成机制解析与绿色生物制造。 E-mail:thui@whu.edu.cn -
基金资助:国家重点研发计划(2023YFA0916200)
CLC Number:
Cite this article
CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids[J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071.
程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-006
| 酶家族 | 成员 | GenBank/UniProt ID | 生物合成途径 | 富含 Asp 的基序 | PDB ID | 参考文献 |
|---|---|---|---|---|---|---|
| TypeⅠsubunit | VenA | AAB81504.1 | venezuelaene A | DxxxxD | 7Y9H | [ |
| Chimeric typeⅠTS | TvTS | P9WER5.1 | talaropentaene | DDxxD NSE | 7VTB | [ |
| UbiA-type cyclase | Tps1A | KAI0942648.1 | (+)-(S,Z)-α-bisabolene | Nxxx(G/A)xxxD QDxxDxxxD | — | [ |
| Cytochrome P450 | SdnB | A0A1B4XBJ9.1 | sordarinane | — | — | [ |
| AriF | WP_092528764.1 | aridacins A-C | — | — | [ | |
| Methyltransferase | SodC | A0A7U3Z1M0 | sodorifen | — | — | [ |
| PchlO6_6045 | EIM17055.1 | chlororaphen | — | — | [ | |
| Vanadium haloperoxidase | LoVBPO2a | BCK50960.1 | snyderol | — | — | [ |
| Haloacid dehalogenase | AncA | THU99223.1 | monocyclofarnesol | — | — | [ |
| AncC | THU99223.1 | antrocin | — | — |
Table 1 Non-canonical terpene synthases
| 酶家族 | 成员 | GenBank/UniProt ID | 生物合成途径 | 富含 Asp 的基序 | PDB ID | 参考文献 |
|---|---|---|---|---|---|---|
| TypeⅠsubunit | VenA | AAB81504.1 | venezuelaene A | DxxxxD | 7Y9H | [ |
| Chimeric typeⅠTS | TvTS | P9WER5.1 | talaropentaene | DDxxD NSE | 7VTB | [ |
| UbiA-type cyclase | Tps1A | KAI0942648.1 | (+)-(S,Z)-α-bisabolene | Nxxx(G/A)xxxD QDxxDxxxD | — | [ |
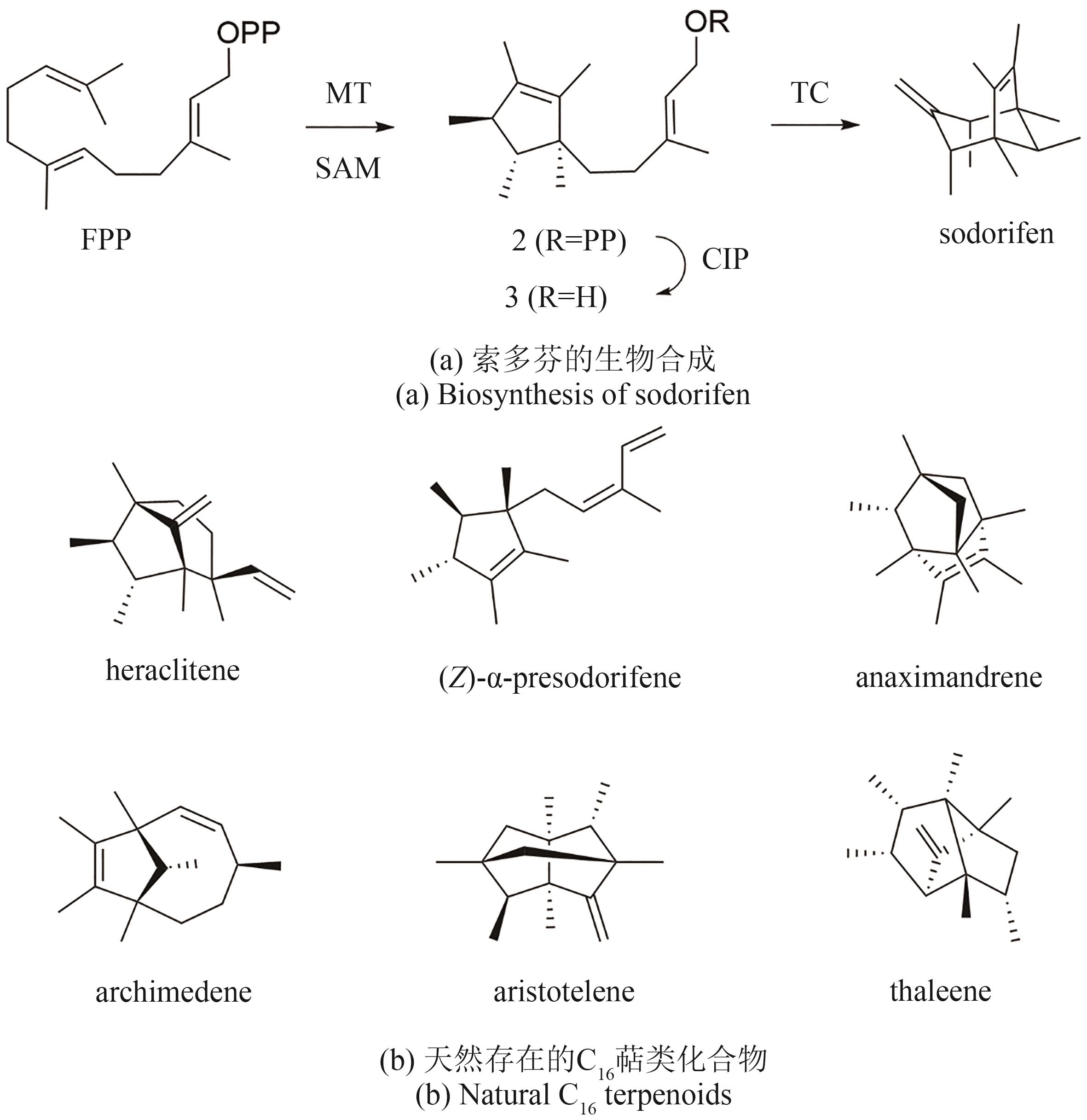
| Cytochrome P450 | SdnB | A0A1B4XBJ9.1 | sordarinane | — | — | [ |
| AriF | WP_092528764.1 | aridacins A-C | — | — | [ | |
| Methyltransferase | SodC | A0A7U3Z1M0 | sodorifen | — | — | [ |
| PchlO6_6045 | EIM17055.1 | chlororaphen | — | — | [ | |
| Vanadium haloperoxidase | LoVBPO2a | BCK50960.1 | snyderol | — | — | [ |
| Haloacid dehalogenase | AncA | THU99223.1 | monocyclofarnesol | — | — | [ |
| AncC | THU99223.1 | antrocin | — | — |
| 1 | BERGMAN M E, DAVIS B, PHILLIPS M A. Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action[J]. Molecules, 2019, 24(21): 3961. |
| 2 | 高铫晖, 王高乾, 黄蕙芸, 等. 真菌三萜及甾体的生物合成研究进展[J]. 有机化学, 2018, 38(9): 2335-2347. |
| GAO Y H, WANG G Q, HUANG H Y, et al. Biosynthesis of fungal triterpenoids and steroids[J]. Chinese Journal of Organic Chemistry, 2018, 38(9): 2335-2347. | |
| 3 | SCESA P D, LIN Z J, SCHMIDT E W. Author Correction: ancient defensive terpene biosynthetic gene clusters in the soft corals[J]. Nature Chemical Biology, 2023, 19(6): 790. |
| 4 | BROCK N L, DICKSCHAT J S. Biosynthesis of terpenoids[M/OL]//RAMAWAT K, MÉRILLON J M. Natural products. Berlin, Heidelberg: Springer, 2013: 2693-2732. (2013-01-01)[2023-12-01]. . |
| 5 | KOZLOV A V, GILLE L, STANIEK K, et al. Dihydrolipoic acid maintains ubiquinone in the antioxidant active form by two-electron reduction of ubiquinone and one-electron reduction of ubisemiquinone[J]. Archives of Biochemistry and Biophysics, 1999, 363(1): 148-154. |
