|
||
|
Biosynthesis of xylo-oligosaccharides from wheat straw xylan via the synergistic hydrolysis by xylanase Xyn11A and arabinofuranosidase Abf62A
Synthetic Biology Journal
DOI: 10.12211/2096-8280.2025-037
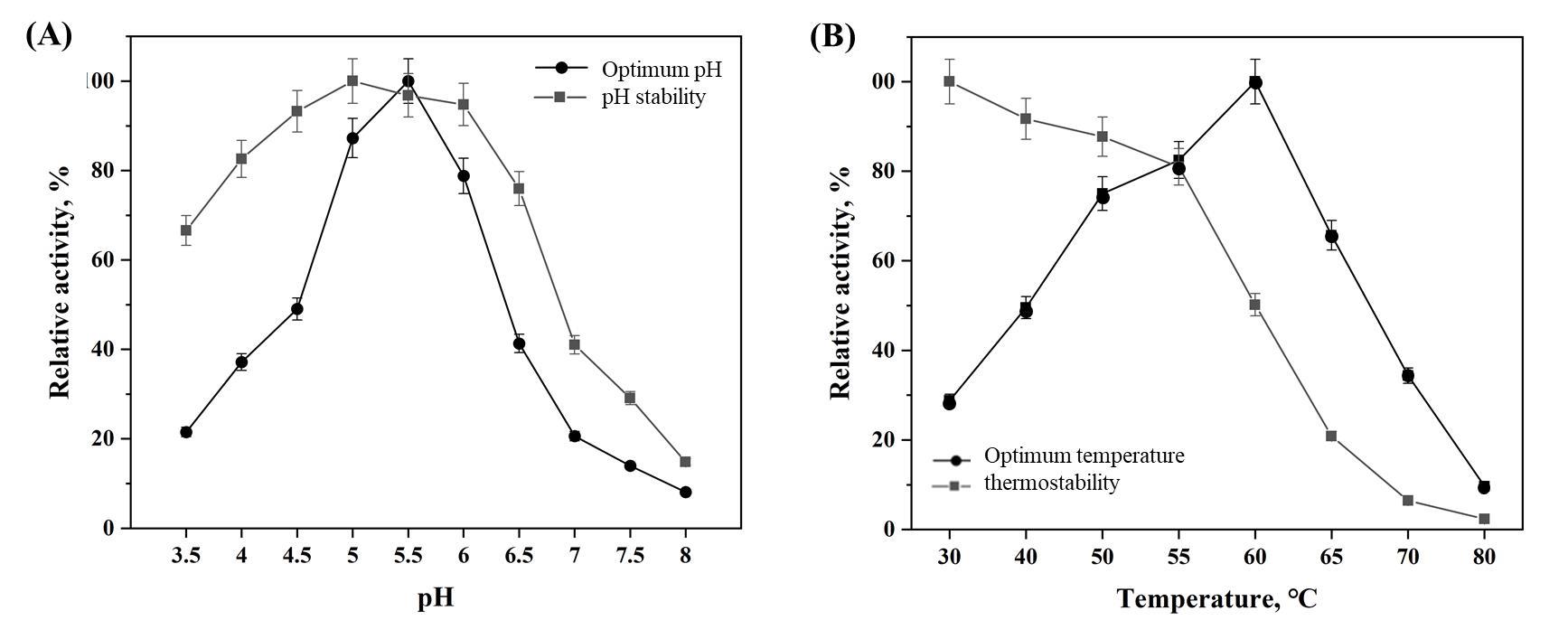
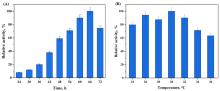
Fig. 5
The optimum pH and pH stability of Abf62A (a) and its optimum temperature and temperature stability (b)
Extracts from the Article
As showcased in Fig. 5(a), it was observed that the enzyme activity of Abf62A varies greatly between pH 3.5 and 8.0. When the pH was between 5.0 and 6.0, the enzyme activity was higher than 80%, and the optimum pH was pH 5.5. However, the enzyme activity under other pH was very low. Especially, the enzyme activity of Abf62A was only less than 10% when the pH was alkaline. This was due to the fact that the enzyme was derived from fungi and the optimum pH was usually in the acidic range [22]. The arabinofuranosidase Abf4980 screened from Bispora sp. MEY-1CGMCC2500 and expressed in yeast GS115 manifested good activity in pH 3.0-6.0 [22-23]. The stability of Abf62A between pH 5.0-6.0 was excellent, all above 90%, and the enzyme activity was above 60% under acidic condition. In contrast, the enzyme activity under alkaline conditions was very low, and the remaining enzyme activity after incubation at pH 8.0 for 1 h was less than 20%. This might be caused by enzyme inactivation under alkaline conditions. The arabinoxylanase from Bacillus subtili was also the most stable in pH under pH 5.5 [24]. However, the pH stability of enzymes from other strains varies greatly, and α-L-arabinosidase in Jiangella alba DSM45237 still maintained high activity under the condition of pH 8.0 [25].
Under the optimum pH conditions, the enzyme activity of arabinofuranosidase Abf62A at different temperatures was assessed. The results were showcased in Fig. 5(b). It was found that the optimum temperature of Abf62A was 60 ℃, and the enzyme activity declined sharply as the temperature continued to rise. 63% of enzyme activity was acquired at 65 ℃, and less than 10% at 80 ℃. Too high temperature can cause the protein denaturation, which would result in the rapid decrease of enzyme activity [25]. α-L-arabinofuranosidase from A. terreus was heterologous expressed in P. pastoris X33 and remained stable at 60-65 °C, which was consistent with the results of this research [26]. In addition, it was obvious that the temperature stability of Abf62A was poor. The enzyme activity reached the maximum at 30 ℃, and the enzyme activity weakened gradually with the increase of temperature, but the remaining enzyme activity was 81% at 55 ℃. After rising to 60 ℃, the enzyme activity was maintained only 50%. Almost all the enzymes were denatured at 80 ℃, and the enzyme activity was only 2.4%. The newly discovered α- L -arabinofuranosidase Abf4980 remained fully active after incubation at 70 ℃ for 24 hours, showing excellent thermal stability [23]. At present, industrial applications are pursuing enzymes with high temperature stability[27-29]. Directional mutagenesis is one of the most powerful tools for improving the biophysical properties of proteins for biopharmaceutical and industrial processes [30-32], which can be used to further improve the thermostability of Abf62A.
Other Images/Table from this Article
|