合成生物学 ›› 2021, Vol. 2 ›› Issue (4): 559-576.DOI: 10.12211/2096-8280.2021-028
多酶催化体系在医药化学品合成中的应用
汤恒, 韩鑫, 邹树平, 郑裕国
- 浙江工业大学生物工程学院手性生物制造国家地方联合工程研究中心,浙江 杭州 310014
-
收稿日期:2021-02-24修回日期:2021-05-25出版日期:2021-08-31发布日期:2021-09-10 -
通讯作者:邹树平 -
作者简介:汤恒 (1989—),男,博士,讲师。研究方向为酶工程。E-mail:tangheng@zjut.edu.cn邹树平 (1980—),男,博士,教授,博士生导师。研究方向为医药化学品生物催化合成。E-mail:zousp@zjut.edu.cn -
基金资助:国家重点研究计划(2019YFA0905000)
Application of multi-enzyme catalytic system in the synthesis of pharmaceutical chemicals
TANG Heng, HAN Xin, ZOU Shuping, ZHENG Yuguo
- National and Local Joint Engineering Research Center for Chiral Biomanufacturing,College of Biotechnology and Bioengineering,Zhejiang University of Technology,Hangzhou 310014,Zhejiang,China
-
Received:2021-02-24Revised:2021-05-25Online:2021-08-31Published:2021-09-10 -
Contact:ZOU Shuping
摘要:
多酶催化体系成为近年来生物催化领域的研究热点。由于过程可控性及下游易分离的特性,越来越多的体外多酶催化体系已成功构建,部分体系还可耦合化学催化步骤,应用于精细化学品的合成。随着多酶催化体系构建技术的逐步成熟,将会给未来化工与医药类产品的生物制造带来广阔的应用前景。本文介绍了多酶催化体系的相关设计原则,通过对反应过程和合成路径进行热力学和动力学分析,设计高价值产物的生物合成路径,挖掘路径中的关键酶,结合各类新型组装策略将功能各异的酶级联组装成一个结构和功能整体,形成“底物通道”,减少中间体损失和降低副反应,实现从简单底物向复杂产物的高效生物转化。系统分析了多酶催化体系在医药化学品(如抗生素、抗癌药物、心血管疾病治疗药物、肝病治疗药物和精神疾病治疗药物及各类活性成分如D-葡萄糖二酸、萜类化合物和5-氨基乙酰丙酸)合成中的应用实例,并总结多酶催化体系仍存在的问题及可能的解决方法。
中图分类号:
引用本文
汤恒, 韩鑫, 邹树平, 郑裕国. 多酶催化体系在医药化学品合成中的应用[J]. 合成生物学, 2021, 2(4): 559-576.
TANG Heng, HAN Xin, ZOU Shuping, ZHENG Yuguo. Application of multi-enzyme catalytic system in the synthesis of pharmaceutical chemicals[J]. Synthetic Biology Journal, 2021, 2(4): 559-576.
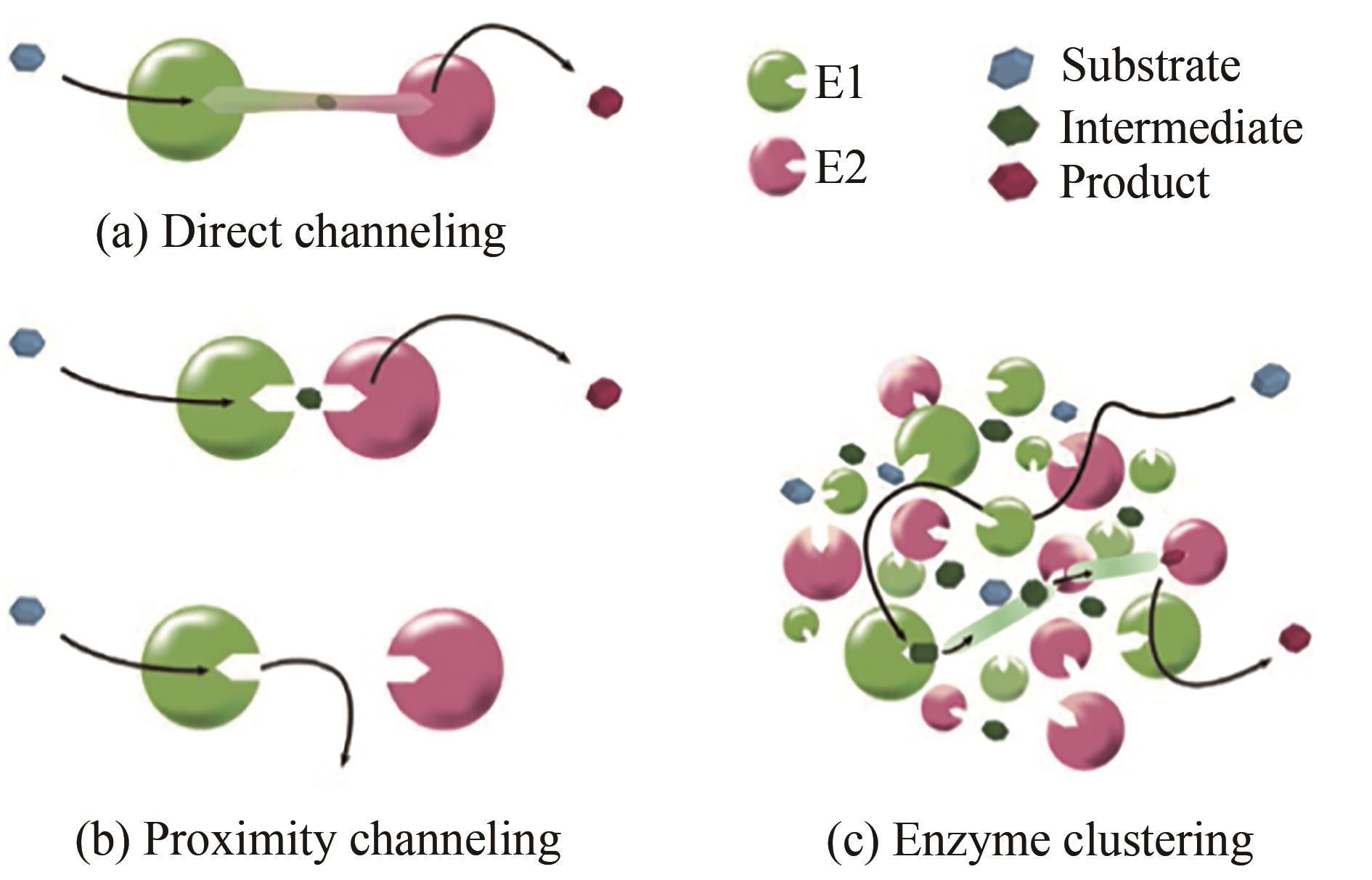
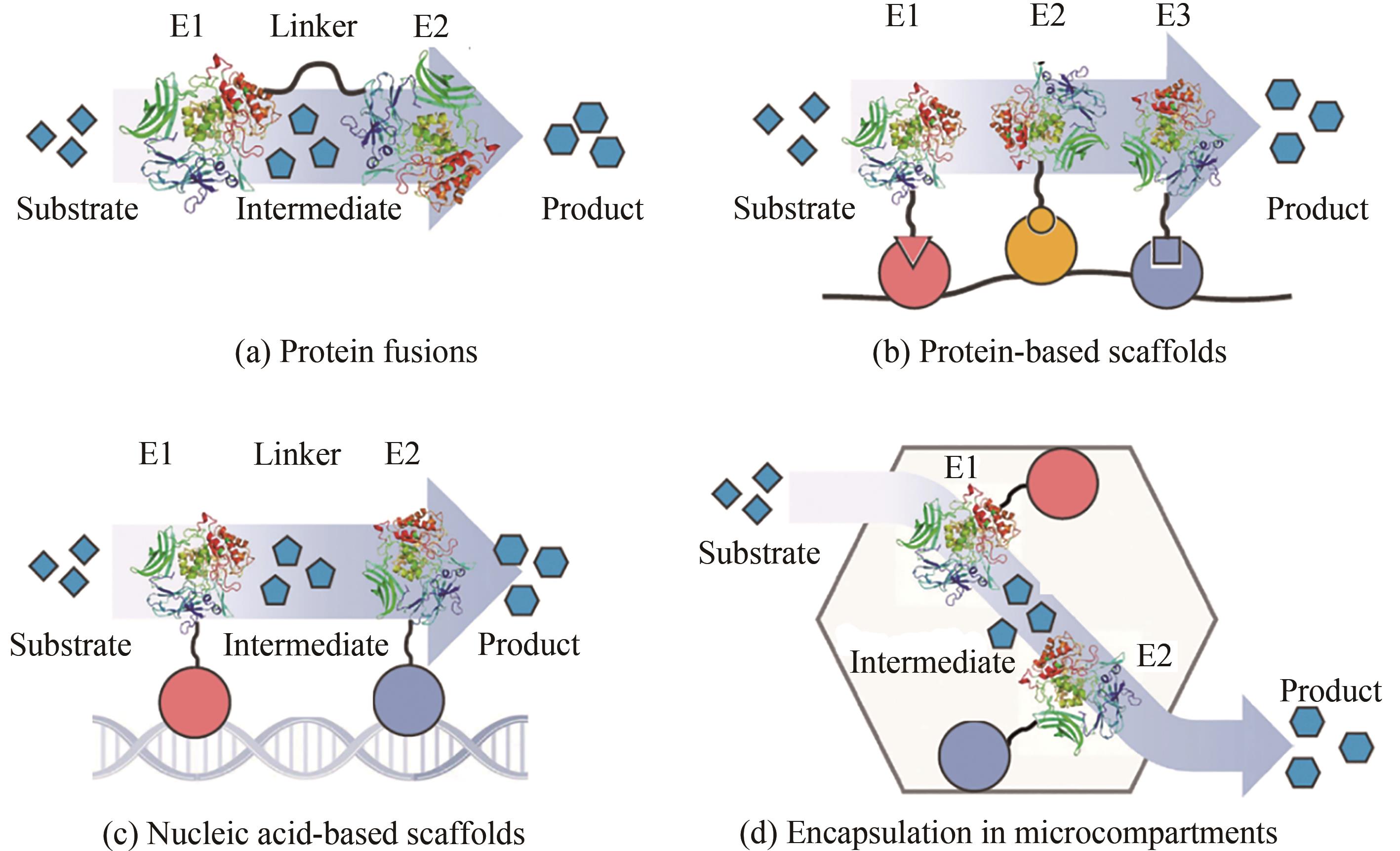
图1 底物通道效应[7](Different types of intermediate channeling in a two-step metabolic pathway, where a substrate is processed by enzyme E1 and turned into intermediate, which is then processed by enzyme E2 and turned into product)
Fig. 1 Substrate channel in a two-step metabolic pathway[7]
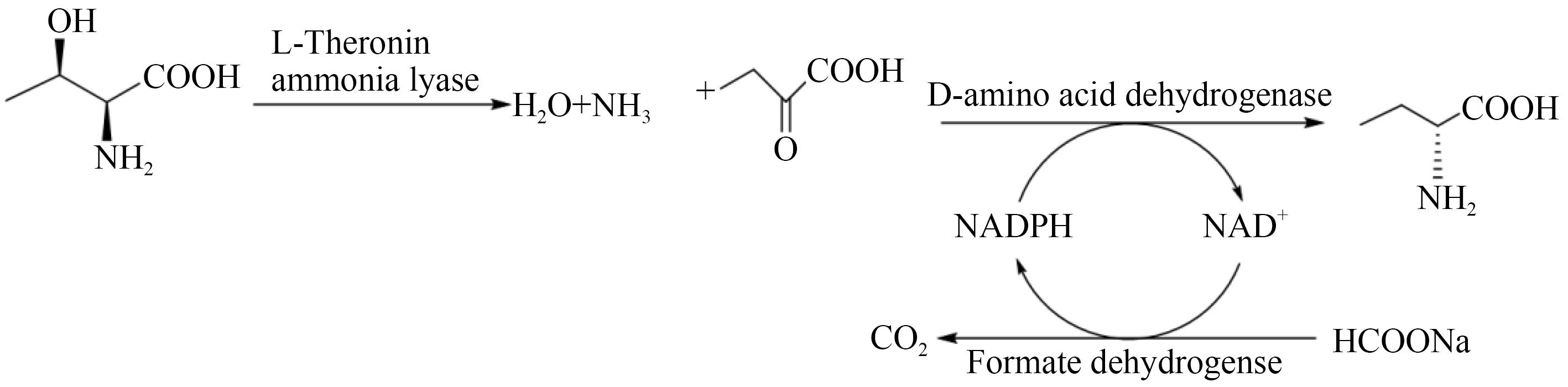
