合成生物学 ›› 2020, Vol. 1 ›› Issue (4): 481-494.DOI: 10.12211/2096-8280.2020-033
2'-岩藻糖基乳糖的酶法合成研究进展和展望
史然, 江正强
- 中国农业大学食品科学与营养工程学院,北京 100083
-
收稿日期:2020-03-23修回日期:2020-05-09出版日期:2020-08-31发布日期:2020-10-09 -
通讯作者:江正强 -
作者简介:史然(1987—),女,博士研究生,研究方向为食品酶的发掘与应用。E-mail:shiranb20153060234@cau.edu.cn
江正强(1971—),男,博士生导师,教授,研究方向为食品酶与发酵工程。E-mail:zhqjiang@cau.edu.cn -
基金资助:国家自然科学基金(31630096);国家优秀青年科学基金(31822037)
Enzymatic synthesis of 2'-fucosyllactose: advances and perspectives
SHI Ran, JIANG Zhengqiang
- College of Food Science and Nutritional Engineering,China Agricultural University,Beijing 100083,China
-
Received:2020-03-23Revised:2020-05-09Online:2020-08-31Published:2020-10-09 -
Contact:JIANG Zhengqiang
摘要:
人乳寡糖(human milk oligosaccharides,HMOs)是人乳中一类结构复杂、非消化性的碳水化合物。2'-岩藻糖基乳糖(2'-fucosyllactose,2'-FL)是人乳中含量最高的寡糖,也是最早被FDA和欧盟批准可添加到婴幼儿奶粉、膳食补充剂以及医疗食品中的HMOs之一。2'-FL具有调节肠道菌群、抵抗病原菌的黏附、免疫调节及促进神经系统发育和修复等多种功能活性。2'-FL的主要合成方法有化学合成法、全细胞合成法及酶催化合成法。全细胞合成法是当前工业上生产2'-FL的主要方法,降低L-岩藻糖的成本、调节合成途径中鸟苷二磷酸-L-岩藻糖(GDP-岩藻糖)的水平与菌体生长之间的平衡、发掘新型高活性的α-1,2-岩藻糖基转移酶是降低全细胞合成2'-FL成本的关键。2'-FL的合成途径在一些更为安全表达宿主(如无抗生素大肠杆菌、枯草芽孢杆菌和酵母菌等)中的构建也面临着挑战。本文重点综述了2'-FL酶法合成的研究现状,利用α-1,2-岩藻糖基转移酶合成2'-FL专一性好,但所需糖基供体GDP-岩藻糖成本较高;利用α-L-岩藻糖苷酶的转糖苷活性也可以合成2'-FL,α-L-岩藻糖苷酶来源广泛,易获得,稳定性好,可利用天然底物作为糖基供体。将α-L-岩藻糖苷酶应用于2'-FL合成的关键在于高效的转糖苷酶以及天然、经济的岩藻糖基供体的发掘。酶法合成将来有望成为工业上生产2'-FL的方法。
中图分类号:
引用本文
史然, 江正强. 2'-岩藻糖基乳糖的酶法合成研究进展和展望[J]. 合成生物学, 2020, 1(4): 481-494.
SHI Ran, JIANG Zhengqiang. Enzymatic synthesis of 2'-fucosyllactose: advances and perspectives[J]. Synthetic Biology Journal, 2020, 1(4): 481-494.
| 分类 | 化合物 | 结构式 | 浓度范围 /g·L-1 | 摩尔 分数/% |
|---|---|---|---|---|
| neutral fucosylated HMOs | 2'-FL | Fucα1,2Galβ1,4Glc | 0.06~3.93 | 31 |
| 3-FL | Galβ1,4(Fucα1,3)Glc | 0.03~1.34 | 5 | |
| DFL (2',3-FL) | Fucα1,2Galβ1,4(Fucα1,3)Glc | 0.28~0.43 | 4 | |
| LNFP Ⅰ | Fucα1,2Galβ1,3GlcNAcβ1,3Galβ1,4Glc | 0.001~2.08 | 8 | |
| LNFP Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4Glc | 0.02~1.79 | 2 | |
| LNFP Ⅲ | Galβ1,4(Fucα1,3)GlcNAcβ1,3Galβ1,4Glc | 0.06~0.78 | 2 | |
| LNFP Ⅴ | Galβ1,3GlcNAcβ1,3Galβ1,4(Fucα1,3)Glc | 0.06 | — | |
| LNFP Ⅵ | Galβ1,4GlcNAcβ1,3Galβ1,4(Fucα1,3)Glc | 0.01 | — | |
| LNDFH Ⅰ | Fucα1,2Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4Glc | 0.43~1.87 | 4 | |
| LNDFH Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4(Fucα1,3)Glc | 0.02~0.25 | — | |
| F-LNH Ⅰ | Fucα1,2Galβ1,3GlcNAcβ1,3(Galβ1,4GlcNAcβ1,6)Galβ1,4Glc | 0.2~2.62 | — | |