| 6 | DELLAPENNA D. A decade of progress in understanding vitamin E synthesis in plants[J]. Journal of Plant Physiology, 2005, 162(7): 729-737. |
| 7 | KATO S. The function of vitamin D receptor in vitamin D action[J]. Journal of Biochemistry, 2000, 127(5): 717-722. |
| 8 | ZHAO M Y, WANG L, WANG J M, et al. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants[J]. Journal of Integrative Plant Biology, 2020, 62(10): 1461-1468. |
| 9 | BHATIA S P, MCGINTY D, LETIZIA C S, et al. Fragrance material review on L-menthol[J]. Food and Chemical Toxicology, 2008, 46(11): S218-S223. |
| 10 | CARAZO A, MACÁKOVÁ K, MATOUŠOVÁ K, et al. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity[J]. Nutrients, 2021, 13(5): 1703. |
| 11 | GOZARI M, ALBORZ M, EL-SEEDI H R, et al. Chemistry, biosynthesis and biological activity of terpenoids and meroterpenoids in bacteria and fungi isolated from different marine habitats[J]. European Journal of Medicinal Chemistry, 2021, 210: 112957. |
| 12 | LI C Y, ZHA W J, LI W, et al. Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance[J]. International Journal of Molecular Sciences, 2023, 24(14): 11561. |
| 13 | MA N, ZHANG Z Y, LIAO F L, et al. The birth of artemisinin[J]. Pharmacology & Therapeutics, 2020, 216: 107658. |
| 14 | GONG X, YANG M, HE C N, et al. Plant pharmacophylogeny: review and future directions[J]. Chinese Journal of Integrative Medicine, 2022, 28(6): 567-574. |
| 15 | SAMAAN T M ABU, SAMEC M, LISKOVA A, et al. Paclitaxel’s mechanistic and clinical effects on breast cancer[J]. Biomolecules, 2019, 9(12): 789. |
| 16 | ZAPPAVIGNA S, COSSU A M, GRIMALDI A, et al. Anti-inflammatory drugs as anticancer agents[J]. International Journal of Molecular Sciences, 2020, 21(7): 2605. |
| 17 | HUANG Y, VALIANTE V. Chemical diversity and biosynthesis of drimane-type sesquiterpenes in the fungal Kingdom[J]. ChemBioChem, 2022, 23(17): e202200173. |
| 18 | WANG C H, HOU J, DENG H K, et al. Microbial production of mevalonate[J]. Journal of Biotechnology, 2023, 370: 1-11. |
| 19 | FRANK A, GROLL M. The methylerythritol phosphate pathway to isoprenoids[J]. Chemical Reviews, 2017, 117(8): 5675-5703. |
| 20 | KARLIC H, VARGA F. Mevalonate Pathway[M/OL]//Encyclopedia of Cancer. Third Edition. New York: Academic Press, 2019, 445-457 [2023-12-01]. . |
| 21 | OGURA K, KOYAMA T. Enzymatic aspects of isoprenoid chain elongation[J]. Chemical Reviews, 1998, 98(4): 1263-1276. |
| 22 | OLDFIELD E, LIN F Y. Terpene biosynthesis: modularity rules[J]. Angewandte Chemie International Edition, 2012, 51(5): 1124-1137. |
| 23 | CROTEAU R, PURKETT P T. Geranyl pyrophosphate synthase: characterization of the enzyme and evidence that this chain-length specific prenyltransferase is associated with monoterpene biosynthesis in sage (Salvia officinalis)[J]. Archives of Biochemistry and Biophysics, 1989, 271(2): 524-535. |
| 24 | PARVIN R, SHAHROKH K O, MOZAFAR S, et al. Biosynthesis, regulation and properties of plant monoterpenoids[J]. Journal of Medicinal Plants Research, 2014, 8(29): 983-991. |