图4 多酶催化合成D-2-氨基丁酸[50][L-threonine deaminase catalyzes production of 2-oxobutyric acid, which is combined with cofactors to be reductively amination by D-amino acid dehydrogenase to obtain D-2-aminobutyric acid, and at same time combined with formate dehydrogenase (FDH) to achieve NADPH of recycling]
Fig. 4 Synthesis of D-2-aminobutyric acid catalyzed by multi-enzyme[50]
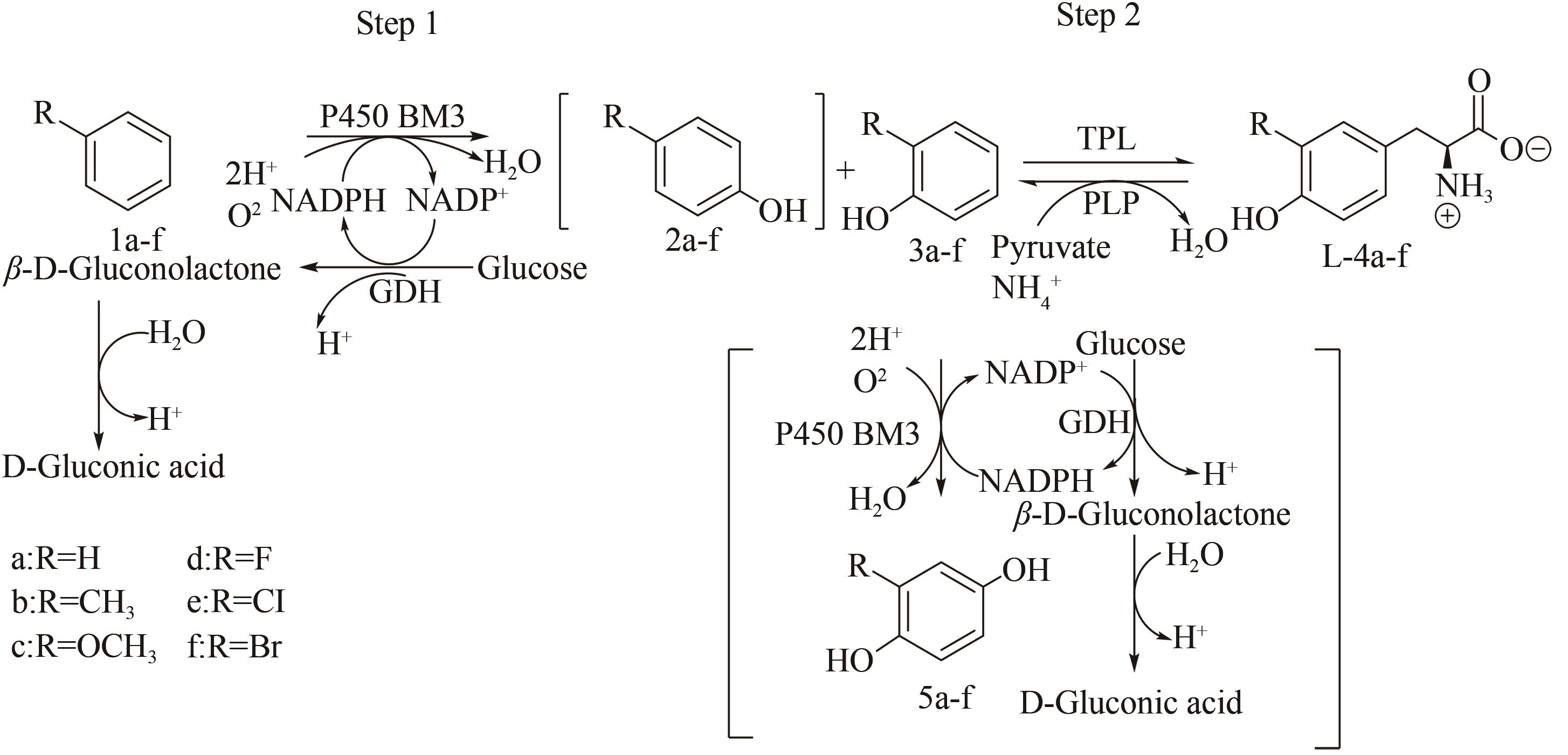
图6 一锅法多酶级联催化合成L-酪氨酸衍生物[66][Regioselective and chemoselective hydroxylation of aromatics (1a-f) is catalyzed by highly selective P450 BM3 mutant to generate o-phenol intermediates (3a-f) and p-phenol intermediates (2a-f). In second step, under condition of consuming NH3, tyrosine phenol lyase (TPL) catalyzes C-C coupling of 3a-f to generate pyruvate to obtain L-4a-f]
Fig. 6 Synthesis of L-tyrosine derivatives by one-pot multi-enzyme cascade catalysis[66]
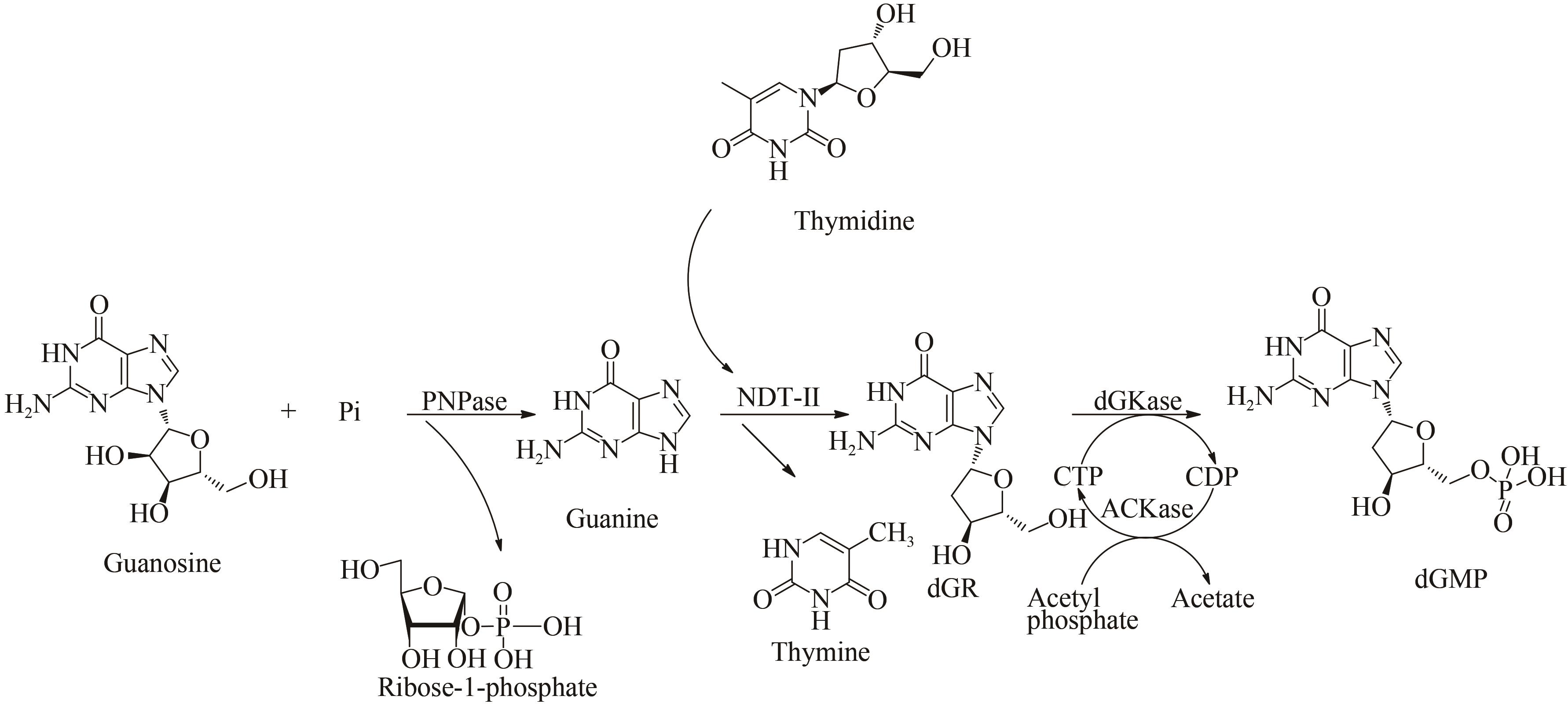
图7 一锅法多酶级联反应生产2'-脱氧鸟苷酸路线[70][Original guanosine substrate is first cleaved by PNPase into guanine and 1-phosphate ribose. Subsequently, NDT-Ⅱ catalyzes reaction of guanine and thymidine to produce deoxyguanosine (dGR). Finally, dGKase and ACKase utilize cytidine triphosphate (CTP) regeneration system of acetyl phosphate to phosphorylate intermediate dGR into dGMP. During reaction, original guanosine substrate is cleaved by PNPase into guanine and ribose-1-phosphate. Then, dGR is subsequently produced by reaction between guanine and thymidine, and NDT-Ⅱ catalyzes reaction to proceed. Finally, intermediate deoxyribonucleic acid is phosphorylated by deoxyglucosidase and CTP to generate deoxyribonucleic acid polysaccharide]
Fig. 7 One-pot multi-enzyme cascade production route of deoxyguanosine-2'-monophosphate[70]
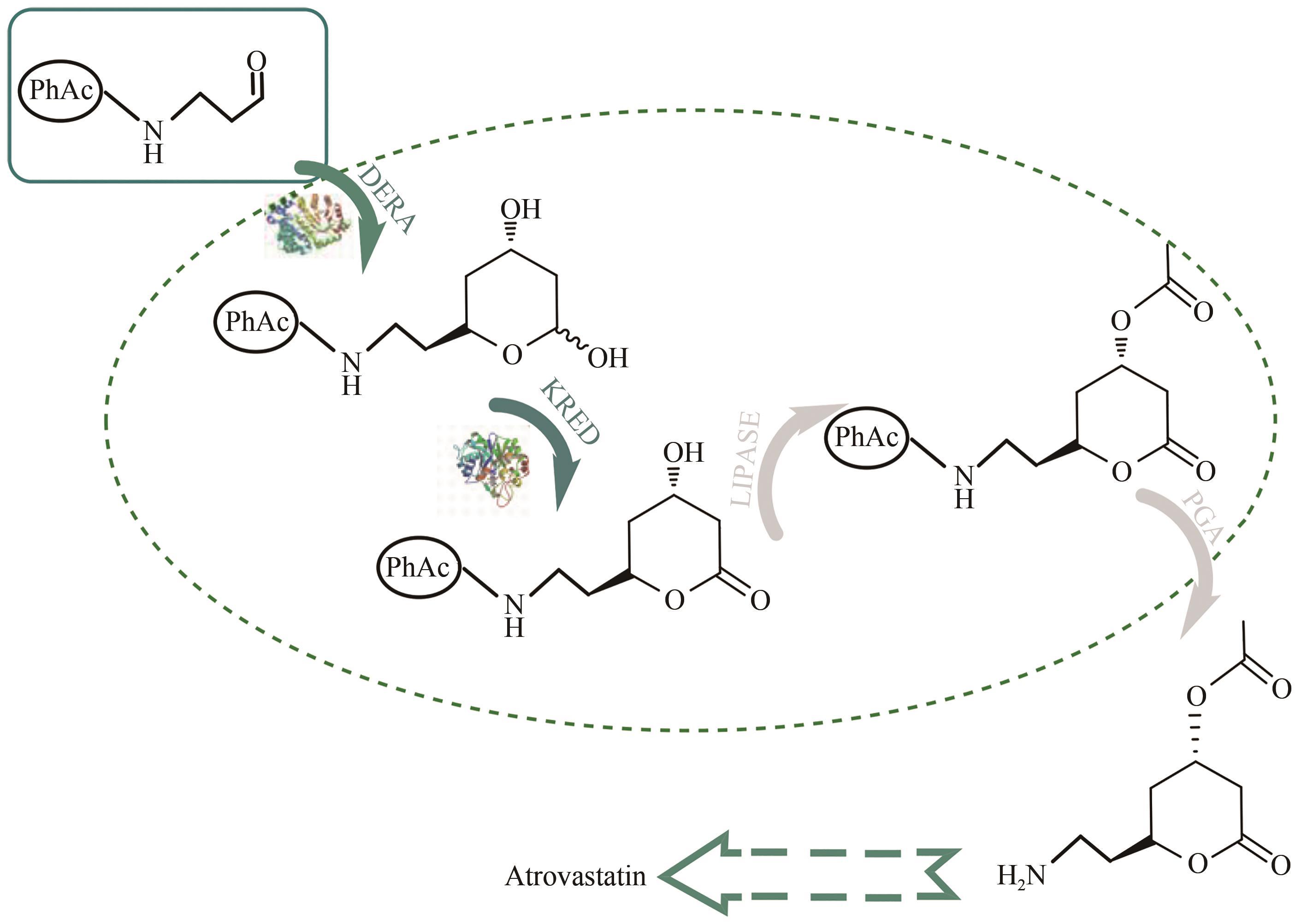
图8 阿托伐他汀侧链前体生产路线[75]DERA—2-deoxyribose-5-phosphate aldolase; KRED—NADPH-dependent dehydrogenases; PGA—penicillin G-acylase
Fig. 8 Atorvastatin side chain precursor production route[75]
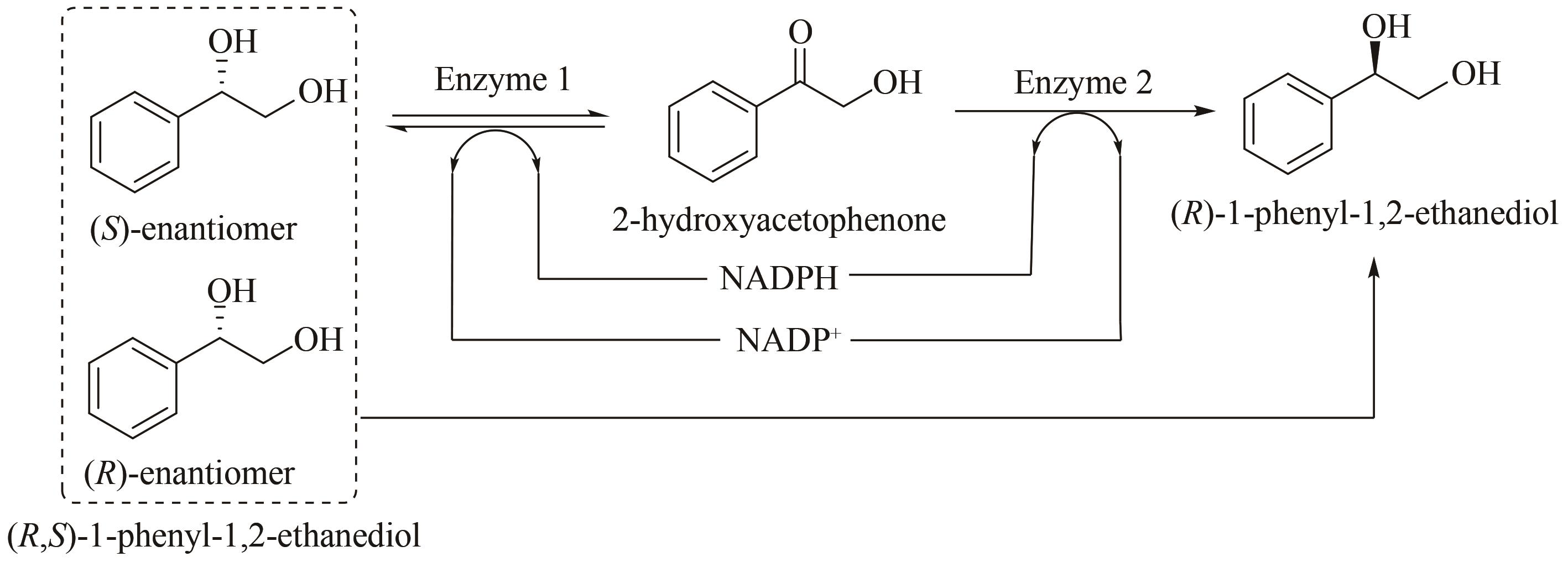
图10 体外多酶催化去消旋化体系生产(R)-1-苯基-1,2-乙二醇[87][Racemic PED is reduced to (R)-enantiomer through selective oxidation and asymmetric reduction involving self-circulation of cofactor. Enzyme 1 oxidizes (S)-PED to 2-HAP and reduces NADP+ to NADPH. Subsequently, by enzyme 2, 2-HAP is reduced to (R)-PED, while NADPH is oxidized to NADP+]
