|
||
|
Applications of the CRISPR/Cas9 editing system in the study of microbial natural products
Synthetic Biology Journal
2024, 5 (3):
658-671.
DOI: 10.12211/2096-8280.2023-110
Microorganisms have consistently been a crucial source for researchers to explore and develop new natural products. Currently, research methods involving gene editing tools for the discovery, biosynthesis, and metabolic engineering of natural products have garnered broad attention in this field. However, traditional methods for gene editing usually rely on the recombination ability of the host or introduced proteins. It’s difficult to establish a general platform for all bacteria mainly because of their complicated genetic background. This genetic diversity often causes laborious experimental operations with low efficiency. The CRISPR/Cas9 gene editing system, with its unique and flexible targeting advantages, overcomes common limitations such as sequence homology or site constraint in other gene editing methods and thus is more likely to function in diverse bacteria species. This simplifies experimental procedures, enhances work efficiency, and promotes the development of natural product research. This article introduces the applications of the CRISPR/Cas9 system for the discovery, biosynthesis, and metabolic engineering of natural products in microorganisms. It covers the development of the CRISPR/Cas9 system, cloning and genetic editing of natural product biosynthetic gene clusters, structural derivatization and metabolic engineering of natural products, and the activation of silenced natural product biosynthetic gene clusters. These aspects highlight the advantages of the CRISPR/Cas9 system in the research of natural products with microorganisms. Finally, solutions are proposed for addressing challenges that the CRISPR/Cas9 system currently faces in overcoming low recombination efficiency and host adaptability issues. Especially the CRISPR/Cas12a system which has broadened applications of the CRISRP/Cas9 system by preferring different PAM sites. In addition