| 25 | MILLER D J, ALLEMANN R K. Sesquiterpene synthases: passive catalysts or active players?[J]. Natural Product Reports, 2012, 29(1): 60-71. |
| 26 | ZERBE P, BOHLMANN J. Plant diterpene synthases: exploring modularity and metabolic diversity for bioengineering[J]. Trends in Biotechnology, 2015, 33(7): 419-428. |
| 27 | FOY N J, PRONIN S V. Synthesis of pleuromutilin[J]. Journal of the American Chemical Society, 2022, 144(23): 10174-10179. |
| 28 | CHEN Q W, LI J X, LIU Z X, et al. Molecular basis for sesterterpene diversity produced by plant terpene synthases[J]. Plant Communications, 2020, 1(5): 100051. |
| 29 | RAZ K, LEVI S, GUPTA P K, et al. Enzymatic control of product distribution in terpene synthases: insights from multiscale simulations[J]. Current Opinion in Biotechnology, 2020, 65: 248-258. |
| 30 | WHITEHEAD J N, LEFERINK N G H, JOHANNISSEN L O, et al. Decoding catalysis by terpene synthases[J]. ACS Catalysis, 2023, 13(19): 12774-12802. |
| 31 | NAGEGOWDA D A, GUPTA P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids[J]. Plant Science, 2020, 294: 110457. |
| 32 | ZHAO Y J, CHENG Q Q, SU P, et al. Research progress relating to the role of cytochrome P450 in the biosynthesis of terpenoids in medicinal plants[J]. Applied Microbiology and Biotechnology, 2014, 98(6): 2371-2383. |
| 33 | CHRISTIANSON D W. Structural biology and chemistry of the terpenoid cyclases[J]. Chemical Reviews, 2006, 106(8): 3412-3442. |
| 34 | CHRISTIANSON D W. Structural and chemical biology of terpenoid cyclases[J]. Chemical Reviews, 2017, 117(17): 11570-11648. |
| 35 | LI Z, ZHANG L L, XU K W, et al. Molecular insights into the catalytic promiscuity of a bacterial diterpene synthase[J]. Nature Communications, 2023, 14(1): 4001. |
| 36 | TAO H, LAUTERBACH L, BIAN G K, et al. Discovery of non-squalene triterpenes[J]. Nature, 2022, 606(7913): 414-419. |
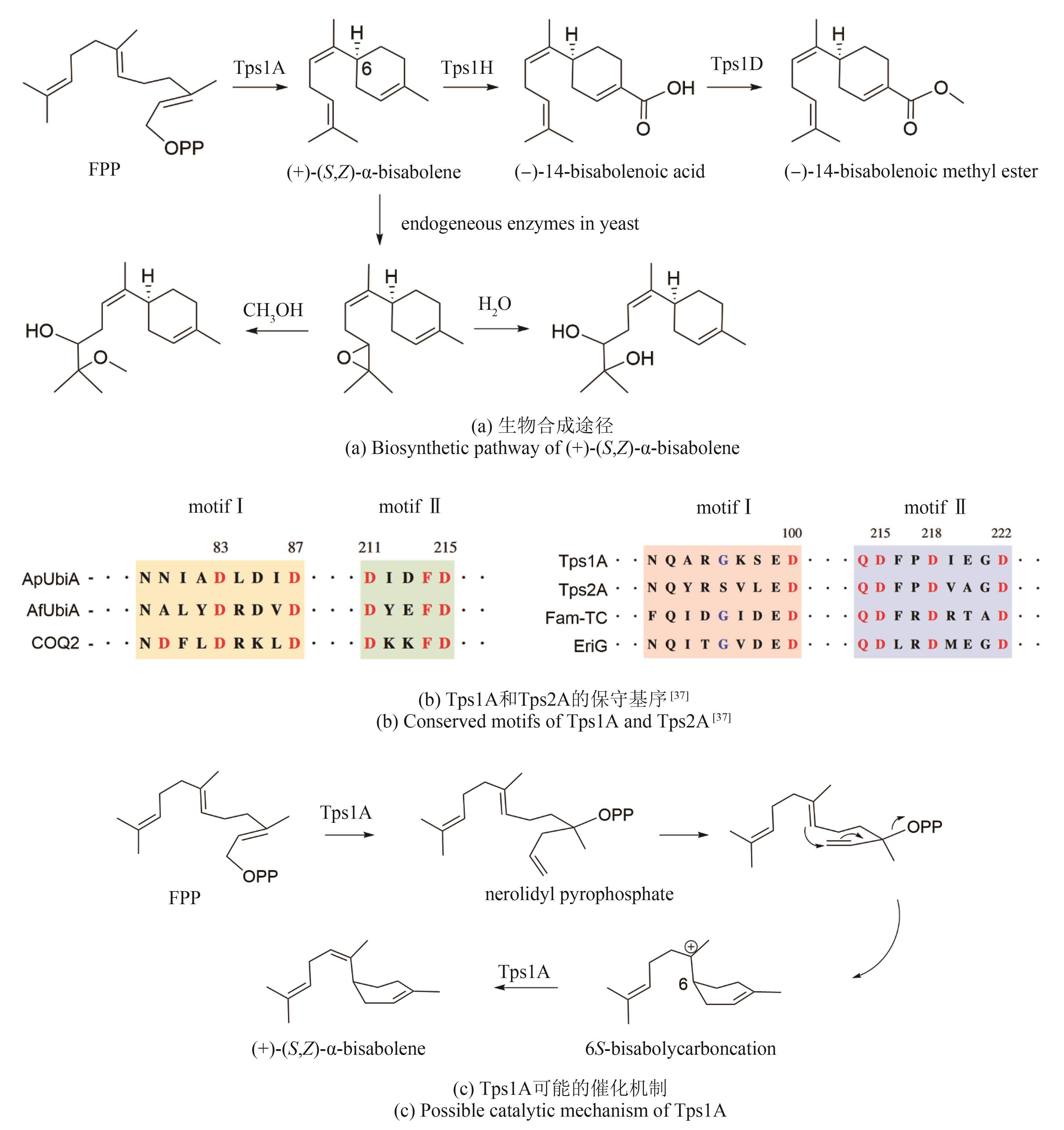
| 37 | HEWAGE R T, TSENG C C, LIANG S Y, et al. Genome mining of cryptic bisabolenes that were biosynthesized by intramembrane terpene synthases from Antrodia cinnamomea [J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2023, 378(1871): 20220033. |
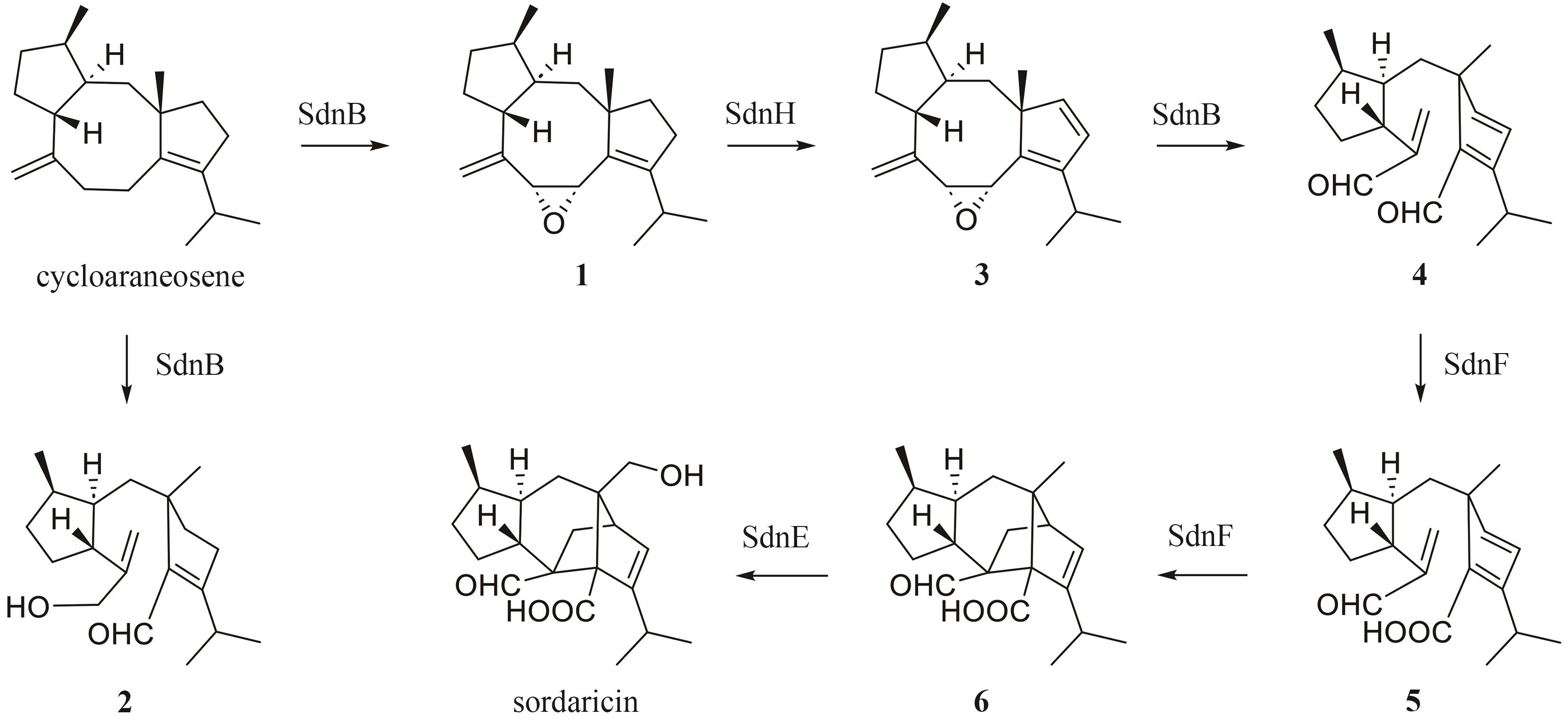
| 38 | CHEN Q B, YUAN G Y, YUAN T, et al. Set of cytochrome P450s cooperatively catalyzes the synthesis of a highly oxidized and rearranged diterpene-class sordarinane architecture[J]. Journal of the American Chemical Society, 2022, 144(8): 3580-3589. |
| 39 | WANG Z Y, YANG Q, HE J Y, et al. Cytochrome P450 mediated cyclization in eunicellane derived diterpenoid biosynthesis[J]. Angewandte Chemie International Edition, 2023, 62(45): e202312490. |
| 40 | XU H C, LAUTERBACH L, GOLDFUSS B, et al. Fragmentation and [4+3] cycloaddition in sodorifen biosynthesis[J]. Nature Chemistry, 2023, 15(8): 1164-1171. |
| 41 | DUAN Y T, KOUTSAVITI A, HARIZANI M, et al. Widespread biosynthesis of 16-carbon terpenoids in bacteria[J]. Nature Chemical Biology, 2023, 19(12): 1532-1539. |