| F-LNH Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3(Galβ1,4GlcNAcβ1-6Galβ1-4Glc | 0.18~1.06 | — | |
| DF-LNH Ⅰ | Fucα1,2Galβ1,3GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1,6)Galβ1,4Glc | 0.31 | — | |
| DF-LNH Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1-6)Galβ1-4Glc | 0.12~1.02 | — | |
| TF-LNH | Fucα1,2Galβ1,3(Fucα1,4)GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1,6)Galβ1,4Glc | 2.60~3.10 | — | |
| neutral non-fucosylated HMOs | LNT | Galβ1,3GlcNAcβ1,3Galβ1,4Glc | 0.16~1.54 | 6 |
| LNnT | Galβ1,4GlcNAcβ1,3Galβ1,4Glc | 0.04~2.04 | 6 | |
| LNH | Galβ1,3GlcNAcβ1,3(Galβ1,4GlcNAcβ1,6)Galβ1,4Glc | 0.05~0.17 | — | |
| LNnH | Galβ1,4GlcNAcβ1,3(Galβ1,4GlcNAcβ1,6)Galβ1,4Glc | 0.09~0.28 | — | |
| sialylated HMOs | 3'-SL | Neu5Acα2,3Galβ1,4Glc | 0.09~0.30 | 2 |
| 6'-SL | Neu5Acα2,6Galβ1,4Glc | 0.07~0.59 | 6 | |
| LSTa | Neu5Acα2,3Galβ1,3GlcNAcβ1,3Galβ1,4Glc | 0.01~0.18 | — | |
| LSTb | Galβ1,3(Neu5Acα2,6)GlcNAcβ1,3Galβ1,4Glc | 0.04~0.25 | — | |
| LSTc | Neu5Acα2,6Galβ1,4GlcNAcβ1,3Galβ1,4Glc | 0.05~1.05 | — | |
| DSLNT | Neu5Acα2,3Galβ1,3(Neu5Acα2,6)GlcNAcβ1,3Galβ1,4Glc | 0.10~0.80 | 2 | |
| FS-LNnH Ⅰ | Neu5Acα2,6Galβ1,4GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1,6)Galβ1,4Glc | 0.26~0.55 | — | |
| other HMOs | 13 |
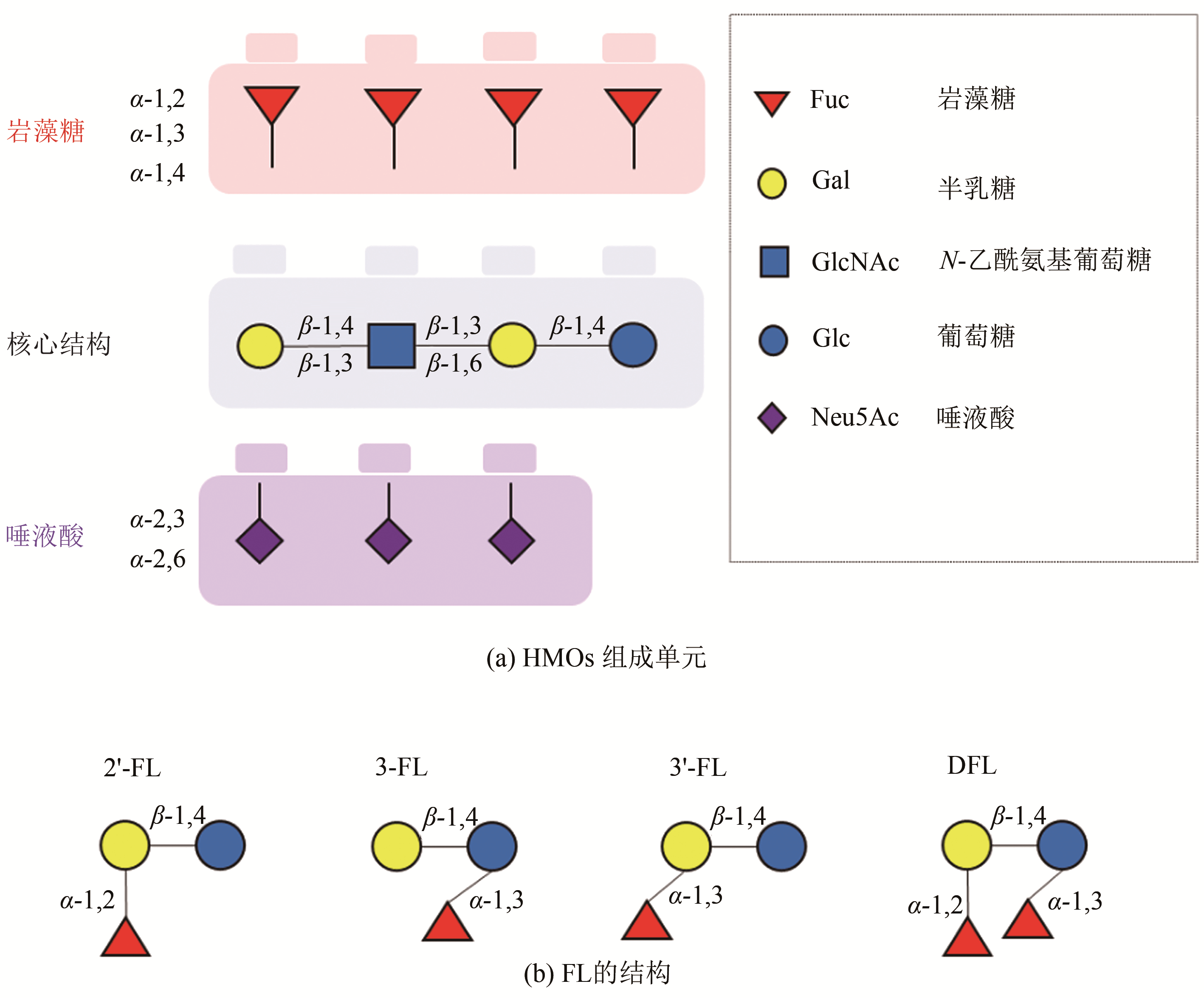
表1 主要HMOs的分类、结构及含量[4,6]
Tab. 1 Structures and contents of major HMOs[4,6]
| 分类 | 化合物 | 结构式 | 浓度范围 /g·L-1 | 摩尔 分数/% |
|---|---|---|---|---|
| neutral fucosylated HMOs | 2'-FL | Fucα1,2Galβ1,4Glc | 0.06~3.93 | 31 |
| 3-FL | Galβ1,4(Fucα1,3)Glc | 0.03~1.34 | 5 | |
| DFL (2',3-FL) | Fucα1,2Galβ1,4(Fucα1,3)Glc | 0.28~0.43 | 4 | |
| LNFP Ⅰ | Fucα1,2Galβ1,3GlcNAcβ1,3Galβ1,4Glc | 0.001~2.08 | 8 | |
| LNFP Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4Glc | 0.02~1.79 | 2 | |
| LNFP Ⅲ | Galβ1,4(Fucα1,3)GlcNAcβ1,3Galβ1,4Glc | 0.06~0.78 | 2 | |
| LNFP Ⅴ | Galβ1,3GlcNAcβ1,3Galβ1,4(Fucα1,3)Glc | 0.06 | — | |
| LNFP Ⅵ | Galβ1,4GlcNAcβ1,3Galβ1,4(Fucα1,3)Glc | 0.01 | — | |
| LNDFH Ⅰ | Fucα1,2Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4Glc | 0.43~1.87 | 4 | |
| LNDFH Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3Galβ1,4(Fucα1,3)Glc | 0.02~0.25 | — | |
| F-LNH Ⅰ | Fucα1,2Galβ1,3GlcNAcβ1,3(Galβ1,4GlcNAcβ1,6)Galβ1,4Glc | 0.2~2.62 | — | |
| F-LNH Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3(Galβ1,4GlcNAcβ1-6Galβ1-4Glc | 0.18~1.06 | — | |
| DF-LNH Ⅰ | Fucα1,2Galβ1,3GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1,6)Galβ1,4Glc | 0.31 | — | |
