合成生物学 ›› 2025, Vol. 6 ›› Issue (4): 715-727.DOI: 10.12211/2096-8280.2025-020
生物制造的PE值与PX值:定义与应用
- 1.中国科学院天津工业生物技术研究所,低碳合成工程生物学全国重点实验室,天津 300308
2.中国科学院天津工业生物技术研究所,体外合成生物学中心,天津 300308
-
收稿日期:2025-03-20修回日期:2025-04-29出版日期:2025-08-31发布日期:2025-09-03 -
通讯作者:张以恒 -
作者简介:张以恒 (1971—),低碳合成工程生物学全国重点实验室主任,中国科学院天津工业生物技术研究所体外合成生物学中心主任,曾经任美国弗吉尼亚理工大学终身正教授。他是体外合成生物学的奠基人之一与产业化领跑者,创建体外生物转化(ivBT)技术平台,率先提出“人工多酶分子机器”概念并实现产业化突破。在秸秆制粮、体外无细胞呼吸作用(无氧呼吸作用产氢、有氧呼吸作用制电)、人工合成淀粉、淀粉制塔格糖与肌醇等方向取得了一系列原创性突破。E-mail:zhang_xw@tib.cas.cn -
基金资助:国家重点研发计划“合成生物学”重点专项(2022YFA0912300);国家自然科学基金面上项目(NSFC32271544);合成生物学海河实验室颠覆性创新项目(22HHSWSS000155);天津市合成生物技术创新能力提升行动项目(TSBICIP-CXRC-067)
PE and PX values in biomanufacturing: definitions and applications
ZHANG Yi-Heng P. Job1,2( ), CHEN Xuemei2, HAN Pingping1,2
), CHEN Xuemei2, HAN Pingping1,2
- 1.State Key Laboratory of Engineering Biology for Low-Carbon Manufacturing,Tianjin Institute of Industrial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
2.In vitro Synthetic Biology Center,Tianjin Institute of Industrial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
-
Received:2025-03-20Revised:2025-04-29Online:2025-08-31Published:2025-09-03 -
Contact:ZHANG Yi-Heng P. Job
摘要:
生物制造是战略新兴产业的典型代表,是生物经济的新质生产力。作者曾提出“道法术器”对工业生物制造的哲学指导意义。为进一步阐述生物制造中“术以立策”的原则,本文首次提出衡量生物催化剂(即“术”)水平的关键经济指标——PE值(product-to-enzyme ratio)与PX值[product-to-X(cell)ratio]。这两个指标具有简单、透明且量化的属性。PE值表示产品与非细胞催化剂(酶分子或多酶分子机器,以下简称“多酶机器”)的质量比值,也可通过技术指标总转换数(total turn-over number,TTN)计算其理论值。PX值应用于细胞工厂发酵,表示产品与细胞催化剂的质量比。基于PE值与PX值,可以快速估算不同生物制造过程中的生物催化剂成本,进而指导降低降本增效的关键路径。作者汇总了生物制造的产业案例及文献数据,展示了酶分子及多酶分子机器PE值、细胞工厂PX值的特点。研究表明,淀粉酶水解淀粉的PE值是纤维素酶水解纤维素的50~100倍;在固态纤维素水解过程中,纤维素酶的超大用量是非粮生物质糖化与利用的最大经济障碍。最后,本文探讨了PE值的技术改进路径,特别是多酶共固定技术的潜力,并明确了工业酶皇冠——纤维素酶研究的新方向。PE值与PX值的分析将为生物制造战略新兴产业的发展提供全新的视角,深化了对生物制造中关键“芯片”——生物催化剂成本的理解,为未来技术的发展提供重要参考与指导。
中图分类号:
引用本文
张以恒, 陈雪梅, 韩平平. 生物制造的PE值与PX值:定义与应用[J]. 合成生物学, 2025, 6(4): 715-727.
ZHANG Yi-Heng P. Job, CHEN Xuemei, HAN Pingping. PE and PX values in biomanufacturing: definitions and applications[J]. Synthetic Biology Journal, 2025, 6(4): 715-727.