Fig. 10 (R)-1-Phenyl-1,2-ethanediol were produced by in vitro multi-enzyme catalytic de-racemization system[87]
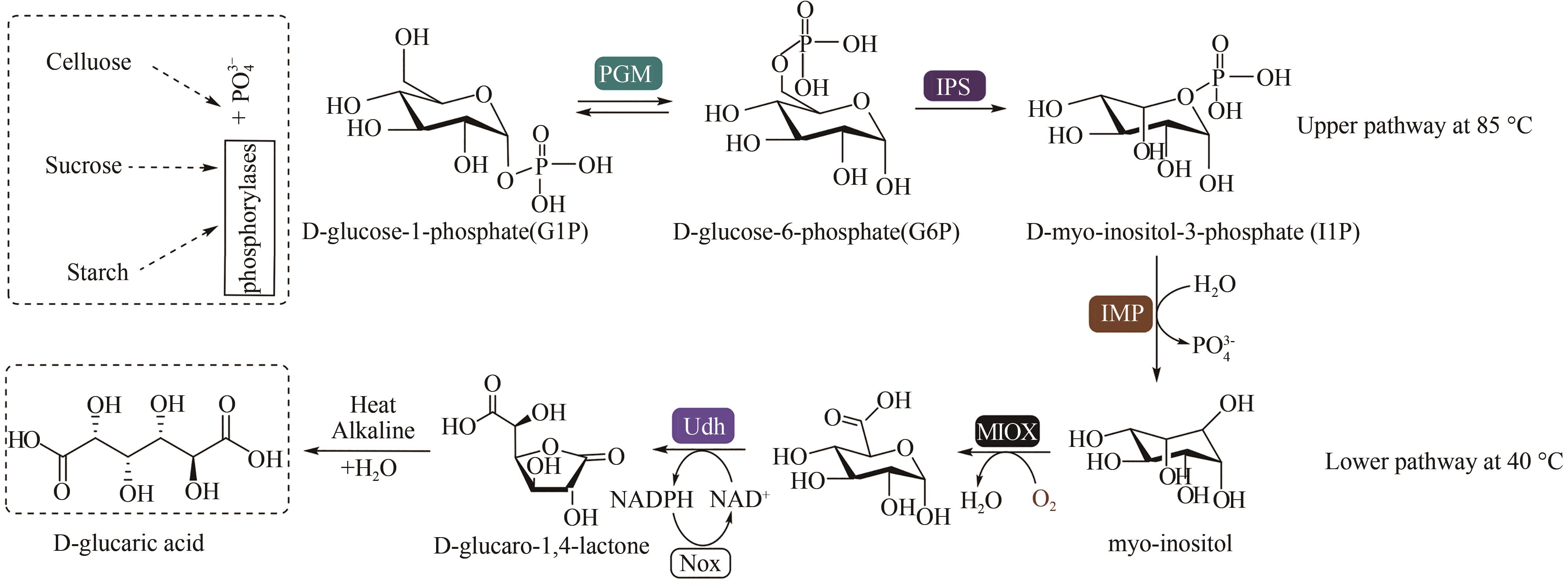
图11 多酶催化生产D-葡萄糖二酸[90][Conversion of G1P to GlucA was achieved by six enzymes: i) a phosphoglucomutase (PGM) which catalyses intermolecular phosphate transfer of G1P to G6P, ii) a myo-inositol-3-phosphate synthase (IPS) which catalyses multi-step oxidation, cyclisation and reduction of G6P to I1P, iii) a inositol-1-monophosphatase (IMP) which catalyses dephosphorylation of I1P to myo-inositol, iv) a myo-inositol oxygenase (MIOX) which mediates oxygen-catalysed oxidation of myo-inositol to GA, v) uronate dehydrogenase (Udh) which catalyses oxidation of GA to glucaro-1,4-lactone (GlucA lactone) under reduction of NAD+ to NADH and vi) a NADH oxidase (Nox) for NAD+ regeneration to minimise supply of expensive coenzyme for continuous GlucA production. GlucA lactone ring is mostly stable in neutral solutions and is hydrolysed to GlucA with heating in alkaline conditions]
Fig. 11 Production of D-gluconic acid by multi-enzyme catalysis from glucose[90]
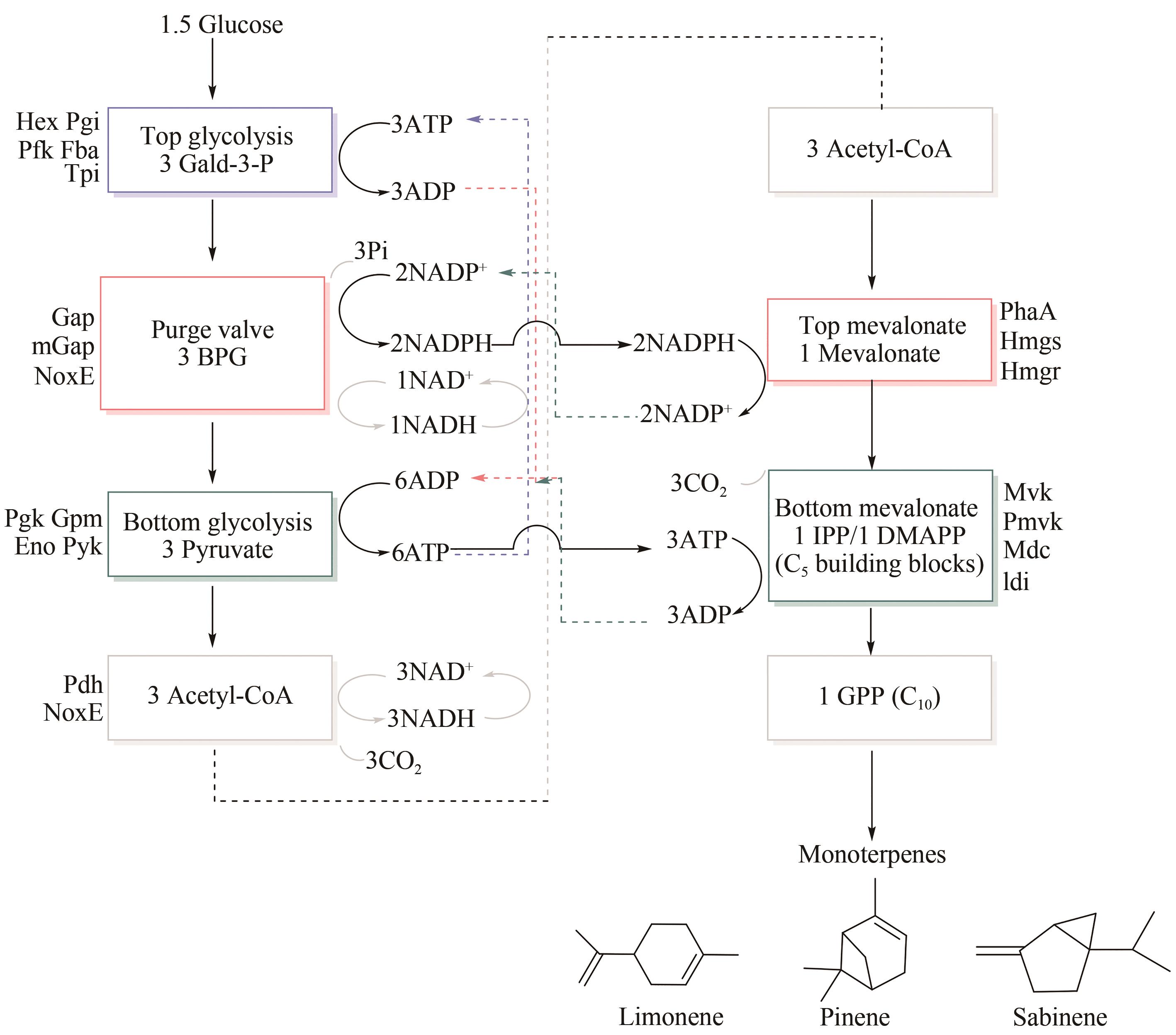
图12 多酶催化生产单萜[102](Enzymes in each sub-module are marked with colored boxes, and steps of consuming and generating ATP are shown in purple and blue respectively. Purge valve that produces NADPH is shown in red, while top of mevalonate pathway that consumes NADPH is shown in orange. Solid arrow represents flow of cofactor, and dotted arrow emphasizes cycle of each step in pathway)
Fig. 12 Production of monoterpenes from glucose by multi-enzyme catalysis[102]
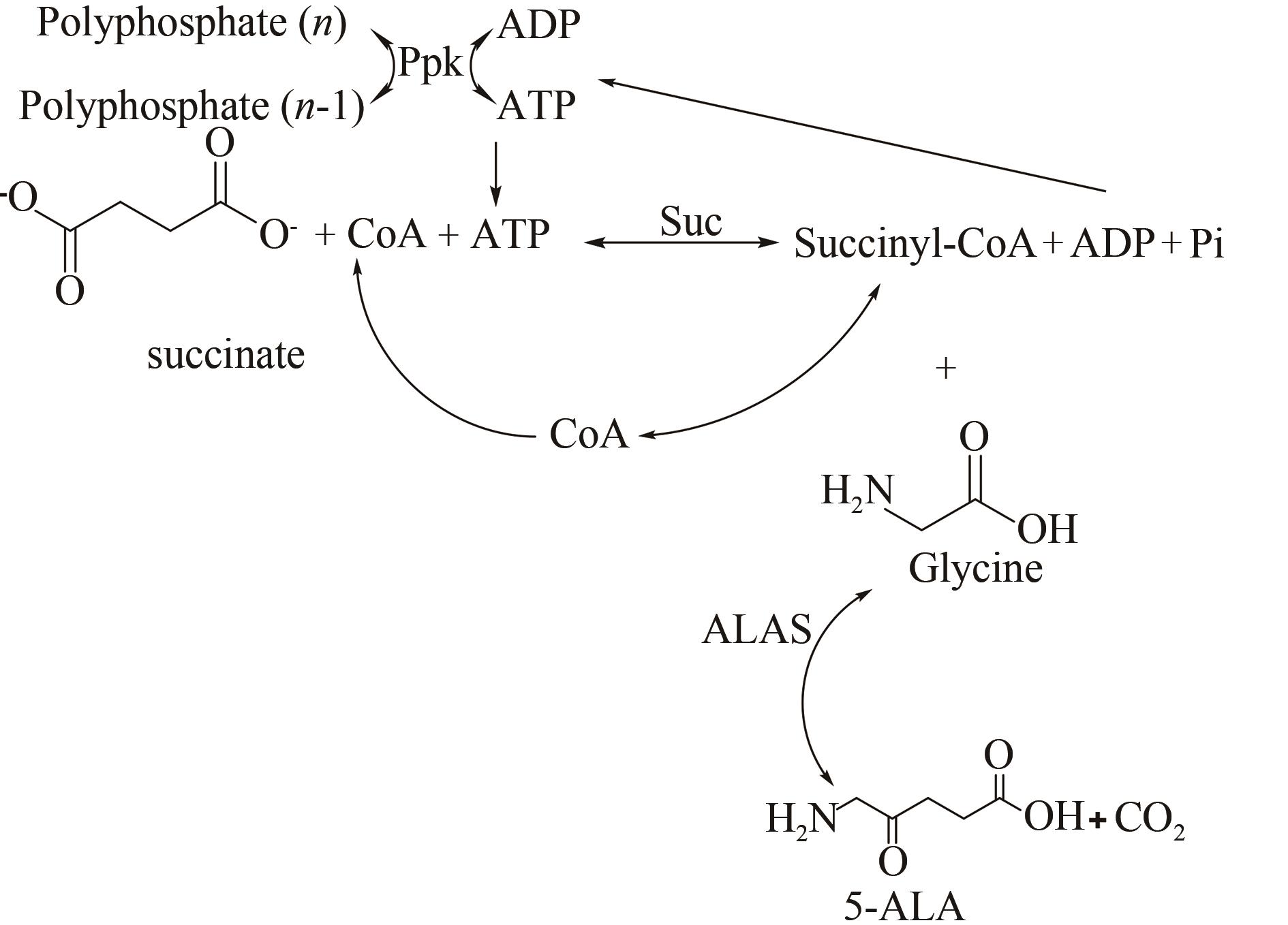
图13 无细胞多酶催化合成5-氨基乙酰丙酸[107][(Succinyl-CoA synthase catalyzes succinate to produce succinyl-CoA, and CoA can be catalyzed by two reactions catalyzed by 5-aminolevulinic acid synthase and succinyl-CoA synthase recycling is carried out, and another required substrate, ATP, provides required ATP from polyphosphate through polyphosphate kinase regeneration system)]