| DF-LNH Ⅱ | Galβ1,3(Fucα1,4)GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1-6)Galβ1-4Glc | 0.12~1.02 | — | |
| TF-LNH | Fucα1,2Galβ1,3(Fucα1,4)GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1,6)Galβ1,4Glc | 2.60~3.10 | — | |
| neutral non-fucosylated HMOs | LNT | Galβ1,3GlcNAcβ1,3Galβ1,4Glc | 0.16~1.54 | 6 |
| LNnT | Galβ1,4GlcNAcβ1,3Galβ1,4Glc | 0.04~2.04 | 6 | |
| LNH | Galβ1,3GlcNAcβ1,3(Galβ1,4GlcNAcβ1,6)Galβ1,4Glc | 0.05~0.17 | — | |
| LNnH | Galβ1,4GlcNAcβ1,3(Galβ1,4GlcNAcβ1,6)Galβ1,4Glc | 0.09~0.28 | — | |
| sialylated HMOs | 3'-SL | Neu5Acα2,3Galβ1,4Glc | 0.09~0.30 | 2 |
| 6'-SL | Neu5Acα2,6Galβ1,4Glc | 0.07~0.59 | 6 | |
| LSTa | Neu5Acα2,3Galβ1,3GlcNAcβ1,3Galβ1,4Glc | 0.01~0.18 | — | |
| LSTb | Galβ1,3(Neu5Acα2,6)GlcNAcβ1,3Galβ1,4Glc | 0.04~0.25 | — | |
| LSTc | Neu5Acα2,6Galβ1,4GlcNAcβ1,3Galβ1,4Glc | 0.05~1.05 | — | |
| DSLNT | Neu5Acα2,3Galβ1,3(Neu5Acα2,6)GlcNAcβ1,3Galβ1,4Glc | 0.10~0.80 | 2 | |
| FS-LNnH Ⅰ | Neu5Acα2,6Galβ1,4GlcNAcβ1,3(Galβ1,4(Fucα1,3)GlcNAcβ1,6)Galβ1,4Glc | 0.26~0.55 | — | |
| other HMOs | 13 |
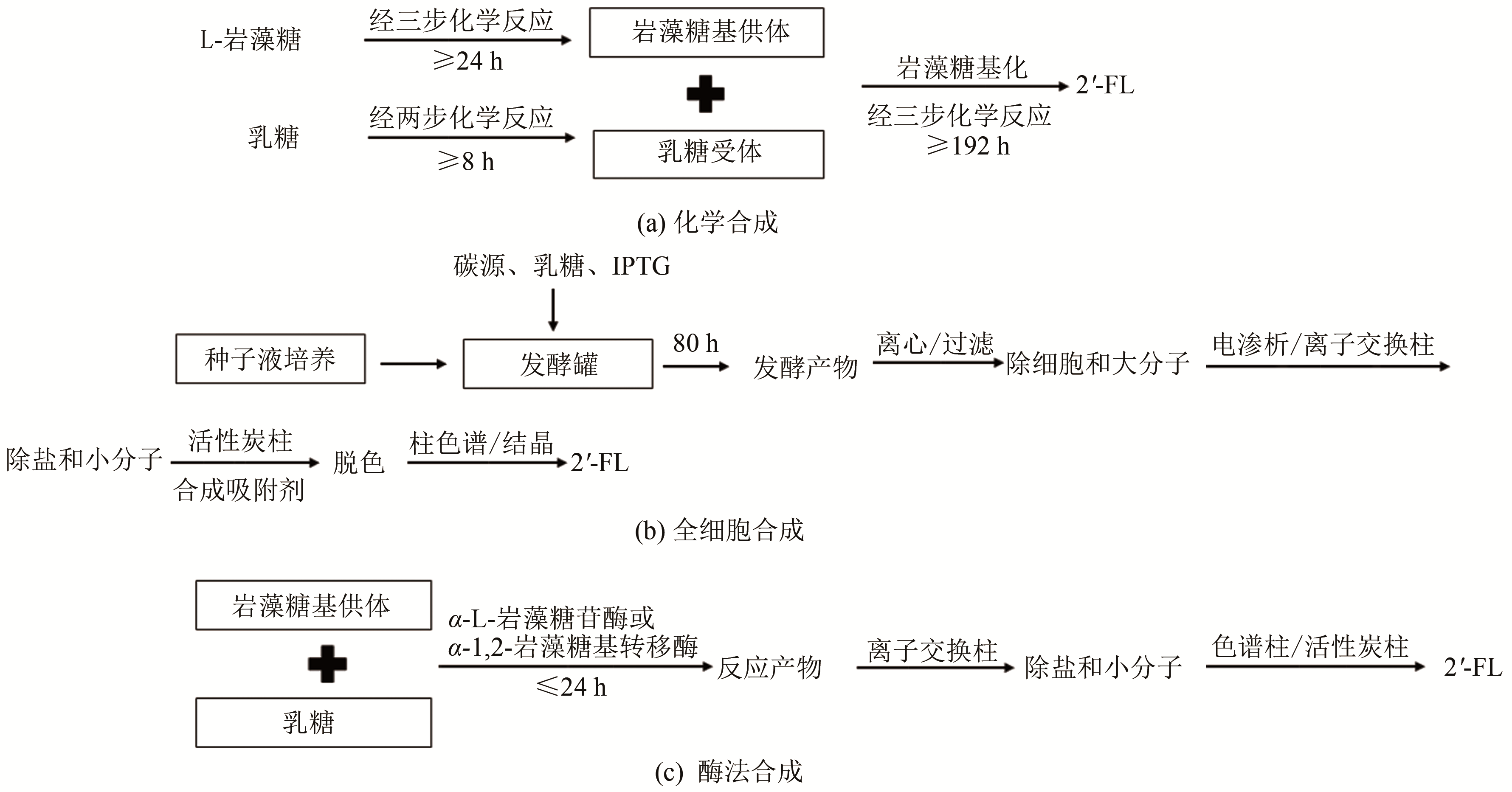
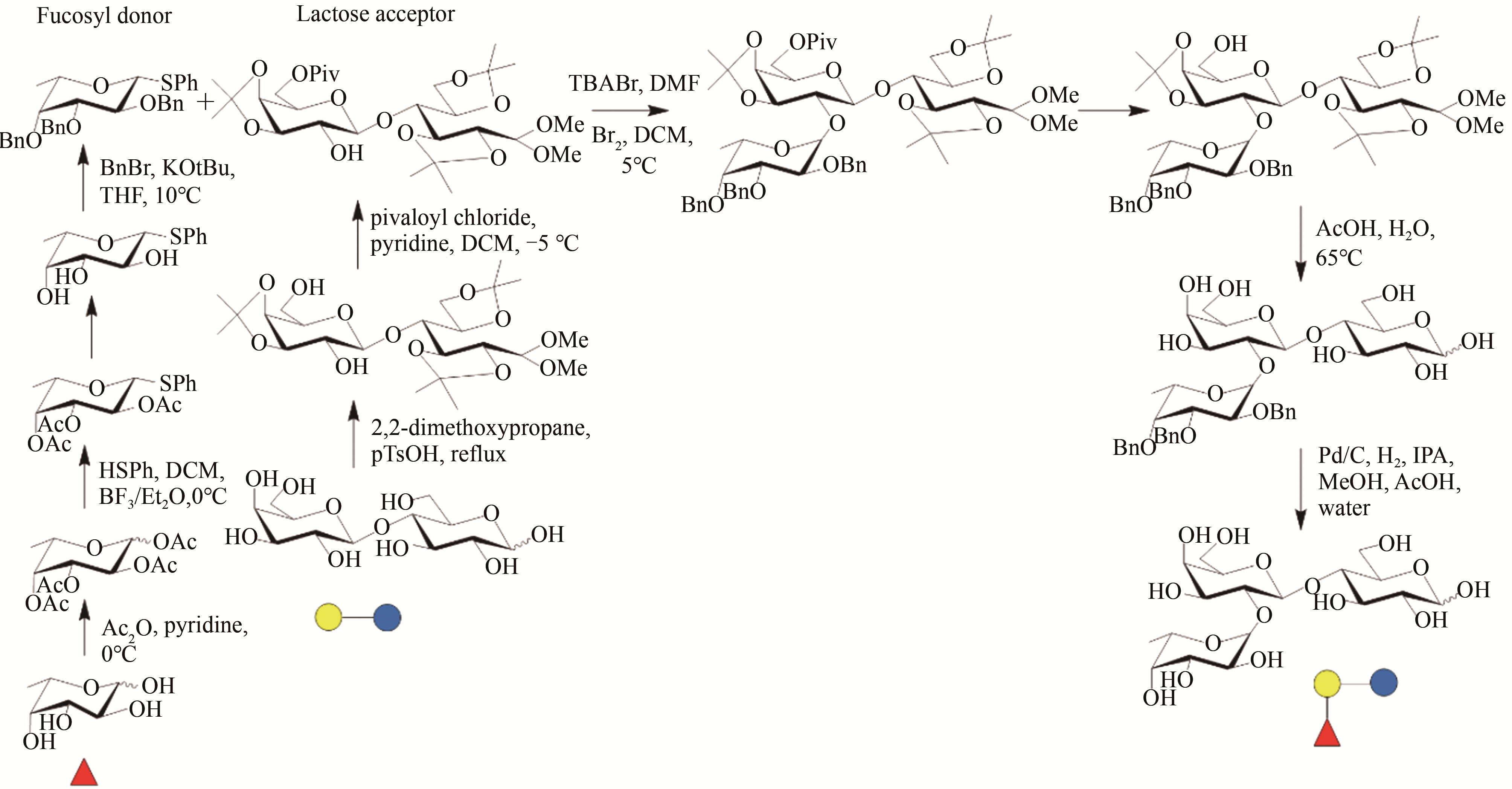
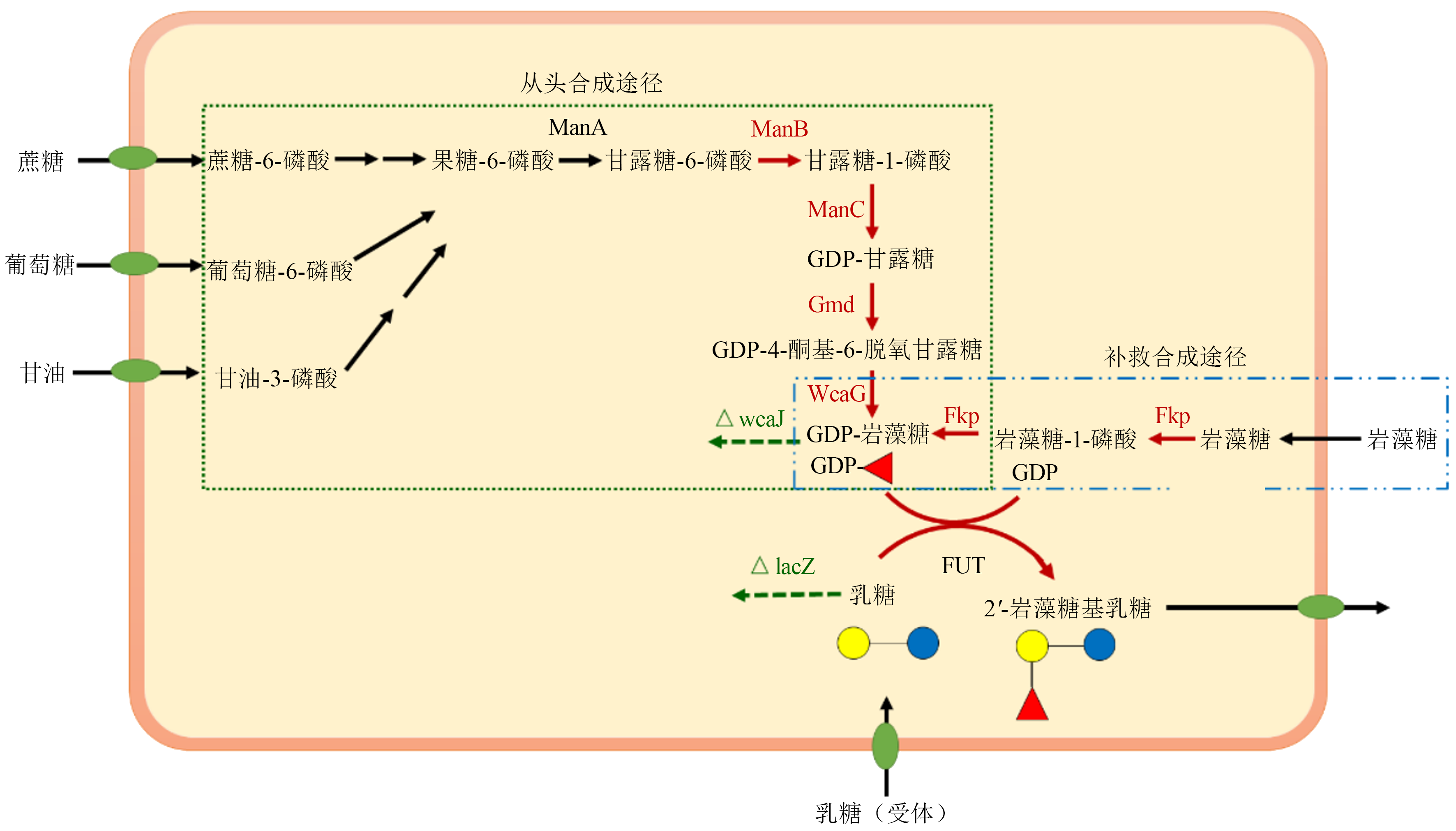
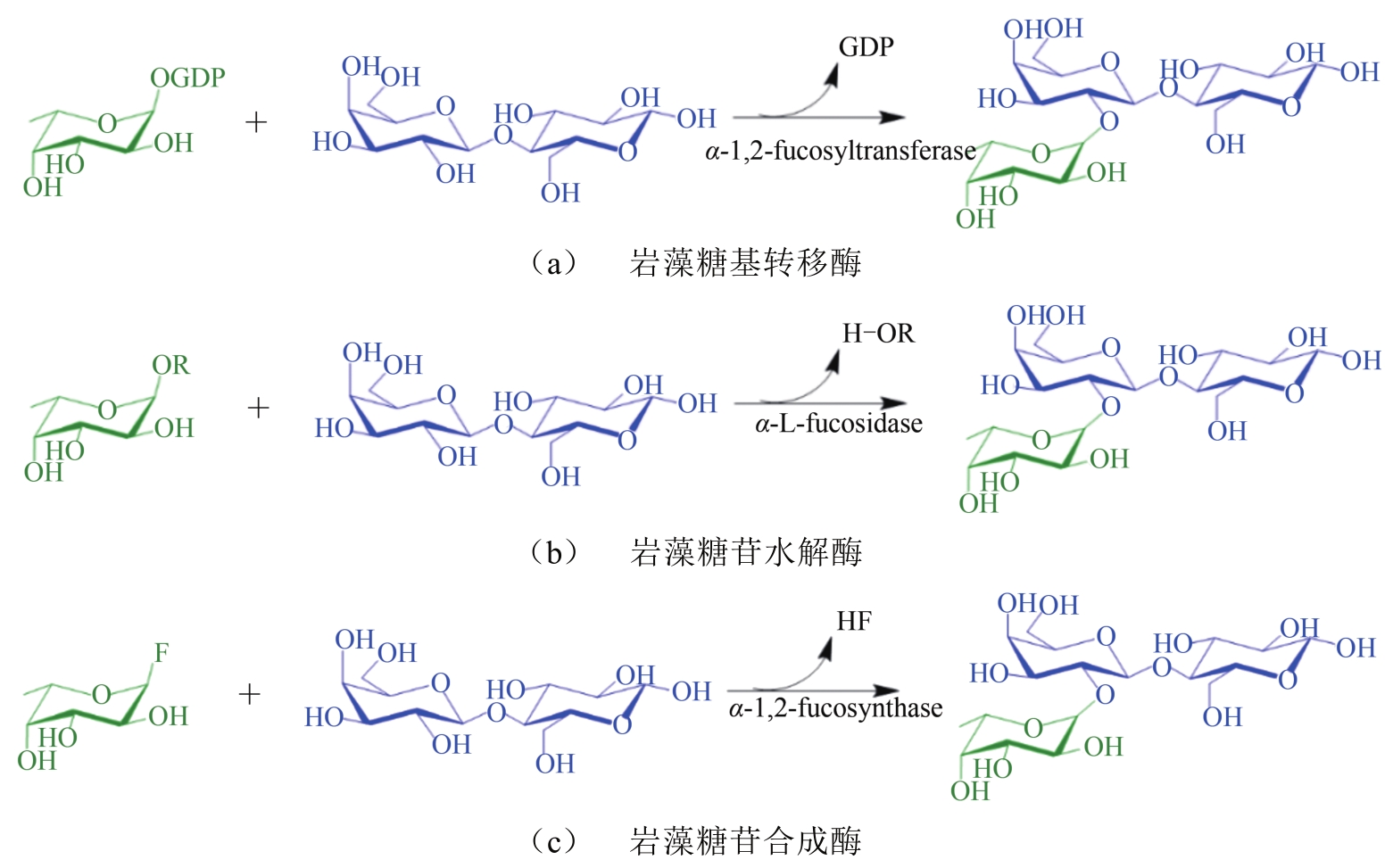
图2 2'-FL的生产工艺流程
Fig. 2 Process flow charts of 2'-FL production by chemical synthesis strategy (a) cell factory approach (b) and enzymatic synthesis method (c)
| 1 | SOUSA Y R F, MEDEIROS L B, PINTADO M M E, et al. Goat milk oligosaccharides: composition, analytical methods and bioactive and nutritional properties [J]. Trends in Food Science & Technology, 2019, 92: 152-161. |
| 2 | HONG Q T, RUHAAK L R, TOTTEN S M, et al. Label free absolute quantitation of oligosaccharides using multiple reaction monitoring [J]. Analytical Chemistry, 2014, 86: 2640-2647. |
| 3 | URASHIMA T, ASAKUMA S, LEO F, et al. The predominance of type I oligosaccharides is a feature specific to human breast milk [J]. Advances in Nutrition, 2012, 3: 473-482. |
| 4 | BYCH K, MIKS M H, MARKUS T J, et al. Production of HMOs using microbial hosts — from cell engineering to large scale production [J]. Current Opinion in Biotechnology, 2019, 56C: 130-137. |
| 5 | VANDENPLAS Y, BERGER B, CARNIELLI V P, et al. Human milk oligosaccharides: 2'-fucosyllactose (2'-FL) and lacto-N-neotetraose (LNnT) in infant formula [J]. Nutrients, 2018, 10(9): 1161. |