| 比较内容 | 分泌酶 | 胞内酶 |
|---|---|---|
| 代表性菌 | 毕赤酵母、里氏木霉、枯草芽孢杆菌、黑曲霉 | 大肠杆菌、枯草芽孢杆菌 |
| 代表性酶制剂 | 植酸酶、纤维素酶、木聚糖酶、蛋白酶、液化酶、糖化酶、脂肪酶、果胶酶、漆酶等 | 木糖异构酶、核酸酶、SOD、甲酸脱氢酶、醇脱氢酶等 |
| 蛋白表达水平 | 200 g/L(最高),一般在10~50 g/L左右 | 5~10 g/L(最高),大多数在1~5 g/L左右 |
| 蛋白制造成本[ | 约100元/kg酶干重 | 约250~1000元/kg酶干重 |
| 酶分离难易程度 | 容易,低成本 | 比较贵 |
| 目标酶 | 有限制,与底盘细胞的分泌同源酶序列高度相似 | 普适性 |
| 技术难点 | 基因操作难,要长期(几年以上)系统优化 | 基因操作相当容易 |
| 蛋白表达的比喻 | 牛产奶 | 杀牛产肉 |
表1 工业酶的分泌与胞内表达生产方法比较
Table 1 Comparison of production methods for industrial enzymes: secretion versus intracellular expression
| 比较内容 | 分泌酶 | 胞内酶 |
|---|---|---|
| 代表性菌 | 毕赤酵母、里氏木霉、枯草芽孢杆菌、黑曲霉 | 大肠杆菌、枯草芽孢杆菌 |
| 代表性酶制剂 | 植酸酶、纤维素酶、木聚糖酶、蛋白酶、液化酶、糖化酶、脂肪酶、果胶酶、漆酶等 | 木糖异构酶、核酸酶、SOD、甲酸脱氢酶、醇脱氢酶等 |
| 蛋白表达水平 | 200 g/L(最高),一般在10~50 g/L左右 | 5~10 g/L(最高),大多数在1~5 g/L左右 |
| 蛋白制造成本[ | 约100元/kg酶干重 | 约250~1000元/kg酶干重 |
| 酶分离难易程度 | 容易,低成本 | 比较贵 |
| 目标酶 | 有限制,与底盘细胞的分泌同源酶序列高度相似 | 普适性 |
| 技术难点 | 基因操作难,要长期(几年以上)系统优化 | 基因操作相当容易 |
| 蛋白表达的比喻 | 牛产奶 | 杀牛产肉 |
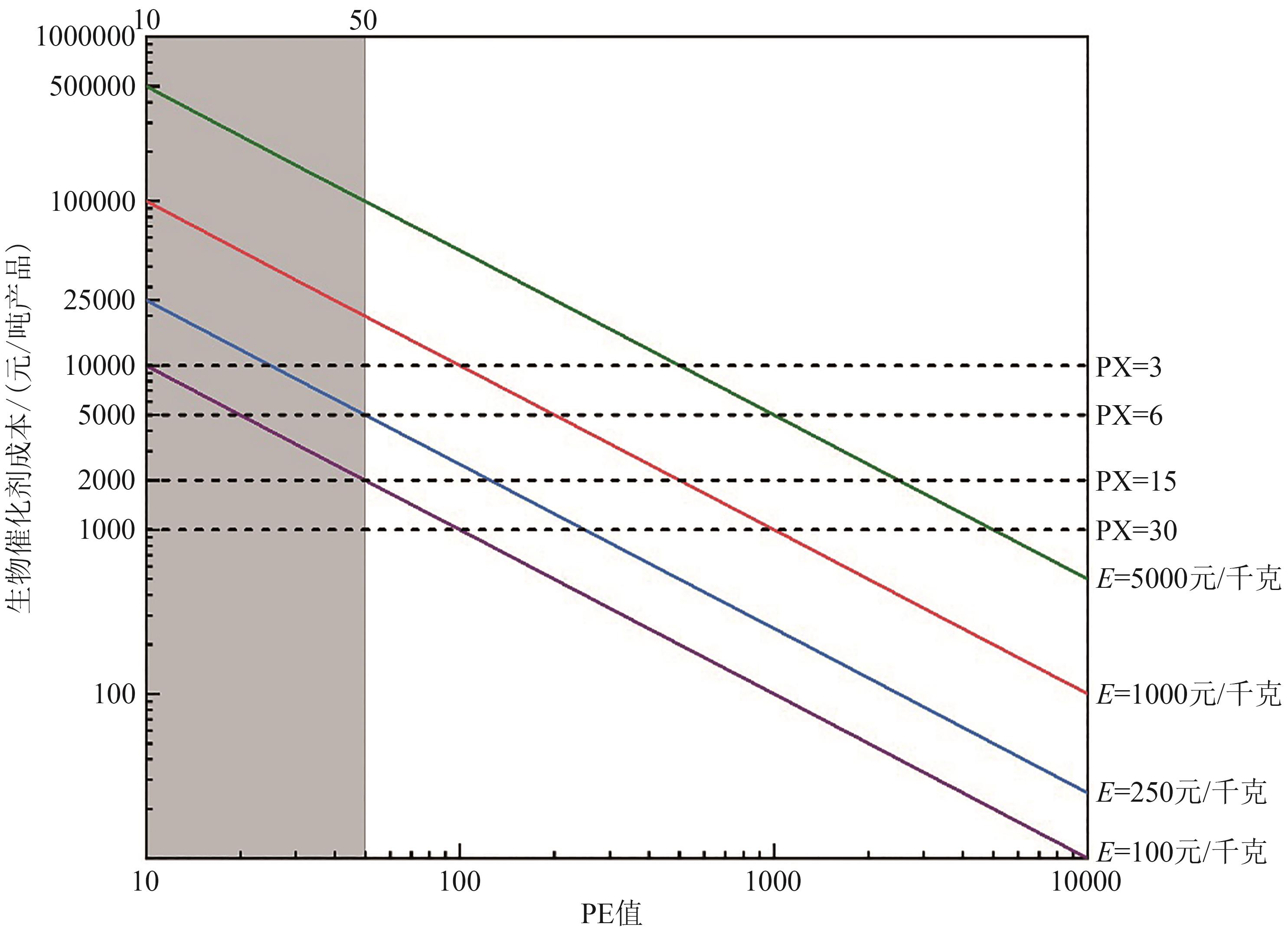
图1 微生物发酵与体外生物转化(ivBT)的生物催化剂成本[x轴—PE值,计算公式为:PE=产品质量/酶干重;y轴—生物催化剂成本(元/吨产品),即细胞催化剂成本或酶成本;虚线—细胞催化剂成本(元/吨产品),计算公式为:细胞催化剂成本=细胞制备成本/PX,其中虚线由上至下PX值分别为3、6、15、30,细胞制备成本为30元/kg;实线—酶成本(元/吨产品),计算公式为:酶成本=酶制备成本/PE,其中实线由上至下酶制备成本(E)分别为5000元/kg、1000元/kg、250元/kg、100元/kg]
Fig. 1 Biocatalyst costs of microbial fermentation and invitro biotransformation (ivBT)[X-axis-PE value, calculated as: PE = product mass/enzyme dry weight. Y-axis-Biocatalyst cost (CNY/tonne of product), referring to either the cell cost or enzyme cost. Dashed line-Cell cost (CNY/tonne of product), calculated as: Cell cost = Cell preparation cost×1000 (kg/tonne)/PX, where PX values are 3, 6, 15, and 30 (top to bottom), and the cell preparation cost is 30 CNY/kg dry cell weight. Solid line-Enzyme cost (CNY/tonne of product), calculated as: Enzyme cost = Enzyme preparation cost×1000 (kg/t)/PE, where enzyme preparation costs (E) are 5000, 1000, 250, and 100 CNY/kg (top to bottom).]