Fig. 13 Synthesis of 5-aminolevulinic acid catalyzed by cell-free multi-enzyme[107]
| 1 | CASTELLANA M, WILSON M Z, XU Y, et al. Enzyme clustering accelerates processing of intermediates through metabolic channeling [J]. Nature Biotechnology, 2014, 32(10): 1011-1018. |
| 2 | WARNECKE T, GILL R T. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications [J]. Microbial Cell Factories, 2005, 4: 25. |
| 3 | GRAHAM J W A, WILLIAMS T C R, MORGAN M, et al. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling [J]. The Plant Cell, 2007, 19(11): 3723-3738. |
| 4 | HAGGIE P M, VERKMAN A S. Diffusion of tricarboxylic acid cycle enzymes in the mitochondrial matrix in vivo. Evidence for restricted mobility of a multienzyme complex [J]. The Journal of Biological Chemistry, 2002, 277(43): 40782-40788. |
| 5 | DING S Y, HIMMEL M E. The maize primary cell wall microfibril: a new model derived from direct visualization [J]. Journal of Agricultural and Food Chemistry, 2006, 54(3): 597-606. |
| 6 | GECK M K, KIRSCH J F. A novel, definitive test for substrate channeling illustrated with the aspartate aminotransferase/malate dehydrogenase system [J]. Biochemistry, 1999, 38(25): 8032-8037. |
| 7 | ZHANG Y H. Substrate channeling and enzyme complexes for biotechnological applications [J]. Biotechnology Advances, 2011, 29(6): 715-725. |
| 8 | WOODLEY J M. Microbial biocatalytic processes and their development [J]. Advances in Applied Microbiology, 2006, 60: 1-15. |
| 9 | 许可, 吕波, 李春. 无细胞的合成生物技术——多酶催化与生物合成[J]. 中国科学:化学, 2015, 45(5): 429-437. |
| XU K, LÜ B, LI C. Cell-free synthetic biotechnology — multi-enzyme catalysis and biosynthesis[J]. Scientia Sinica Chimica, 2015, 45(5): 429-437. | |
| 10 | SCHOFFELEN S, HEST J C M VAN. Multi-enzyme systems: bringing enzymes together in vitro [J]. Soft Matter, 2012, 8(6): 1736-1746. |
| 11 | FEIST A M, PALSSON B Ø. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli [J]. Nature Biotechnology, 2008, 26(6): 659-667. |