| 6 | FAIJES M, CASTEJON-VILATERSANA M, VAL-CID C, et al. Enzymatic and cell factory approaches to the production of human milk oligosaccharides [J]. Biotechnology Advances, 2019, 37: 667-697. |
| 7 | MURATA T, MORIMOTO S, ZENG X X, et al. Enzymatic synthesis of α-L-fucosyl-N-acetyllac-tosamines and 3'-O-α-L-fucosyllactose utilizing α-L-fucosidases [J]. Carbohydrate Research, 1999, 320(3/4): 192-199. |
| 8 | YAMASHITA K, TACHIBANA Y, KOBATA A. Oligosaccharides of human milk: isolation and characterization of three new disialylfucosyl hexasaccharides [J]. Archives of Biochemistry and Biophysics, 1976, 174(2): 582-591. |
| 9 | MATSUKI T, YAHAGI K, MORI H, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development [J]. Nature Communications, 2016, 7: 11939. |
| 10 | ASAKUMA S, HATAKEYAMA E, URASHIMA T, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated Bifidobacteria [J]. Journal of Biological Chemistry, 2011, 286: 34583-34592. |
| 11 | FERGUSON S A, SIMS I M, BISWAS A, et al. Bifidobacterium bifidum ATCC 15696 and Bifidobacterium breve 24b metabolic interaction based on 2'-O-fucosyl-lactose studied in steady-state cultures in a freter-style chemostat [J]. Applied and Environmental Microbiology, 2019, 85(7): e02783-18. |
| 12 | THONGARAM T, HOEFLINGER J L, CHOW J M, et al. Human milk oligosaccharide consumption by probiotic and human-associated Bifidobacteria and Lactobacilli [J]. Journal of Dairy Science, 2017, 100(10): 7825-7833. |
| 13 | SAKANAKA M, GOTOH A, YOSHIDA K, et al. Varied pathways of infant gut-associated Bifidobacterium to assimilate human milk oligosaccharides: prevalence of the gene set and its correlation with Bifidobacteria-rich microbiota formation [J]. Nutrients, 2019, 12(1). |
| 14 | GARRIDO D, BARILE D, MILLS D A. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract [J]. Advances in Nutrition, 2012, 3(3): 415S-421S. |
| 15 | ZABEL B, YDE C C, ROOS P, et al. Novel genes and metabolite trends in Bifidobacterium longum subsp. infantis Bi-26 metabolism of human milk oligosaccharide 2'-fucosyllactose [J]. Scientific Reports, 2019, 9(1): 7983. |
| 16 | HOEFLINGER J L, DAVIS S R, CHOW J M, et al. In vitro impact of human milk oligosaccharides on Enterobacteriaceae growth [J]. Journal of Agricultural and Food Chemistry, 2015, 63: 3295-3302. |
| 17 | BODE L. The functional biology of human milk oligosaccharides [J]. Early Human Development, 2015, 91(11): 619-622. |