| 产物 | 细胞工厂 | PX值 |
|---|---|---|
| 重组蛋白 | 大肠杆菌 | 0.1~0.2 |
| 全蛋白(可食用) | SCP生产菌 | 0.4~0.65 |
| α-淀粉酶 | 枯草芽孢杆菌 | 0.5~2 |
| 糖化酶 | 黑曲霉 | 0.5~2 |
| 植酸酶 | 毕赤酵母 | 1 |
| 青霉素 | 产黄青霉 | 1~2 |
| 蛋白酶 | 枯草芽孢杆菌 | 1~3 |
| 丁二酸 | 大肠杆菌 | 2~3 |
| 纤维素酶 | 里氏木霉 | 2~4 |
| 赖氨酸 | 大肠杆菌 | 2~4 |
| 谷氨酸 | 谷氨酸棒杆菌 | 3~4 |
| 乙醇 | 酿酒酵母 | 5~10 |
| 乳酸 | 大肠杆菌 | 5~10 |
| 柠檬酸 | 黑曲霉 | 8~10 |
| L-丙氨酸 | 大肠杆菌 | 约10 |
表2 细胞工厂生物制造代表性产品的PX值现状
Table 2 Current PX values for representative products in cell factory biomanufacturing
| 产物 | 细胞工厂 | PX值 |
|---|---|---|
| 重组蛋白 | 大肠杆菌 | 0.1~0.2 |
| 全蛋白(可食用) | SCP生产菌 | 0.4~0.65 |
| α-淀粉酶 | 枯草芽孢杆菌 | 0.5~2 |
| 糖化酶 | 黑曲霉 | 0.5~2 |
| 植酸酶 | 毕赤酵母 | 1 |
| 青霉素 | 产黄青霉 | 1~2 |
| 蛋白酶 | 枯草芽孢杆菌 | 1~3 |
| 丁二酸 | 大肠杆菌 | 2~3 |
| 纤维素酶 | 里氏木霉 | 2~4 |
| 赖氨酸 | 大肠杆菌 | 2~4 |
| 谷氨酸 | 谷氨酸棒杆菌 | 3~4 |
| 乙醇 | 酿酒酵母 | 5~10 |
| 乳酸 | 大肠杆菌 | 5~10 |
| 柠檬酸 | 黑曲霉 | 8~10 |
| L-丙氨酸 | 大肠杆菌 | 约10 |
| 酶类别 | 酶名 | 底物 | 产物 | 酶形式 | 酶数量 | kcat/s-1 | kd/s-1 | TTN | MWP/Da | MWE/kDa | PE值① (理论) | PE值② (实际) | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 异构酶 | 葡萄糖磷酸异构酶 | 果糖-6-磷酸 | 葡萄糖-6-磷酸 | 游离 | 1 | 2765 | 8.0×10-5 | 3.4E×107 | 260.14 | 48 | 1.9×105 | NA | [ |
| 固定化 | 1 | 2198 | 1.0×10-6 | 2.2×109 | 260.14 | 67 | 8.4×106 | NA | [ | ||||
| D-塔格糖-3-差向异构酶 | D-果糖 | D-阿洛酮糖 | 游离 | 1 | 55.1 | 1.7×10-4 | 3.3×105 | 180 | 33 | 1.8×103 | NA | [ | |
| 固定化 | 1 | 93.4 | 3.9×10-5 | 2.4×106 | 180 | 33 | 1.3×104 | 1000 | [ | ||||
| 木糖异构酶 | D-葡萄糖 | D-果糖 | 游离 | 1 | 30 | 7.7×10-6 | 3.9×106 | 180 | 46 | 1.5×104 | NA | [ | |
| 固定化 | 1 | 27 | 1.0×10-6 | 2.7×107 | 180 | 46 | 1.1×105 | 1.0×105 | [ | ||||
| 水解酶 | 青霉素G酰胺酶 | 青霉素G | 6-氨基青霉烷酸 | 游离 | 1 | 44.6 | 1.1×10-4 | 4.0×105 | 216.25 | 88 | 9.8×102 | NA | [ |
| 固定化 | 1 | NA | NA | NA | NA | NA | NA | 600 | [ | ||||
| 脂肪酶 | 油脂+醇类 | 脂肪酸酯 | 游离 | 1 | 500 | 4.1×10-5 | 1.2×107 | 250 | 43 | 6.9×104 | 150 | [ | |
| 固定化 | 1 | NA | NA | NA | NA | NA | NA | 1000~5000 | [ | ||||
| 异构酶 | 核糖磷酸异构酶 | 核酮糖-5-磷酸 | 核糖-5-磷酸 | 游离 | 1 | 540 | 2.7×10-6 | 2.0×108 | 230.1 | 15.9 | 2.9×106 | NA | [ |
| 葡萄糖磷酸变位酶 | 葡萄糖-1-磷酸 | 葡萄糖-6-磷酸 | 游离 | 1 | 190 | 2.7×10-6 | 7.1×107 | 260.14 | 64.9 | 2.8×105 | NA | [ | |
| 水解酶 | 果糖-1,6-二磷酸酶 | 果糖-1,6-二磷酸 | 果糖-6-磷酸 | 游离 | 1 | 8.57 | 1.4×10-7 | 5.9×107 | 260.14 | 28 | 5.5×105 | NA | [ |
| β-糖苷酶 | 纤维二糖 | 葡萄糖 | 游离 | 1 | 208 | 3.5×10-5 | 6.0×106 | 180 | 62.78 | 1.7×104 | NA | [ | |
| 氧化还原酶 | 肉桂醇脱氢酶 | 肉桂醇 | 肉桂醛 | 游离 | 1 | 18 | 2.00×10-3 | 9.0×103 | 132.159 | 80 | 15 | NA | [ |
| 氢酶 | H+ | 氢气 | 游离 | 1 | 1030 | 2.7×10-6 | 3.9×108 | 2 | 110 | 7.0×103 | NA | [ | |
| 胺脱氢酶 | NAD+ | NADH | 游离 | 1 | 167 | 1.1×10-6 | 1.5×108 | 663.43 | 45 | 2.1×106 | NA | [ | |
| 6-磷酸葡萄糖酸脱氢酶 | 葡萄糖6-磷酸 | 6-磷酸葡萄糖酸 | 游离 | 1 | 325 | 1.4×10-6 | 2.4×108 | 495.3 | 53 | 2.2×106 | NA | [ | |
| 转移酶 | 转醛缩酶 | 果糖-6-磷酸,赤藓糖-4-磷酸 | 景天糖-7-磷酸 | 游离 | 1 | 22.3 | 1.5×10-5 | 1.5×106 | 290.16 | 24 | 1.8×104 | NA | [ |
| 混合酶 | 伊沙替韦酶 | 2-乙酰甘油 | 伊沙替韦 | 游离 | 9 | NA | NA | NA | NA | NA | NA | 1 | [ |
| PHB合成 | 淀粉 | PHB | 游离 | 19~20 | NA | NA | NA | NA | NA | NA | 1 | [ | |
| 合成淀粉多酶机器 | CO2+H2 | 合成淀粉 | 游离 | 13 | NA | NA | NA | NA | NA | NA | 1 | [ | |
| 单萜烯酶 | 葡萄糖 | 单萜烯 | 游离 | 27 | NA | NA | NA | NA | NA | NA | 2 | [ | |
| 肌醇多酶机器 | 淀粉 | 肌醇 | 游离 | 4~6 | 1.1 | 2.0×10-5 | 5.5×104 | 180 | 50 | 196 | 13 | [ | |
| 固定化 | 4~6 | 1.2 | 3.5×10-6 | 3.4×105 | 180 | 50 | 1242 | 60~80 | [ | ||||
| D-塔格糖多酶机器 | 淀粉 | D-塔格糖 | 游离 | 5~6 | NA | NA | NA | NA | NA | NA | 35 | [ | |
| 固定化 | 4~6 | NA | NA | NA | NA | NA | NA | 103 | [ | ||||
| 纤维素酶(混合) | (预处理)生物质 | D-葡萄糖 | 游离 | 3~10 | NA | NA | NA | NA | NA | NA | 50~100 | [ | |
| 淀粉酶(混合) | (糊化)淀粉 | D-葡萄糖 | 游离 | 2~3 | NA | NA | NA | NA | NA | NA | 5000 | [ |
表3 酶催化生物制造代表性产品的PE值现状
Table 3 Current PE values for representative products in enzyme-catalyzed biomanufacturing