| 42 | MAGNUS N, VON REUSS S H, BRAACK F, et al. Non-canonical biosynthesis of the Brexane-type bishomosesquiterpene chlororaphen through two consecutive methylation steps in Pseudomonas chlororaphis O6 and Variovorax boronicumulans PHE5-4[J]. Angewandte Chemie International Edition, 2023, 62(29): e202303692. |
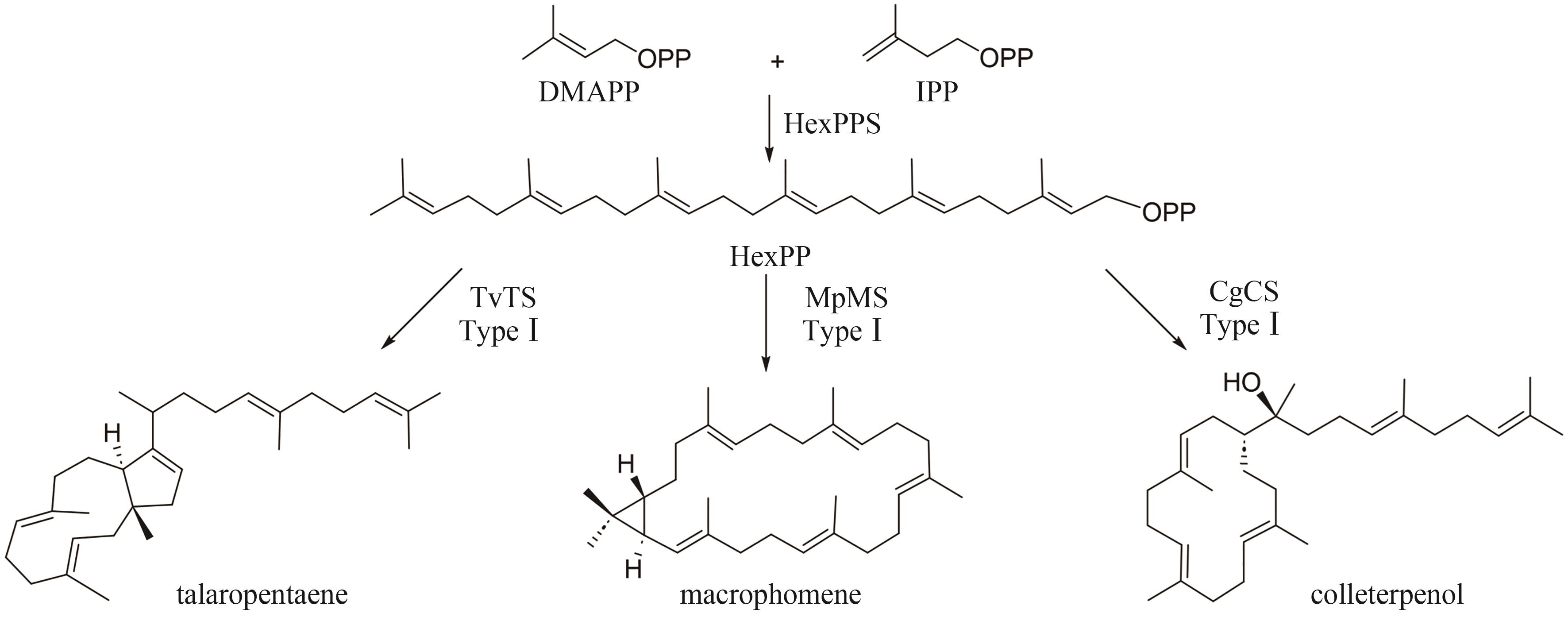
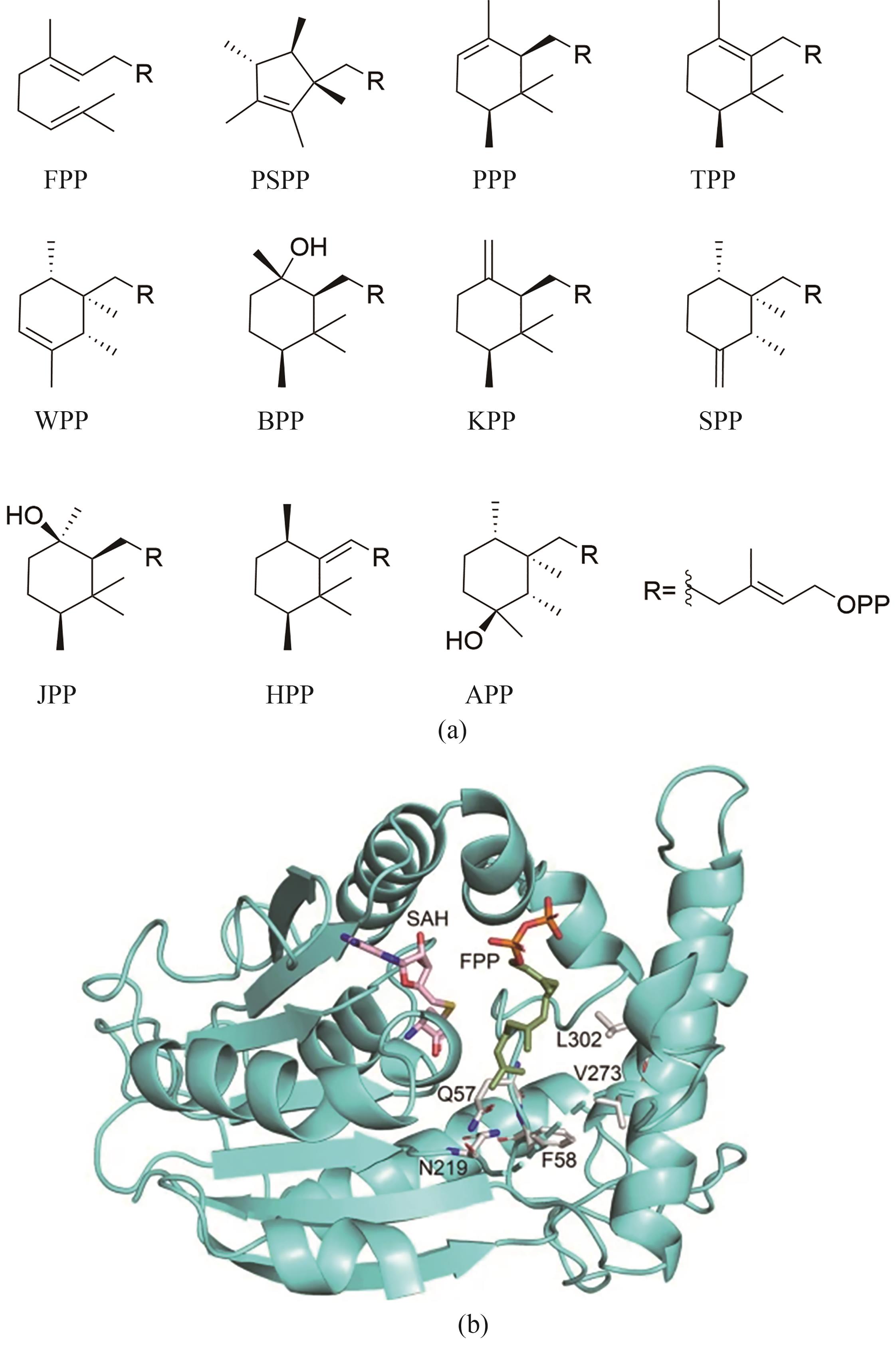
| 43 | ISHIKAWA T, WASHIO K, KANEKO K, et al. Characterization of vanadium-dependent bromoperoxidases involved in the production of brominated sesquiterpenes by the red alga Laurencia okamurae [J]. Applied Phycology, 2022, 3(1): 120-131. |
| 44 | IGNEA C, PONTINI M, MOTAWIA M S, et al. Synthesis of 11-carbon terpenoids in yeast using protein and metabolic engineering[J]. Nature Chemical Biology, 2018, 14(12): 1090-1098. |
| 45 | GENNADIOS H A, GONZALEZ V, COSTANZO L D, et al. Crystal structure of (+)-delta-cadinene synthase from Gossypium arboreum and evolutionary divergence of metal binding motifs for catalysis[J]. Biochemistry, 2009, 48(26): 6175-6183. |
| 46 | CANE D E, KANG I. Aristolochene synthase: purification, molecular cloning, high-level expression in Escherichia coli, and characterization of the Aspergillus terreus cyclase[J]. Archives of Biochemistry and Biophysics, 2000, 376(2): 354-364. |
| 47 | BAER P, RABE P, FISCHER K, et al. Induced-fit mechanism in classⅠterpene cyclases[J]. Angewandte Chemie International Edition, 2014, 53(29): 7652-7656. |
| 48 | KÖKSAL M, JIN Y H, COATES R M, et al. Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis[J]. Nature, 2011, 469(7328): 116-120. |
| 49 | THOMA R, SCHULZ-GASCH T, D′ARCY B, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase[J]. Nature, 2004, 432(7013): 118-122. |
| 50 | LENHART A, WEIHOFEN W A, PLESCHKE A E, et al. Crystal structure of a squalene cyclase in complex with the potential anticholesteremic drug Ro48-8071[J]. Chemistry & Biology, 2002, 9(5): 639-645. |
| 51 | PAN X M, RUDOLF J D, DONG L B. Class Ⅱ terpene cyclases: structures, mechanisms, and engineering[J]. Natural Product Reports, 2024, 41(3): 402-433. |
| 52 | WANG Y H, XU H C, ZOU J, et al. Catalytic role of carbonyl oxygens and water in selinadiene synthase[J]. Nature Catalysis, 2022, 5: 128-135. |
| 53 | JIA Q D, BROWN R, KÖLLNER T G, et al. Origin and early evolution of the plant terpene synthase family[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(15): e2100361119. |
| 54 | VOGEL B S, WILDUNG M R, VOGEL G, et al. Abietadiene synthase from grand fir (Abies grandis). cDNA isolation, characterization, and bacterial expression of a bifunctional diterpene cyclase involved in resin acid biosynthesis[J]. Journal of Biological Chemistry, 1996, 271(38): 23262-23268. |
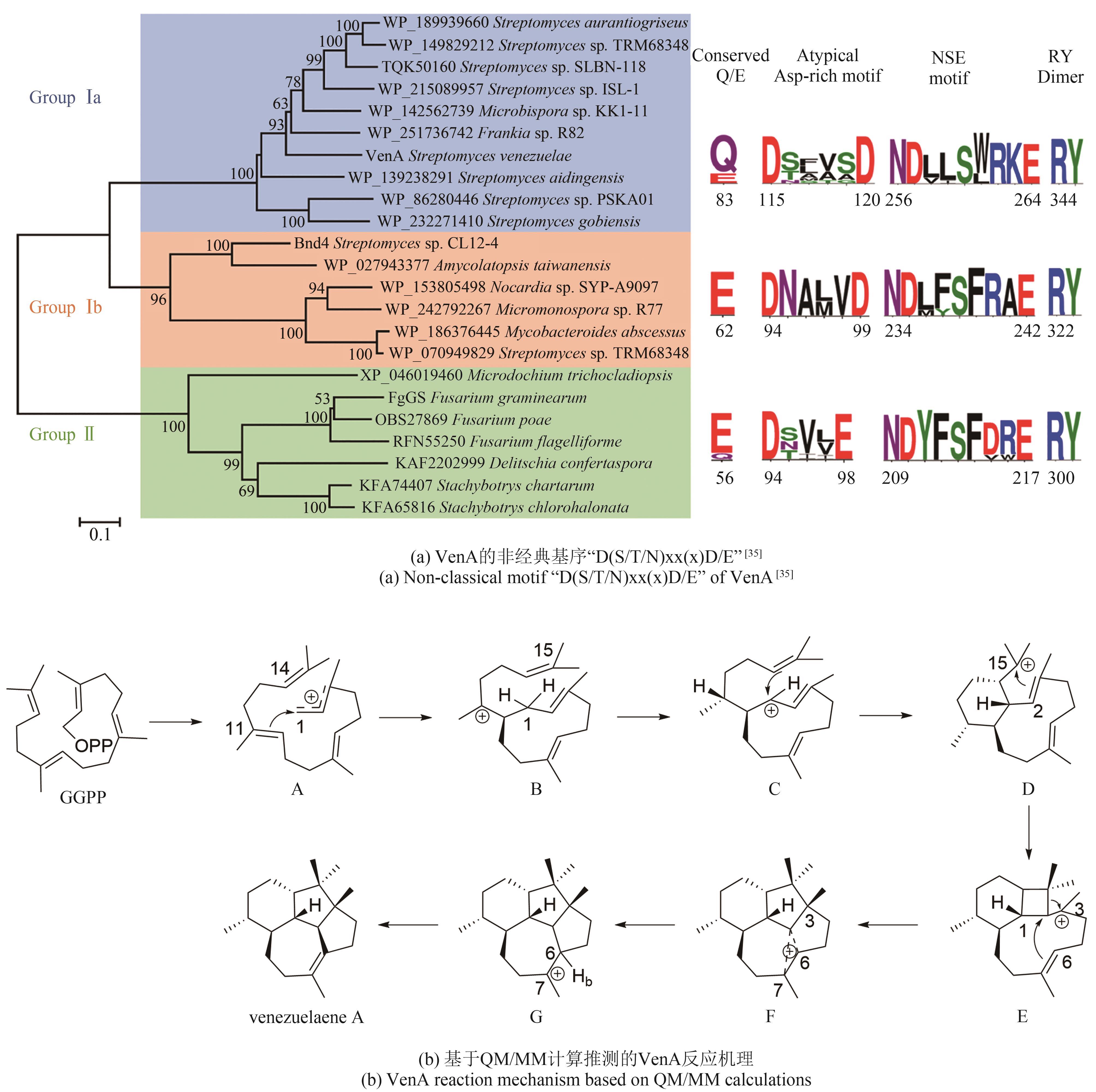
| 55 | LI Z, JIANG Y Y, ZHANG X W, et al. Fragrant venezuelaenes A and B with A 5-5-6-7 tetracyclic skeleton: discovery, biosynthesis, and mechanisms of central catalysts[J]. ACS Catalysis, 2020, 10(10): 5846-5851. |