| 18 | YU Z T, NANTHAKUMAR N N, NEWBURG D S. The human milk oligosaccharide 2'-fucosyllactose quenches Campylobacter jejuni-induced inflammation in human epithelial cells HEp-2 and HT-29 and in mouse intestinal mucosa [J]. Journal of Nutrition, 2016, 146: 1980-1990. |
| 19 | MORROW A L, RUIZ-PALACIOS G M, ALTAYE M, et al. Human milk oligosaccharide blood group epitopes and innate immune protection against Campylobacter and calicivirus diarrhea in breastfed infants [J]. Advances in Experimental Medicine and Biology, 2004, 554: 443-446. |
| 20 | KONG C L, ELDERMAN M, CHENG L H, et al. Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non-digestible carbohydrates [J]. Molecular Nutrition & Food Research, 2019, 63(17): e1900303. |
| 21 | WEICHERT S, JENNEWEIN S, HUFNER E, et al. Bioengineered 2'-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines [J]. Nutrition Research, 2013, 33(10): 831-838. |
| 22 | GOOD M, SODHI C P, YAMAGUCHI Y, et al. The human milk oligosaccharide 2'-fucosyllactose attenuates the severity of experimental NEC by enhancing mesenteric perfusion in the neonatal intestine [J]. British Journal of Nutrition, 2016, 116: 1175-1187. |
| 23 | CASTILLO-COURTADE L, HAN S, LEE S, et al. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model [J]. Allergy, 2015, 70: 1091-1102. |
| 24 | CHARBONNEAU M R, ÓDONNELL D, BLANTON L V, et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition [J]. Cell, 2016, 164: 859-871. |
| 25 | WU K J, CHEN Y H, BAE E K, et al. Human milk oligosaccharide 2'-fucosyllactose reduces neurodegeneration in stroke brain [J]. Translational Stroke Research, 2020, 11: 1001-1011. |
| 26 | VAZQUEZ E, BARRANCO A, RAMIREZ M, et al. Effects of a human milk oligosaccharide, 2'-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents [J]. Journal of Nutritional Biochemistry, 2015, 26: 455-465. |
| 27 | AGOSTION K, HEDEROS M J, BAJZA I, et al. Kilogram scale chemical synthesis of 2'-fucosyllactose [J]. Carbohydrate Research, 2019, 476: 71-77. |
| 28 | JUNG S M, CHIN Y W, LEE Y G. Enhanced production of 2'-fucosyllactose from fucose by elimination of rhamnose isomerase and arabinose isomerase in engineered Escherichia coli [J]. Biotechnology & Bioengineering, 2019, 116(9): 2412-2417. |
| 29 | ALBERMANN C, PIEPERSBERG W, WEHMEIER U F. Synthesis of the milk oligosaccharide 2'-fucosyllactose using recombinant bacterial enzymes [J]. Carbohydrate Research, 2001, 334: 97-103. |
| 30 | 陈坚, 邓洁莹, 李江华, 等. 母乳寡糖的生物合成研究进展[J]. 中国食品学报, 2016, 16(11): 1-8. |