| 酶类别 | 酶名 | 底物 | 产物 | 酶形式 | 酶数量 | kcat/s-1 | kd/s-1 | TTN | MWP/Da | MWE/kDa | PE值① (理论) | PE值② (实际) | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 异构酶 | 葡萄糖磷酸异构酶 | 果糖-6-磷酸 | 葡萄糖-6-磷酸 | 游离 | 1 | 2765 | 8.0×10-5 | 3.4E×107 | 260.14 | 48 | 1.9×105 | NA | [ |
| 固定化 | 1 | 2198 | 1.0×10-6 | 2.2×109 | 260.14 | 67 | 8.4×106 | NA | [ | ||||
| D-塔格糖-3-差向异构酶 | D-果糖 | D-阿洛酮糖 | 游离 | 1 | 55.1 | 1.7×10-4 | 3.3×105 | 180 | 33 | 1.8×103 | NA | [ | |
| 固定化 | 1 | 93.4 | 3.9×10-5 | 2.4×106 | 180 | 33 | 1.3×104 | 1000 | [ | ||||
| 木糖异构酶 | D-葡萄糖 | D-果糖 | 游离 | 1 | 30 | 7.7×10-6 | 3.9×106 | 180 | 46 | 1.5×104 | NA | [ | |
| 固定化 | 1 | 27 | 1.0×10-6 | 2.7×107 | 180 | 46 | 1.1×105 | 1.0×105 | [ | ||||
| 水解酶 | 青霉素G酰胺酶 | 青霉素G | 6-氨基青霉烷酸 | 游离 | 1 | 44.6 | 1.1×10-4 | 4.0×105 | 216.25 | 88 | 9.8×102 | NA | [ |
| 固定化 | 1 | NA | NA | NA | NA | NA | NA | 600 | [ | ||||
| 脂肪酶 | 油脂+醇类 | 脂肪酸酯 | 游离 | 1 | 500 | 4.1×10-5 | 1.2×107 | 250 | 43 | 6.9×104 | 150 | [ | |
| 固定化 | 1 | NA | NA | NA | NA | NA | NA | 1000~5000 | [ | ||||
| 异构酶 | 核糖磷酸异构酶 | 核酮糖-5-磷酸 | 核糖-5-磷酸 | 游离 | 1 | 540 | 2.7×10-6 | 2.0×108 | 230.1 | 15.9 | 2.9×106 | NA | [ |
| 葡萄糖磷酸变位酶 | 葡萄糖-1-磷酸 | 葡萄糖-6-磷酸 | 游离 | 1 | 190 | 2.7×10-6 | 7.1×107 | 260.14 | 64.9 | 2.8×105 | NA | [ | |
| 水解酶 | 果糖-1,6-二磷酸酶 | 果糖-1,6-二磷酸 | 果糖-6-磷酸 | 游离 | 1 | 8.57 | 1.4×10-7 | 5.9×107 | 260.14 | 28 | 5.5×105 | NA | [ |
| β-糖苷酶 | 纤维二糖 | 葡萄糖 | 游离 | 1 | 208 | 3.5×10-5 | 6.0×106 | 180 | 62.78 | 1.7×104 | NA | [ | |
| 氧化还原酶 | 肉桂醇脱氢酶 | 肉桂醇 | 肉桂醛 | 游离 | 1 | 18 | 2.00×10-3 | 9.0×103 | 132.159 | 80 | 15 | NA | [ |
| 氢酶 | H+ | 氢气 | 游离 | 1 | 1030 | 2.7×10-6 | 3.9×108 | 2 | 110 | 7.0×103 | NA | [ | |
| 胺脱氢酶 | NAD+ | NADH | 游离 | 1 | 167 | 1.1×10-6 | 1.5×108 | 663.43 | 45 | 2.1×106 | NA | [ | |
| 6-磷酸葡萄糖酸脱氢酶 | 葡萄糖6-磷酸 | 6-磷酸葡萄糖酸 | 游离 | 1 | 325 | 1.4×10-6 | 2.4×108 | 495.3 | 53 | 2.2×106 | NA | [ | |
| 转移酶 | 转醛缩酶 | 果糖-6-磷酸,赤藓糖-4-磷酸 | 景天糖-7-磷酸 | 游离 | 1 | 22.3 | 1.5×10-5 | 1.5×106 | 290.16 | 24 | 1.8×104 | NA | [ |
| 混合酶 | 伊沙替韦酶 | 2-乙酰甘油 | 伊沙替韦 | 游离 | 9 | NA | NA | NA | NA | NA | NA | 1 | [ |
| PHB合成 | 淀粉 | PHB | 游离 | 19~20 | NA | NA | NA | NA | NA | NA | 1 | [ | |
| 合成淀粉多酶机器 | CO2+H2 | 合成淀粉 | 游离 | 13 | NA | NA | NA | NA | NA | NA | 1 | [ | |
| 单萜烯酶 | 葡萄糖 | 单萜烯 | 游离 | 27 | NA | NA | NA | NA | NA | NA | 2 | [ | |
| 肌醇多酶机器 | 淀粉 | 肌醇 | 游离 | 4~6 | 1.1 | 2.0×10-5 | 5.5×104 | 180 | 50 | 196 | 13 | [ | |
| 固定化 | 4~6 | 1.2 | 3.5×10-6 | 3.4×105 | 180 | 50 | 1242 | 60~80 | [ | ||||
| D-塔格糖多酶机器 | 淀粉 | D-塔格糖 | 游离 | 5~6 | NA | NA | NA | NA | NA | NA | 35 | [ | |
| 固定化 | 4~6 | NA | NA | NA | NA | NA | NA | 103 | [ | ||||
| 纤维素酶(混合) | (预处理)生物质 | D-葡萄糖 | 游离 | 3~10 | NA | NA | NA | NA | NA | NA | 50~100 | [ | |
| 淀粉酶(混合) | (糊化)淀粉 | D-葡萄糖 | 游离 | 2~3 | NA | NA | NA | NA | NA | NA | 5000 | [ |
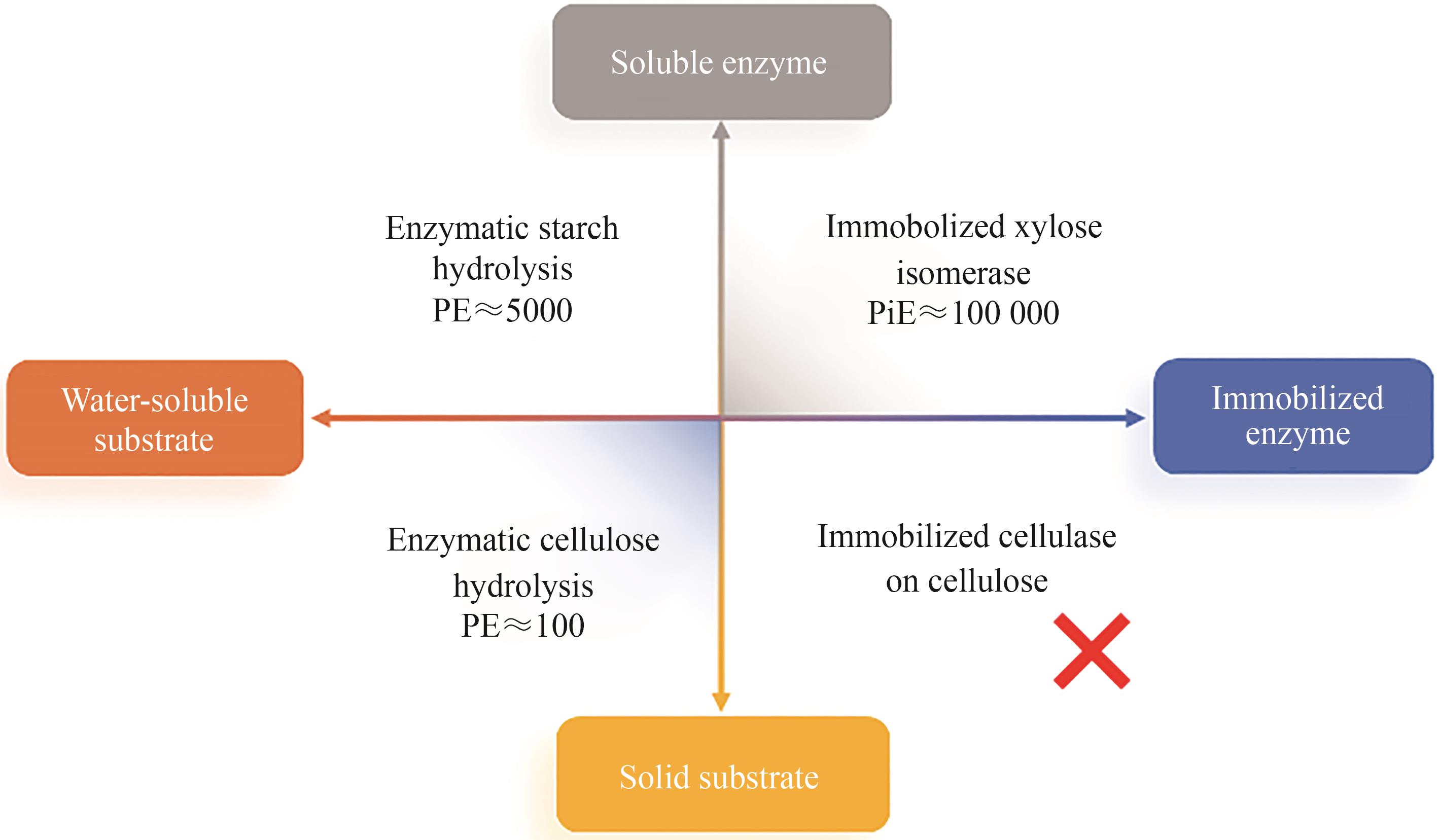
图2 酶催化产品的代表性PE值与底物-酶的可溶性(Soluble enzyme—游离酶;Water-soluble substrate—水溶性底物;Immobilized enzyme—固定化酶;Solid substrate—固态底物;Enzymatic starch hydrolysis—淀粉酶水解;Immobolized xylose isomerase—葡萄糖-果糖异构酶;Enzymatic cellulose hydrolysis—纤维素酶水解;Immobilized cellulase on cellulose—固定化纤维素酶水解纤维素,数据来源参见表3)
Fig. 2 Representative PE values of enzyme-catalyzed products and the solubility of substrate-enzyme(Data source: refer to Table 3)
| [1] | ZHANG Y H P, SUN J B, MA Y H. Biomanufacturing: history and perspective[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(4/5): 773-784. |
| [2] | 马延和. 生物制造产业是生物经济重点发展方向[J]. 中国生物工程杂志, 2022, 42(5): 4-5. |
| MA Y H. Biomanufacturing industry: a priority development direction in bioeconomy [J]. China Biotechnology, 2022, 42(5): 4-5. | |
| [3] | 李寅. 合成生物制造2022[J]. 生物工程学报, 2023, 39(3): 807-841. |
| LI Y. Biomanufacturing driven by engineered organisms(2022)[J]. Chinese Journal of Biotechnology, 2023, 39(3): 807-841. | |