| 56 | GALAPPATHTHI M C A, PATABENDIGE N M, PREMARATHNE B M, et al. A review of Ganoderma triterpenoids and their bioactivities[J]. Biomolecules, 2022, 13(1): 24. |
| 57 | GAO X Y, LIU G C, ZHANG J X, et al. Pharmacological properties of ginsenoside Re[J]. Frontiers in Pharmacology, 2022, 13: 754191. |
| 58 | VANE J R, BOTTING R M. Anti-inflammatory drugs and their mechanism of action[J]. Inflammation Research, 1998, 47(): S78-S87. |
| 59 | WELANDER P V. Deciphering the evolutionary history of microbial cyclic triterpenoids[J]. Free Radical Biology & Medicine, 2019, 140: 270-278. |
| 60 | LI Y L, WANG J, LI L Y, et al. Natural products of pentacyclic triterpenoids: from discovery to heterologous biosynthesis[J]. Natural Product Reports, 2023, 40(8): 1303-1353. |
| 61 | CHEN L L. Linking long noncoding RNA localization and function[J]. Trends in Biochemical Sciences, 2016, 41(9): 761-772. |
| 62 | SMANSKI M J, YU Z G, CASPER J, et al. Dedicated ent-kaurene and ent-atiserene synthases for platensimycin and platencin biosynthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(33): 13498-13503. |
| 63 | LIN H C, CHOOI Y H, DHINGRA S, et al. The fumagillin biosynthetic gene cluster in Aspergillus fumigatus encodes a cryptic terpene cyclase involved in the formation of β-trans-bergamotene[J]. Journal of the American Chemical Society, 2013, 135(12): 4616-4619. |
| 64 | YANG Y L, ZHANG Y T, ZHANG S S, et al. Identification and characterization of a membrane-bound sesterterpene cyclase from Streptomyces somaliensis [J]. Journal of Natural Products, 2018, 81(4): 1089-1092. |
| 65 | RUDOLF J D, CHANG C Y. Terpene synthases in disguise: enzymology, structure, and opportunities of non-canonical terpene synthases[J]. Natural Product Reports, 2020, 37(3): 425-463. |
| 66 | BRANDT W, BRÄUER L, GÜNNEWICH N, et al. Molecular and structural basis of metabolic diversity mediated by prenyldiphosphate converting enzymes[J]. Phytochemistry, 2009, 70(15/16): 1758-1775. |
| 67 | LU M C, EL-SHAZLY M, WU T Y, et al. Recent research and development of Antrodia cinnamomea [J]. Pharmacology & Therapeutics, 2013, 139(2): 124-156. |
| 68 | HUANG H, LEVIN E J, LIU S A, et al. Structure of a membrane-embedded prenyltransferase homologous to UBIAD1[J]. PLoS Biology, 2014, 12(7): e1001911. |
| 69 | MANIKANDAN P, NAGINI S. Cytochrome P450 structure, function and clinical significance: a review[J]. Current Drug Targets, 2018, 19(1): 38-54. |
| 70 | ZUO H L, HUANG H Y, LIN Y C, et al. Enzyme activity of natural products on cytochrome P450[J]. Molecules, 2022, 27(2): 515. |
| 71 | ZHU D Q, SEO M J, IKEDA H, et al. Genome mining in streptomyces. Discovery of an unprecedented P450-catalyzed oxidative rearrangement that is the final step in the biosynthesis of pentalenolactone[J]. Journal of the American Chemical Society, 2011, 133(7): 2128-2131. |
| 72 | HANSEN N L, KJAERULFF L, HECK Q K, et al. Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide[J]. Nature Communications, 2022, 13(1): 5011. |