| 12 | HAWKINS K M, SMOLKE C D. Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae [J]. Nature Chemical Biology, 2008, 4(9): 564-573. |
| 13 | FRANCE S P, HEPWORTH L J, TURNER N J, et al. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways [J]. ACS Catalysis, 2016, 7(1): 710-724. |
| 14 | BACHMANN B O. Biosynthesis: Is it time to go retro? [J]. Nature Chemical Biology, 2010, 6(6): 390-393. |
| 15 | DELÉPINE B, DUIGOU T, CARBONELL P, et al. RetroPath2.0: A retrosynthesis workflow for metabolic engineers [J]. Metabolic Engineering, 2018, 45: 158-170. |
| 16 | KUMAR A, WANG L, NG C Y, et al. Pathway design using de novo steps through uncharted biochemical spaces [J]. Nature Communications, 2018, 9(1): 184. |
| 17 | SHI J F, WU Y Z, ZHANG S H, et al. Bioinspired construction of multi-enzyme catalytic systems [J]. Chemical Society Reviews, 2018, 47(12): 4295-4313. |
| 18 | MUTTI F G, KNAUS T, SCRUTTON N S, et al. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades [J]. Science, 2015, 349(6255): 1525-1529. |
| 19 | KIM Y H, CAMPBELL E, YU J, et al. Complete oxidation of methanol in biobattery devices using a hydrogel created from three modified dehydrogenases[J]. Angewandte Chemie (International Ed in English), 2013, 52(5): 1437-1440. |
| 20 | ANDRE C, KIM S W, YU X H, et al. Fusing catalase to an alkane-producing enzyme maintains enzymatic activity by converting the inhibitory byproduct H2O2 to the cosubstrate O2 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(8): 3191-3196. |
| 21 | QUIN M B, WALLIN K K, ZHANG G, et al. Spatial organization of multi-enzyme biocatalytic cascades [J]. Organic & Biomolecular Chemistry, 2017, 15(20): 4260-4271. |
| 22 | ZHANG G Q, QUIN M B, SCHMIDT-DANNERT C. Self-assembling protein scaffold system for easy in vitro coimmobilization of biocatalytic cascade enzymes [J]. ACS Catalysis, 2018, 8(6): 5611-5620. |