| [4] | 石婷, 宋展, 宋世怡, 等. 体外生物转化(ivBT): 生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| SHI T, SONG Z, SONG S Y, et al. In vitro bio transformation(ivBT): a new frontier of industrial biomanufacturing[J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. | |
| [5] | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6): 1231-1241. |
| ZHANG Y H P. The enlightenment of the Chinese philosophy “Tao-Fa-Shu-Qi” to industrial biomanufacturing[J]. Synthetic Biology Journal, 2024, 5(6): 1231-1241. | |
| [6] | 张以恒, 陈雪梅, 石婷. 生物制造的市本率(PC值): 定义与应用[J]. 合成生物学, 2025, 6(1): 8-17. |
| ZHANG Y H P, CHEN X M, SHI T. Price to cost-of-raw-materials ratio (PC) of biomanufacturing: definition and application[J]. Synthetic Biology Journal, 2025, 6(1): 8-17. | |
| [7] | YE J, LI Y J, BAI Y Q, et al. A facile and robust T7-promoter-based high-expression of heterologous proteins in Bacillus subtilis [J]. Bioresources and Bioprocessing, 2022, 9(1): 56. |
| [8] | LIU G, ZHANG J, BAO J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling[J]. Bioprocess and Biosystems Engineering, 2016, 39(1): 133-140. |
| [9] | BAILEY J E, OLLIS D F. Biochemical engineering fundamentals[M]. New York: McGraw-Hill, 1986. |
| [10] | CLARK D S, BLANCH H W. Biochemical Engineering[M/OL]. 2nd Edition. Boca Raton, FL, USA: Taylor & Francis, CRC Press, 1997. (1997-02-14)[2025-01-01]. . |
| [11] | ROGERS T A, BOMMARIUS A S. Utilizing simple biochemical measurements to predict lifetime output of biocatalysts in continuous isothermal processes[J]. Chemical Engineering Science, 2010, 65(6): 2118-2124. |
| [12] | MYUNG S, ZHANG X Z, PERCIVAL ZHANG Y H P. Ultra-stable phosphoglucose isomerase through immobilization of cellulose-binding module-tagged thermophilic enzyme on low-cost high-capacity cellulosic adsorbent[J]. Biotechnology Progress, 2011, 27(4): 969-975. |
| [13] | GAO X, FANG S B, MA X Z, et al. Customized self-assembled bimetallic hybrid nanoflowers promoting the robustness of D-allulose 3-epimerase[J]. Chemical Engineering Journal, 2024, 484: 149453. |
| [14] | JIA D X, ZHOU L, ZHENG Y G. Properties of a novel thermostable glucose isomerase mined from Thermus oshimai and its application to preparation of high fructose corn syrup[J]. Enzyme and Microbial Technology, 2017, 99: 1-8. |
| [15] | JIA D X, WANG T, LIU Z J, et al. Whole cell immobilization of refractory glucose isomerase using tris(hydroxymethyl)phosphine as crosslinker for preparation of high fructose corn syrup at elevated temperature[J]. Journal of Bioscience and Bioengineering, 2018, 126(2): 176-182. |
| [16] | WANG L H, LIU X L, JIANG Y J, et al. Silica nanoflowers-stabilized Pickering emulsion as a robust biocatalysis platform for enzymatic production of biodiesel[J]. Catalysts, 2019, 9(12): 1026. |
| [17] | RAJENDHRAN J, GUNASEKARAN P. Molecular cloning and characterization of thermostable β-lactam acylase with broad substrate specificity from Bacillus badius [J]. Journal of Bioscience and Bioengineering, 2007, 103(5): 457-463. |
| [18] | PERCIVAL ZHANG Y H P. Production of biocommodities and bioelectricity by cell-free synthetic enzymatic pathway biotransformations: challenges and opportunities[J]. Biotechnology and Bioengineering, 2010, 105(4): 663-677. |
| [19] | MOSBAH H, SAYARI A, HORCHANI H, et al. Involvement of Gly 311 residue on substrate discrimination, pH and temperature dependency of recombinant Staphylococcus xylosus lipase: a study with emulsified substrate[J]. Protein Expression and Purification, 2007, 55(1): 31-39. |
| [20] | SUN F F, ZHANG X Z, MYUNG S, et al. Thermophilic Thermotoga maritima ribose-5-phosphate isomerase RpiB: optimized heat treatment purification and basic characterization[J]. Protein Expression and Purification, 2012, 82(2): 302-307. |