| 73 | CHOOI Y H, HONG Y J, CACHO R A, et al. A cytochrome P450 serves as an unexpected terpene cyclase during fungal meroterpenoid biosynthesis[J]. Journal of the American Chemical Society, 2013, 135(45): 16805-16808. |
| 74 | ABDELRAHEEM E, THAIR B, VARELA R F, et al. Methyltransferases: functions and applications[J]. ChemBioChem, 2022, 23(18): e202200212. |
| 75 | LASHLEY A, MILLER R, PROVENZANO S, et al. Functional diversification and structural origins of plant natural product methyltransferases[J]. Molecules, 2022, 28(1): 43. |
| 76 | DEWICK P M. Medicinal natural products: a biosynthetic approach[M/OL]. 3rd Edition. Hoboken, New Jersey: Wiley, 2009[2023-12-01]. . |
| 77 | VON REUSS S H, KAI M, PIECHULLA B, et al. Octamethylbicyclo[3.2.1]octadienes from the rhizobacterium Serratia odorifera [J]. Angewandte Chemie International Edition, 2010, 49(11): 2009-2010. |
| 78 | VON REUSS S, DOMIK D, LEMFACK M C, et al. Sodorifen biosynthesis in the rhizobacterium Serratia plymuthica involves methylation and cyclization of MEP-derived farnesyl pyrophosphate by a SAM-dependent C-methyltransferase[J]. Journal of the American Chemical Society, 2018, 140(37): 11855-11862. |
| 79 | DOMIK D, THÜRMER A, WEISE T, et al. A terpene synthase is involved in the synthesis of the volatile organic compound sodorifen of Serratia plymuthica 4Rx13[J]. Frontiers in Microbiology, 2016, 7: 737. |
| 80 | DUELL E R, D′AGOSTINO P M, SHAPIRO N, et al. Direct pathway cloning of the sodorifen biosynthetic gene cluster and recombinant generation of its product in E. coli [J]. Microbial Cell Factories, 2019, 18(1): 32. |
| 81 | DOMIK D, MAGNUS N, PIECHULLA B. Analysis of a new cluster of genes involved in the synthesis of the unique volatile organic compound sodorifen of Serratia plymuthica 4Rx13[J]. FEMS Microbiology Letters, 2016, 363(14): fnw139. |
| 82 | WEVER R, KRENN B E, RENIRIE R. Marine vanadium-dependent haloperoxidases, their isolation, characterization, and application[M/OL]//Methods in Enzymology, 2018, 605: 141-201 [2023-12-01]. . |
| 83 | CARTER-FRANKLIN J N, BUTLER A. Vanadium bromoperoxidase-catalyzed biosynthesis of halogenated marine natural products[J]. Journal of the American Chemical Society, 2004, 126(46): 15060-15066. |
| 84 | MCKINNIE S M K, MILES Z D, JORDAN P A, et al. Total enzyme syntheses of napyradiomycins A1 and B1[J]. Journal of the American Chemical Society, 2018, 140(51): 17840-17845. |
| 85 | DIETHELM S, TEUFEL R, KAYSSER L, et al. A multitasking vanadium-dependent chloroperoxidase as an inspiration for the chemical synthesis of the merochlorins[J]. Angewandte Chemie International Edition, 2014, 53(41): 11023-11026. |
| 86 | HARIZANI M, IOANNOU E, ROUSSIS V. The Laurencia paradox: an endless source of chemodiversity[J]. Progress in the Chemistry of Organic Natural Products, 2016, 102: 91-252. |
| 87 | GRESSLER M, LÖHR N A, SCHÄFER T, et al. Mind the mushroom: natural product biosynthetic genes and enzymes of Basidiomycota[J]. Natural Product Reports, 2021, 38(4): 702-722. |