| 23 | DELEBECQUE C J, LINDNER A B, SILVER P A, et al. Organization of intracellular reactions with rationally designed RNA assemblies [J]. Science, 2011, 333(6041): 470-474. |
| 24 | MYHRVOLD C, POLKA J K, SILVER P A. Synthetic lipid-containing scaffolds enhance production by colocalizing enzymes[J]. ACS Synthetic Biology, 2016, 5(12): 1396-1403. |
| 25 | POLKA J K, HAYS S G, SILVER P A. Building spatial synthetic biology with compartments, scaffolds, and communities [J]. Cold Spring Harbor Perspectives in Biology, 2016, 8(8): a024018. |
| 26 | AGAPAKIS C M, BOYLE P M, SILVER P A. Natural strategies for the spatial optimization of metabolism in synthetic biology [J]. Nature Chemical Biology, 2012, 8(6): 527-535. |
| 27 | GIESSEN T W, SILVER P A. Encapsulation as a strategy for the design of biological compartmentalization[J]. Journal of Molecular Biology, 2016, 428(5pt b): 916-927. |
| 28 | LEE H, DELOACHE W C, DUEBER J E. Spatial organization of enzymes for metabolic engineering [J]. Metabolic Engineering, 2012, 14(3): 242-251. |
| 29 | YEATES T O, CROWLEY C S, TANAKA S. Bacterial microcompartment organelles: protein shell structure and evolution [J]. Annual Review of Biophysics, 2010, 39: 185-205. |
| 30 | BOBIK T A, LEHMAN B P, YEATES T O. Bacterial microcompartments: widespread prokaryotic organelles for isolation and optimization of metabolic pathways [J]. Molecular Microbiology, 2015, 98(2): 193-207. |
| 31 | LAWRENCE A D, FRANK S, NEWNHAM S, et al. Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor [J]. ACS Synthetic Biology, 2014, 3(7): 454-465. |
| 32 | LI C, ZHANG R, WANG J, et al. Protein engineering for improving and diversifying natural product biosynthesis [J]. Trends in Biotechnology, 2020, 38(7): 729-744. |
| 33 | XUE R, WOODLEY J M. Process technology for multi-enzymatic reaction systems [J]. Bioresource Technology, 2012, 115: 183-195. |
| 34 | WAHL C, HIRTZ D, ELLING L. Multiplexed capillary electrophoresis as analytical tool for fast optimization of multi-enzyme cascade reactions - synthesis of nucleotide sugars: Dedicated to Prof. Dr. Vladimir Křen on the occasion of his 60th birthday [J]. Biotechnology Journal, 2016, 11(10): 1298-1308. |