| [21] | WANG Y, ZHANG Y H P. A highly active phosphoglucomutase from Clostridium thermocellum: cloning, purification, characterization and enhanced thermostability[J]. Journal of Applied Microbiology, 2010, 108(1): 39-46. |
| [22] | MYUNG S, WANG Y R, ZHANG Y H P. Fructose-1,6-bisphosphatase from a hyper-thermophilic bacterium Thermotoga maritima: characterization, metabolite stability, and its implications[J]. Process Biochemistry, 2010, 45(12): 1882-1887. |
| [23] | DAS A, PAUL T, GHOSH P, et al. Kinetic study of a glucose tolerant β-glucosidase from Aspergillus fumigatus ABK9 entrapped into alginate beads[J]. Waste and Biomass Valorization, 2015, 6(1): 53-61. |
| [24] | PATEL P, GUPTA N, GAIKWAD S, et al. Leucaena sp. recombinant cinnamyl alcohol dehydrogenase: purification and physicochemical characterization[J]. International Journal of Biological Macromolecules, 2014, 63: 254-260. |
| [25] | YOON K S, FUKUDA K, FUJISAWA K, et al. Purification and characterization of a highly thermostable, oxygen-resistant, respiratory [NiFe]-hydrogenase from a marine, aerobic hydrogen-oxidizing bacterium Hydrogenovibrio marinus [J]. International Journal of Hydrogen Energy, 2011, 36(12): 7081-7088. |
| [26] | KONG W X, ZHANG J W, ZHOU L Y, et al. Activation and stabilization of engineered amine dehydrogenase by fatty acids for bioprocess intensification of asymmetric reductive amination[J]. ACS Catalysis, 2025, 15(1): 34-43. |
| [27] | WANG Y R, ZHANG Y H P. Overexpression and simple purification of the Thermotoga maritima 6-phosphogluconate dehydrogenase in Escherichia coli and its application for NADPH regeneration[J]. Microbial Cell Factories, 2009, 8: 30. |
| [28] | HUANG S Y, ZHANG Y H P, ZHONG J J. A thermostable recombinant transaldolase with high activity over a broad pH range[J]. Applied Microbiology and Biotechnology, 2012, 93(6): 2403-2410. |
| [29] | HUFFMAN M A, FRYSZKOWSKA A, ALVIZO O, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir[J]. Science, 2019, 366(6470): 1255-1259. |
| [30] | WEI X L, YANG X, HU C C, et al. ATP-free in vitro biotransformation of starch-derived maltodextrin into poly-3-hydroxybutyrate via acetyl-CoA[J]. Nature Communications, 2024, 15: 3267. |
| [31] | CAI T, SUN H B, QIAO J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide[J]. Science, 2021, 373(6562): 1523-1527. |
| [32] | KORMAN T P, OPGENORTH P H, BOWIE J U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose[J]. Nature Communications, 2017, 8: 15526. |
| [33] | YOU C, SHI T, LI Y J, et al. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch[J]. Biotechnology and Bioengineering, 2017, 114(8): 1855-1864. |
| [34] | HAN P P, YOU C, LI Y J, et al. High-titer production of myo-inositol by a co-immobilized four-enzyme cocktail in biomimetic mineralized microcapsules[J]. Chemical Engineering Journal, 2023, 461: 141946. |
| [35] | HAN P P, WANG X Y, LI Y J, et al. Synthesis of a healthy sweetener D-tagatose from starch catalyzed by semiartificial cell factories[J]. Journal of Agricultural and Food Chemistry, 2023, 71(8): 3813-3820. |
| [36] | LEE D, YU A H C, SADDLER J N. Evaluation of cellulase recycling strategies for the hydrolysis of lignocellulosic substrates[J]. Biotechnology and Bioengineering, 1995, 45(4): 328-336. |
| [37] | QI B K, CHEN X R, SU Y, et al. Enzyme adsorption and recycling during hydrolysis of wheat straw lignocellulose[J]. Bioresource Technology, 2011, 102(3): 2881-2889. |
| [38] | JARBOE L R, ZHANG X L, WANG X, et al. Metabolic engineering for production of biorenewable fuels and chemicals: contributions of synthetic biology[J]. BioMed Research International, 2010, 2010(1): 761042. |
| [39] | XU J R, HE L Y, LIU C G, et al. Genome sequence of the self-flocculating strain Saccharomyces cerevisiae SPSC01[J]. Genome Announcements, 2018, 6(20): e00367-18. |