| 88 | SANDARGO B, CHEPKIRUI C, CHENG T, et al. Biological and chemical diversity go hand in hand: basidiomycota as source of new pharmaceuticals and agrochemicals[J]. Biotechnology Advances, 2019, 37(6): 107344. |
| 89 | LU M Y J, FAN W L, WANG W F, et al. Genomic and transcriptomic analyses of the medicinal fungus Antrodia cinnamomea for its metabolite biosynthesis and sexual development[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(44): E4743-E4752. |
| 90 | IGNEA C, RAADAM M H, KOUTSAVITI A, et al. Expanding the terpene biosynthetic code with non-canonical 16 carbon atom building blocks[J]. Nature Communications, 2022, 13(1): 5188. |
| 91 | TANG M C, SHEN C, DENG Z X, et al. Combinatorial biosynthesis of terpenoids through mixing-and-matching sesquiterpene cyclase and cytochrome P450 pairs[J]. Organic Letters, 2022, 24(26): 4783-4787. |
| 92 | FREY M, BATHE U, MEINK L, et al. Combinatorial biosynthesis in yeast leads to over 200 diterpenoids[J]. Metabolic Engineering, 2024, 82: 193-200. |
| 93 | URLACHER V B, GIRHARD M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology[J]. Trends in Biotechnology, 2019, 37(8): 882-897. |
| 94 | XIAO H, ZHANG Y, WANG M. Discovery and engineering of cytochrome P450s for terpenoid biosynthesis[J]. Trends in Biotechnology, 2019, 37(6): 618-631. |
| 95 | EBRECHT A C, VAN DER BERGH N, HARRISON S T L, et al. Biochemical and structural insights into the cytochrome P450 reductase from Candida tropicalis [J]. Scientific Reports, 2019, 9(1): 20088. |
| 96 | LIN G M, VOIGT C A. Design of a redox-proficient Escherichia coli for screening terpenoids and modifying cytochrome P450s[J]. Nature Catalysis, 2023, 6: 1016-1029. |
| 97 | KEY H M, DYDIO P, LIU Z N, et al. Beyond iron: iridium-containing P450 enzymes for selective cyclopropanations of structurally diverse alkenes[J]. ACS Central Science, 2017, 3(4): 302-308. |
| 98 | LELYVELD V S, BRUSTAD E, ARNOLD F H, et al. Metal-substituted protein MRI contrast agents engineered for enhanced relaxivity and ligand sensitivity[J]. Journal of the American Chemical Society, 2011, 133(4): 649-651. |
| 99 | BORDEAUX M, SINGH R, FASAN R. Intramolecular C(sp3)H amination of arylsulfonyl azides with engineered and artificial myoglobin-based catalysts[J]. Bioorganic & Medicinal Chemistry, 2014, 22(20): 5697-5704. |
| 100 | BLOOMER B, NATOLI S, GARCIA-BORRÀS M, et al. Mechanistic and structural characterization of an iridium-containing cytochrome reveals kinetically relevant cofactor dynamics[J]. Nature Catalysis, 2023, 6: 39-51. |
| 101 | HU Y L, ZHANG Q, LIU S H, et al. Building Streptomyces albus as a chassis for synthesis of bacterial terpenoids[J]. Chemical Science, 2023, 14(13): 3661-3667. |
| 102 | CHEN R, JIA Q D, MU X, et al. Systematic mining of fungal chimeric terpene synthases using an efficient precursor-providing yeast chassis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(29): e2023247118. |