| CHEN Jian, DENG Jieying, LI Jianghua, et al. Advances in biosynthesis of breast milk oligosaccharides [J]. Journal of Chinese Institute of Food Science and Technology, 2016, 16(11): 1-8. | |
| 31 | HUANG Di, YANG Kexin, LIU Jia, et al. Metabolic engineering of Escherichia coli for the production of 2'-fucosyllactose and 3-fucosyllactose through modular pathway enhancement [J]. Metabolic Engineering, 2017, 41: 23-38. |
| 32 | DUMON C, PRIEM B, MARTIN S L, et al. In vivo fucosylation of lacto-N-neotetraose and lacto-N-neohexaose by heterologous expression of Helicobacter pylori α-1,3 fucosyltransferase in engineered Escherichia coli [J]. Glycoconjugate Journal, 2001, 18: 465-474. |
| 33 | CHIN Y W, SEO N, KIM J H, et al. Metabolic engineering of Escherichia coli to produce 2'-fucosyllactose via salvage pathway of guanosine 5'-diphosphate (GDP)-L-fucose [J]. Biotechnology and Bioengineering, 2016, 113: 2443-2452. |
| 34 | BAUMGÄRTNER F, SEITZ L, SPRENGER G A, et al. Construction of Escherichia coli strains with chromosomally integrated expression cassettes for the synthesis of 2'-fucosyllactose [J]. Microbial Cell Factories, 2013, 12: 40. |
| 35 | 王永胜, 王硕, 张慧林, 等. L-岩藻糖对母乳寡糖(HMOs)合成的意义及其产业化研究进展[J]. 中国农学通报, 2019, 35(11): 127-132. |
| WANG Yongsheng, WANG Shuo, ZHANG Huilin, et al. L-fucose: the significance to synthesis of human milk oligosaccharides (HMOs) and its research progress of industrialization [J]. Chinese Agricultural Science Bulletin, 2019, 35(11): 127-132. | |
| 36 | PETSCHACHER B, NIDETZKY B. Biotechnological production of fucosylated human milk oligosaccharides: prokaryotic fucosyltransferases and their use in biocatalytic cascades or whole cell conversion systems [J]. Journal of Biotechnology, 2016, 235: 61-83. |
| 37 | DROUILLARD S, DRIGUEZ H, SAMAIN E. Large-scale synthesis of H-antigen oligosaccharides by expressing Helicobacter pylori α1,2-fucosyltransferase in metabolically engineered Escherichia coli cells [J]. Angewandte Chemie International Edition, 2006, 45: 1778-1780. |
| 38 | MERIGHI M, MCCOY J M, HEIDTMAN M, et al. Biosynthesis of human milk oligosaccharides in engineered bacteria: US9970018 [P]. 2018-07-05. |
| 39 | CHIN Y W, KIM J Y, LEE W H, et al. Enhanced production of 2'-fucosyllactose in engineered Escherichia coli BL21 star (DE3) by modulation of lactose metabolism and fucosyltransferase [J]. Journal of Biotechnology, 2015, 210: 107-115. |