| [40] | CHANG W L, HOU W J, XU M M, et al. High-rate continuous n-butanol production by Clostridium acetobutylicum from glucose and butyric acid in a single-pass fibrous-bed bioreactor[J]. Biotechnology and Bioengineering, 2022, 119(12): 3474-3486. |
| [41] | BURKHARDT C, BARUTH L, MEYER-HEYDECKE N, et al. Mining thermophiles for biotechnologically relevant enzymes: evaluating the potential of European and Caucasian hot springs[J]. Extremophiles, 2023, 28(1): 5. |
| [42] | WOHLGEMUTH R, LITTLECHILD J, MONTI D, et al. Discovering novel hydrolases from hot environments[J]. Biotechnology Advances, 2018, 36(8): 2077-2100. |
| [43] | XU K J, FU H R, CHEN Q M, et al. Engineering thermostability of industrial enzymes for enhanced application performance[J]. International Journal of Biological Macromolecules, 2025, 291: 139067. |
| [44] | BERGESON A R, ALPER H S. Advancing sustainable biotechnology through protein engineering[J]. Trends in Biochemical Sciences, 2024, 49(11): 955-968. |
| [45] | MYUNG S, ZHANG Y H P. Non-complexed four cascade enzyme mixture: simple purification and synergetic co-stabilization[J]. PLoS One, 2013, 8(4): e61500. |
| [46] | SPERL J M, SIEBER V. Multienzyme cascade reactions: status and recent advances[J]. ACS Catalysis, 2018, 8(3): 2385-2396. |
| [47] | REN S Z, LI C H, JIAO X B, et al. Recent progress in multienzymes co-immobilization and multienzyme system applications[J]. Chemical Engineering Journal, 2019, 373: 1254-1278. |
| [48] | XU K L, CHEN X X, ZHENG R C, et al. Immobilization of multi-enzymes on support materials for efficient biocatalysis[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 660. |
| [49] | HWANG E T, LEE S. Multienzymatic cascade reactions via enzyme complex by immobilization[J]. ACS Catalysis, 2019, 9(5): 4402-4425. |
| [50] | BILAL M, HUSSAIN N, AMÉRICO-PINHEIRO J H P, et al. Multi-enzyme co-immobilized nano-assemblies: bringing enzymes together for expanding bio-catalysis scope to meet biotechnological challenges[J]. International Journal of Biological Macromolecules, 2021, 186: 735-749. |
| [51] | WANG X L, LI Z, SHI J F, et al. Bioinspired approach to multienzyme cascade system construction for efficient carbon dioxide reduction[J]. ACS Catalysis, 2014, 4(3): 962-972. |
| [52] | PENG F, CHEN Q S, ZONG M H, et al. Sequential co-immobilization of multienzyme nanodevices based on SpyCatcher and SpyTag for robust biocatalysis[J]. Molecular Catalysis, 2021, 510: 111673. |
| [53] | WILSON L, ILLANES A, OTTONE C, et al. Co-immobilized carrier-free enzymes for lactose upgrading[J]. Current Opinion in Green and Sustainable Chemistry, 2022, 33: 100553. |
| [54] | ZHANG Y H P. Simpler is better: high-yield and potential low-cost biofuels production through cell-free synthetic pathway biotransformation (SyPaB)[J]. ACS Catalysis, 2011, 1(9): 998-1009. |
| [55] | SUN L Q, XU C Z, TONG S S, et al. Enhancing cellulose hydrolysis via cellulase immobilization on zeolitic imidazolate frameworks using physical adsorption[J]. Bioprocess and Biosystems Engineering, 2024, 47(7): 1071-1080. |
| [56] | XU C Z, TONG S S, SUN L Q, et al. Cellulase immobilization to enhance enzymatic hydrolysis of lignocellulosic biomass: an all-inclusive review[J]. Carbohydrate Polymers, 2023, 321: 121319. |
| [57] | TÉBÉKA I R M, SILVA A G L, PETRI D F S. Hydrolytic activity of free and immobilized cellulase[J]. Langmuir, 2009, 25(3): 1582-1587. |
| [58] | CHANG R H, JANG J, WU K C W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion[J]. Green Chemistry, 2011, 13(10): 2844-2850. |
| [59] | AHMED I N, YANG X L, DUBALE A A, et al. Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks[J]. Bioresource Technology, 2018, 270: 377-382. |