| 35 | ARANAZ I, ACOSTA N, FéRNANDEZ-VALLE M E, et al. Optimization of D-amino acid production catalyzed by immobilized multi-enzyme system in polyelectrolyte complex gel capsules [J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 121: 45-52. |
| 36 | KIM Y, YOON K, KHANG Y, et al. The 2.0 Å crystal structure of cephalosporin acylase [J]. Structure, 2000, 8(10): 1059-1068. |
| 37 | HAMAD B. The antibiotics market [J]. Nature Reviews Drug Discovery, 2010, 9(9): 675-676. |
| 38 | DING J M, ZHOU Y, ZHU H J, et al. Characterization of EstZY: a new acetylesterase with 7-aminocephalosporanic acid deacetylase activity from Alicyclobacillus tengchongensis [J]. International Journal of Biological Macromolecules, 2020, 148: 333-341. |
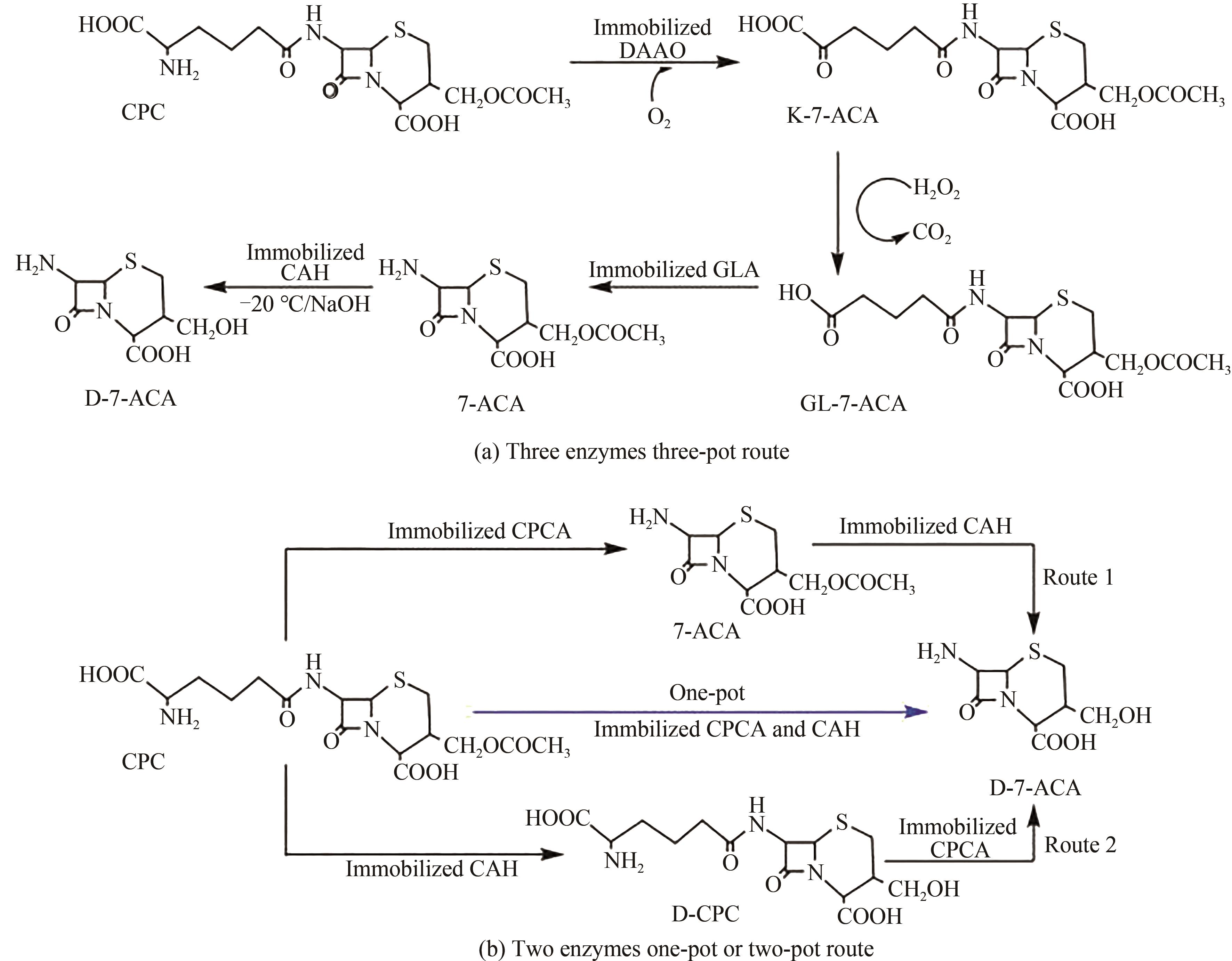
| 39 | TAKIMOTO A, TAKAKURA T, TANI H, et al. Batch production of deacetyl 7-aminocephalosporanic acid by immobilized cephalosporin-C deacetylase [J]. Applied Microbiology and Biotechnology, 2004, 65(3): 263-267. |
| 40 | YAMANAKA H, CHIBA T, KAWABATA K, et al. Studies on β-lactam antibiotics IX. Synthesis and biological activity of a new orally active cephalosporin, cefixime (FK027) [J]. The Journal of Antibiotics, 1985, 38(12): 1738-1751. |
| 41 | GONZÁLEZ M, RODRÍGUEZ Z, TOLÓN B, et al. An alternative procedure for preparation of cefdinir [J]. Farmaco, 2003, 58(6): 409-418. |
| 42 | MA X Q, DENG S W, SU E Z, et al. One-pot enzymatic production of deacetyl-7-aminocephalosporanic acid from cephalosporin C via immobilized cephalosporin C acylase and deacetylase [J]. Biochemical Engineering Journal, 2015, 95: 1-8. |
| 43 | JIANG J J, CHEN X, ZHANG D L, et al. Characterization of (R)-selective amine transaminases identified by in silico motif sequence blast [J]. Applied Microbiology and Biotechnology, 2015, 99(6): 2613-2621. |
| 44 | LEY S V, PRIOUR A. Total synthesis of the cyclic peptide Argyrin B [J]. European Journal of Organic Chemistry, 2002(23): 3995-4004. |