to functions that CRISPR/Cas9 system has realized, its potent multiple targeting ability further enhances the efficiency of target editing. It is believed that with the development of synthetic biology and information technology, an increasing number of genetic manipulation tools and methods related to the CRISPR/Cas9 system will be developed, continually driving progress in the research of natural products.
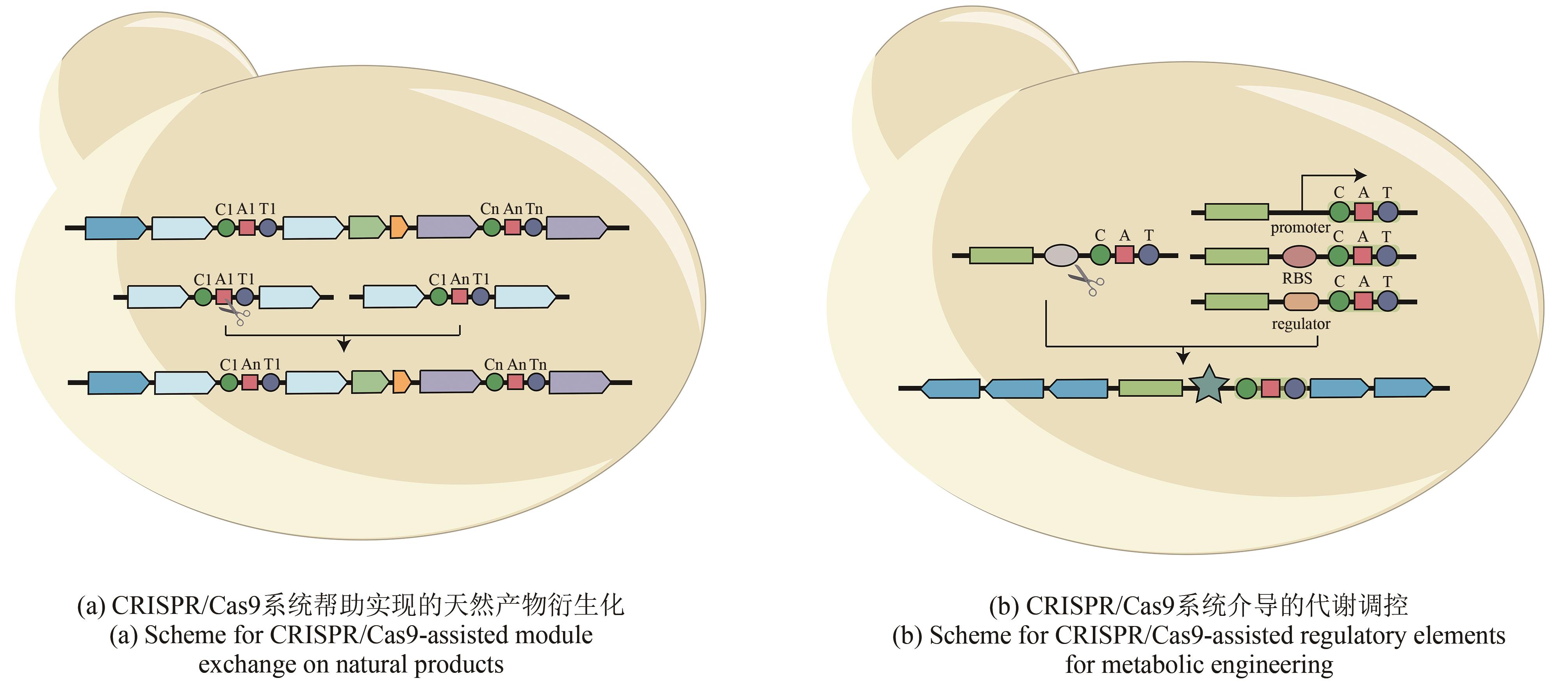
Fig. 3
CRISPR/Cas9-related methods for assembly and genetic engineering of biosynthetic gene clusters
Extracts from the Article
CRISPR/Cas9在基因编辑方面展现出强大的可拓展的能力,因此很多研究人员开始借助该系统来产生新颖天然产物衍生物、调控天然产物产量和激活沉默的天然产物生物合成基因簇(表1)。天然产物药物的衍生化是优化先导化合物的主要和关键步骤。将生物合成基因簇作为天然产物的遗传单元进行修改,为研究天然产物药物衍生物提供了一个易于操作的平台。日本AIST研究所的Kazuo Shin-ya团队[41]通过体外Cas9反应和Gibson组装在rapamycin生物合成基因簇上成功地实现了不同AT结构域(acyltransferase domain)的替换并对应获得了多个不同的rapamycin衍生物[图3(a)]。此外,该团队进一步通过模块删除插入、结构域的编辑和交换也获得了对应的rapamycin衍生物。该研究表明CRISPR/Cas9可帮助实现模块化天然产物生物合成基因簇的编辑,并实现目的天然产物的衍生化。基于蛋白结构活性位点的突变策略,针对合成脂肽类抗生素enduracidin的生物合成基因簇,Jason Micklefield团队[42]通过替换存在于A结构域(adenylation domain)中的高度保守的类黄素氧还蛋白单元实现了A结构域底物选择性靶向突变,从而合成了多个新的脂肽类抗生素。
原核生物的生物合成基因簇通常组织为一个多顺反子操纵子,其表达水平主要由一组共同的调控元件所控制。根据原核生物这一转录翻译特征可知,同一操纵子下的编码序列会同时转录并受相同的调控单元控制,但各个编码序列的翻译会受到其自身核糖体结合位点(ribosome binding site)的调控[63]。启动子工程作为一类可靠的调节方法,可实现不同强度的基因表达调控。许多研究已经利用该技术实现了天然产物的多样化和产量优化[64]。然而天然产物的生物合成是一个非常复杂的过程,通常需要多基因协作完成。所以仅仅通过强的组成型启动子来调节整个生物合成基因簇或限速酶的表达,尚不能实现最佳的产物产量。因此,Hahk-Soo Kang团队[43]设计并优化了一个生物合成的诱导调节系统,该团队将该系统拆分成3个独立的功能模块,经过一系列筛选和综合评估,获得了包含CMT模块(cumate,CymR/cmtO)和A26RS的最优组合,并用于优化act生物合成基因簇的表达。在CRISPR/Cas9系统的帮助下,将组成型RS(promoter/RBS)替换为CMT模块,同时通过更强的RS优化了抑制子表达模块SF14 RS。该系统对三株S. albus菌株的异源表达能力的调节完全超过了阳性对照,表明这种可诱导调节系统和模块化设计方法在生物合成基因簇的启动子工程中具有潜在的应用价值[图3(b)]。除了启动子工程外,中国科学院芦银华和姜卫红团队[44]借助噬菌体整合酶系统,通过CRISPR/Cas9系统在宿主染色体上引入多个attB(attachment site)位点,从而实现生物合成基因簇的多拷贝整合,最终实现次级代谢产物pristinamycin Ⅱ在S. pristinaespirali中的高产量表达。Will J. Quax团队[45]借助精准高效的CRISPR/Cas9系统,通过多拷贝核心基因,遗传突变弱化旁路通路,利用启动子工程调节三羧酸循环和MEP (2C-methyl-D-erythritol-4-phosphate)通路,最终在枯草芽孢杆菌内实现了萜类前体Amorphadiene的最高产量。
Other Images/Table from this Article
|