| [60] | XU X X, ZHANG W, YOU C, et al. Biosynthesis of artificial starch and microbial protein from agricultural residue[J]. Science Bulletin, 2023, 68(2): 214-223. |
| [61] | BORGES S, BRASSESCO M E, CUNHA S A, et al. Recent trends in biocatalysis and its application in the food industry[M]//Enzymatic processes for food valorization. Amsterdam: Elsevier, 2024: 265-284. (2024-06-28)[2025-01-01]. . |
| [62] | ZHANG Y H P. Next generation biorefineries will solve the food, biofuels, and environmental trilemma in the energy-food-water nexus[J]. Energy Science & Engineering, 2013, 1(1): 27-41. |
| [63] | ZHANG Y H P, HIMMEL M E, MIELENZ J R. Outlook for cellulase improvement: screening and selection strategies[J]. Biotechnology Advances, 2006, 24(5): 452-481. |
| [64] | HONG J, YE X H, ZHANG Y H P. Quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications[J]. Langmuir, 2007, 23(25): 12535-12540. |
| [65] | ROLLIN J A, ZHU Z G, SATHITSUKSANOH N, et al. Increasing cellulose accessibility is more important than removing lignin: a comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia[J]. Biotechnology and Bioengineering, 2011, 108(1): 22-30. |
| [66] | ZHANG Y H P, LYND L R. A functionally based model for hydrolysis of cellulose by fungal cellulase[J]. Biotechnology and Bioengineering, 2006, 94(5): 888-898. |
| [67] | ZHANG Y H P, DING S Y, MIELENZ J R, et al. Fractionating recalcitrant lignocellulose at modest reaction conditions[J]. Biotechnology and Bioengineering, 2007, 97(2): 214-223. |
| [68] | SATHITSUKSANOH N, GEORGE A, ZHANG Y H P. New lignocellulose pretreatments using cellulose solvents: a review[J]. Journal of Chemical Technology & Biotechnology, 2013, 88(2): 169-180. |
| [69] | ZHANG Y H P, LYND L R. Determination of the number-average degree of polymerization of cellodextrins and cellulose with application to enzymatic hydrolysis[J]. Biomacromolecules, 2005, 6(3): 1510-1515. |
| [70] | SATHITSUKSANOH N, ZHU Z G, HO T J, et al. Bamboo saccharification through cellulose solvent-based biomass pretreatment followed by enzymatic hydrolysis at ultra-low cellulase loadings[J]. Bioresource Technology, 2010, 101(13): 4926-4929. |
| [71] | ZHANG Y H P, CUI J B, LYND L R, et al. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure[J]. Biomacromolecules, 2006, 7(2): 644-648. |
| [72] | SANTOS C A, MORAIS M A B, MANDELLI F, et al. A metagenomic ‘dark matter’ enzyme catalyses oxidative cellulose conversion[J]. Nature, 2025, 639(8056): 1076-1083. |
| [1] | 钟奶才, 陈缘, 潘文锋, 苏小凤, 廖景文, 翟英雷, 钟近艺. 等离子体微生物育种技术在生物制造中的应用进展[J]. 合成生物学, 2025, 6(4): 789-805. |
| [2] | 吴晓燕, 宋琪, 许睿, 丁陈君, 陈方, 郭勍, 张波. 合成生物学研发竞争态势对比分析[J]. 合成生物学, 2025, 6(4): 940-955. |
| [3] | 马牧青, 吴彦, 曲茂华, 卢夏锋, 曹敏, 杜峰, 季荣涛, 董磊迟, 罗志波. 体外多酶组装与生物级联催化:进展与展望[J]. 合成生物学, 2025, 6(4): 920-939. |
| [4] | 黄瑜晴, 吴涵, 李晓彬, 刘君禹, 马少华, 戈钧, 邢新会, 张灿阳. 非生物元件增强的合成生物杂合体系研究进展[J]. 合成生物学, 2025, 6(4): 764-788. |
| [5] | 黄怡, 司同, 陆安静. 生物制造标准体系建设的现状、问题与建议[J]. 合成生物学, 2025, 6(3): 701-714. |
| [6] | 应汉杰, 柳东, 王振宇, 沈涛, 庄伟, 朱晨杰. 工业生物制造与“碳中和”目标探讨[J]. 合成生物学, 2025, 6(1): 1-7. |
| [7] | 张以恒, 陈雪梅, 石婷. 生物制造的市本率(PC值):定义与应用[J]. 合成生物学, 2025, 6(1): 8-17. |
| [8] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [9] | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6): 1231-1241. |
| [10] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [11] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [12] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [13] | 刘建明, 张炽坚, 张冰, 曾安平. 巴氏梭菌作为工业底盘细胞高效生产1,3-丙二醇——从代谢工程和菌种进化到过程工程和产品分离[J]. 合成生物学, 2024, 5(6): 1386-1403. |
| [14] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [15] | 李怡霏, 陈艾, 孙俊松, 张以恒. 体外多酶分子机器产氢应用中的氢酶研究[J]. 合成生物学, 2024, 5(6): 1461-1484. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