| 40 | DENG Jieying, GU Liuyan, CHEN Taichi, et al. Engineering the substrate transport and cofactor regeneration systems for enhancing 2'-fucosyllactose synthesis in Bacillus subtilis [J]. ACS Synthetic Biology, 2019, 8(10): 2418-2427. |
| 41 | HOLLANDS K, BARONA C M, GIBSONB K J. Engineering two species of yeast as cell factories for 2'-fucosyllactose [J]. Metabolic Engineering, 2019, 52: 232-242. |
| 42 | PALCIC M M. Glycosyltransferases as biocatalysts [J]. Current Opinion in Chemical Biology, 2011, 15: 226-233. |
| 43 | CIPOLLA L. Carbohydrate chemistry: state of the art and challenges for drug development [M]. London: Imperial College Press, 2015: 215-245. |
| 44 | CHOI Y H, KIM J H, PARK B S, et al. Solubilization and iterative saturation mutagenesis of α1,3-fucosyltransferase from Helicobacter pylori to enhance its catalytic efficiency [J]. Biotechnology and Bioengineering, 2016, 113(8): 1666-1675. |
| 45 | TAN Yumeng, ZHANG Yong, HAN Yunbin, et al. Directed evolution of an α1,3-fucosyltransferase using a single-cell ultrahigh-throughput screening method [J]. Science Advances, 2019, 5(10): eaaw8451. |
| 46 | ZHAO Chao, WU Yijing, YU Hai, et al. One-pot multienzyme (OPME) synthesis of human blood group H antigens and a human milk oligosaccharide (HMOS) with highly active Thermosynechococcus elongatus α1,2-fucosyltransferase [J]. Chemical Communications, 2016, 52(20): 3899-3902. |
| 47 | GUZMAN-RODRIGUEZ F, ALATORRE-SANTAMARIA S, GOMEZ-RUI L, et al. Employment of fucosidases for the synthesis of fucosylated oligosaccharides with biological potential [J]. Applied Biochemistry and Biotechnology, 2019, 66: 172-191. |
| 48 | ESCAMILLA-LOZANO Y, GUZMAN-RODRIGUEZ F, ALATORRE-SANTAMARIA S, et al. Synthesis of fucosyl-oligosaccharides using α-L-fucosidase from Lactobacillus rhamnosus GG [J]. Molecules, 2019, 24(13): 2402. |
| 49 | GUZMAN-RODRIGUEZ F, ALATORRE-SANTAMARIA S, GOMEZ-RUI L, et al. Synthesis of a fucosylated trisaccharide via transglycosylation by α-L-fucosidase from Thermotoga maritima [J]. Applied Biochemistry and Biotechnology, 2018, 186: 681-691. |
| 50 | ZEUNER B, MUSCHIOL J, HOLCK J, et al. Substrate specificity and transfucosylation activity of GH 29 α-L-fucosidases for enzymatic production of human milk oligosaccharides [J]. New Biotechnology, 2018, 41: 34-45. |
| 51 | LEZYK M, JERS C, KJAERULFF L, et al. Novel α-L-fucosidases from a soil metagenome for production of fucosylated human milk oligosaccharides [J]. PLoS One, 2016, 11(1): e0147438. |