| 103 | BURKHARDT I, ROND T D, CHEN P Y, et al. Ancient plant-like terpene biosynthesis in corals[J]. Nature Chemical Biology, 2022, 18(6): 664-669. |
| 104 | JUNG Y, MITSUHASHI T, SATO S, et al. Function and structure of a terpene synthase encoded in a giant virus genome[J]. Journal of the American Chemical Society, 2023, 145(48): 25966-25970. |
| 105 | ZHAO Y, LIANG F Y, XIE Y M, et al. Oxetane ring formation in taxol biosynthesis is catalyzed by a bifunctional cytochrome P450 enzyme[J]. Journal of the American Chemical Society, 2024, 146(1): 801-810. |
| 106 | JIANG B, GAO L, WANG H J, et al. Characterization and heterologous reconstitution of Taxus biosynthetic enzymes leading to baccatin Ⅲ[J]. Science, 2024, 383(6683): 622-629. |
| 107 | YUAN Y J, CHENG S, BIAN G K, et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi[J]. Nature Catalysis, 2022, 5: 277-287. |
| 108 | TSUTSUMI H, MORIWAKI Y, TERADA T, et al. Structural and molecular basis of the catalytic mechanism of geranyl pyrophosphate C6-methyltransferase: creation of an unprecedented farnesyl pyrophosphate C6-methyltransferase[J]. Angewandte Chemie International Edition, 2022, 61(1): e202111217. |
| 109 | XING B Y, XU H C, LI A N, et al. Crystal structure based mutagenesis of cattleyene synthase leads to the generation of rearranged polycyclic diterpenes[J]. Angewandte Chemie International Edition, 2022, 61(36): e202209785. |
| 110 | YE Z L, HUANG Y L, SHI B, et al. Coupling cell growth and biochemical pathway induction in Saccharomyces cerevisiae for production of (+)-valencene and its chemical conversion to (+)-nootkatone[J]. Metabolic Engineering, 2022, 72: 107-115. |
| 111 | SAMUSEVICH R, HEBRA T, BUSHUIEV R, et al. Discovery and characterization of terpene synthases powered by machine learning[EB/OL]. bioRxiv, 2024: 2024.01.29.577750. (2024-02-01)[2024-02-28]. . |
| 112 | ZHANG X, KING-SMITH E, DONG L B, et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach[J]. Science, 2020, 369(6505): 799-806. |
| [1] | LEI Ru, TAO Hui, LIU Tiangang. Deep genome mining boosts the discovery of microbial terpenoids [J]. Synthetic Biology Journal, 2024, 5(3): 507-526. |
| [2] | ZHOU Qiang, ZHOU Dawei, SUN Jingxiang, WANG Jingnan, JIANG Wankui, ZHANG Wenming, JIANG Yujia, XIN Fengxue, JIANG Min. Research progress in synthesis of astaxanthin by microbial fermentation [J]. Synthetic Biology Journal, 2024, 5(1): 126-143. |
| [3] | Shichao REN, Qiuyan SUN, Xudong FENG, Chun LI. Biosynthesis of pentacyclic triterpenoid saponins in microbial cell factories [J]. Synthetic Biology Journal, 2022, 3(1): 168-183. |
| [4] | Zhen FAN, Haixue PAN, Gongli TANG. Engineered yeast facilitates rapid and systematic mining of fungal chimeric terpene synthases [J]. Synthetic Biology Journal, 2021, 2(5): 666-673. |
| [5] | Xiaodong LI, Chengshuai YANG, Pingping WANG, Xing YAN, Zhihua ZHOU. Production of sesquiterpenoids α-neoclovene and β-caryophyllene by engineered Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2021, 2(5): 792-803. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||