| 52 | MACKENZIE L F, WANG Q P, WARREN R A J, et al. Glycosynthases: mutant glycosidases for oligosaccharide synthesis [J]. Journal of the American Chemical Society, 1998, 7863: 5583-5584. |
| 53 | WADA J, HONDA Y, NAGAE M. 1, 2-α-L-Fucosynthase: a glycosynthase derived from an inverting α-glycosidase with an unusual reaction mechanism [J]. FEBS Letters, 2008, 582: 3739-3743. |
| 54 | SUGIYAMA Y, GOTOH A, KATOH T. Introduction of H-antigens into oligosaccharides and sugar chains of glycoproteins using highly efficient 1,2-α-L-fucosynthase [J]. Glycobiology, 2016, 26(11): 1235-1247. |
| 55 | OSANJO G, DION M, DRONE J, et al. Directed evolution of the α-L-fucosidase from Thermotoga maritima into an α-L-transfucosidase [J]. Biochemistry, 2007, 46(4): 1022-1033. |
| 56 | SAUMONNEAU A, CHAMPION E, PELTIER-PAIN P, et al. Design of an α-L-transfucosidase for the synthesis of fucosylated HMOs [J]. Glycobiology, 2016, 26(3): 261-269. |
| 57 | PAULY M, KEEGSTRA K. Biosynthesis of the plant cell wall matrix polysaccharide xyloglucan [J]. Annual Review of Plant Biology, 2016, 67: 235-59. |
| 58 | KUSAYKIN M I, SILCHENKO A S, ZAKHARENKO A M, et al. Fucoidanases [J]. Glycobiology, 2016, 26(1): 3-12. |
| 59 | BERTEAU O, MCCORT I, GOASDOUE N, et al. Characterization of a new α-L-fucosidase isolated from the marine mollusk Pecten maximus that catalyzes the hydrolysis of α-L-fucose from algal fucoidan (Ascophyllum nodosum) [J]. Glycobiology, 2002, 12(4): 273-282. |
| 60 | BERTEAU O, BIELICKI J, KILONDA A, et al. α-L-fucosidases: exoglycosidases with unusual transglycosylation properties [J]. Biochemistry, 2004, 43(24): 7881-7891. |
| [1] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [2] | 程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980. |
| [3] | 杨皓然, 叶发荣, 黄平, 王平. 糖蛋白合成的研究进展[J]. 合成生物学, 2024, 5(5): 1072-1101. |
| [4] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [5] | 康里奇, 谈攀, 洪亮. 人工智能时代下的酶工程[J]. 合成生物学, 2023, 4(3): 524-534. |
| [6] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [7] | 崔馨予, 吴冉冉, 王园明, 朱之光. 酶促生物电催化系统的设计构建与强化[J]. 合成生物学, 2022, 3(5): 1006-1030. |
| [8] | 杨璐, 瞿旭东. 亚胺还原酶在手性胺合成中的应用[J]. 合成生物学, 2022, 3(3): 516-529. |
| [9] | 张发光, 曲戈, 孙周通, 马军安. 从化学合成到生物合成——天然产物全合成新趋势[J]. 合成生物学, 2021, 2(5): 674-696. |
| [10] | 张以恒. 忆王义翘教授对生物炼制的贡献和我对此领域未来发展的观点[J]. 合成生物学, 2021, 2(4): 497-508. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||