| 45 | SHAGINIAN A, ROSEN M C, BINKOWSKI B F, et al. Solid-phase synthesis of dihydrovirginiamycin S1, a streptogramin B antibiotic [J]. Chemistry, 2004, 10(17): 4334-4340. |
| 46 | DEATON D N, GRAHAM K P, GROSS J W, et al. Thiol-based angiotensin-converting enzyme 2 inhibitors: P1' modifications for the exploration of the S1' subsite [J]. Bioorganic & Medicinal Chemistry Letters, 2008, 18(5): 1681-1687. |
| 47 | AUBELE D L, HOM R K, ADLER M, et al. Selective and brain-permeable polo-like kinase-2 (Plk-2) inhibitors that reduce α-synuclein phosphorylation in rat brain [J]. ChemMedChem, 2013, 8(8): 1295-1313. |
| 48 | SCHAROW A, KNAPPE D, REINDL W, et al. Development of bifunctional inhibitors of polo-like Kinase 1 with low-nanomolar activities against the polo-box domain [J]. ChemBioChem, 2016, 17(8): 759-767. |
| 49 | BEHRENDS M, WAGNER S, KOPKA K, et al. New matrix metalloproteinase inhibitors based on γ-fluorinated α-aminocarboxylic and α-aminohydroxamic acids [J]. Bioorganic & Medicinal Chemistry, 2015, 23(13): 3809-3818. |
| 50 | CHEN X, CUI Y F, CHENG X K, et al. Highly atom economic synthesis of d-2-aminobutyric acid through an in vitro tri-enzymatic catalytic system [J]. ChemistryOpen, 2017, 6(4): 534-540. |
| 51 | SHINDE P, BANERJEE P, MANDHARE A. Marine natural products as source of new drugs: a patent review (2015—2018) [J]. Expert Opinion on Therapeutic Patents, 2019, 29(4): 283-309. |
| 52 | LIU J, HU K F, QU J P, et al. Organopromoted selectivity-switchable synthesis of polyketones [J]. Organic Letters, 2017, 19(20): 5593-5596. |
| 53 | BRAÏEK O BEN, SMAOUI S, SMAOUI S. Enterococci: between emerging pathogens and potential probiotics [J]. BioMed Research International, 2019, 2019: 5938210. |
| 54 | CHENG Q, XIANG L K, IZUMIKAWA M, et al. Enzymatic total synthesis of enterocin polyketides [J]. Nature Chemical Biology, 2007, 3(9): 557-558. |
| 55 | LEE G E, JOSHI B V, CHEN W, et al. Synthesis and structure-activity relationship studies of tyrosine-based antagonists at the human P2X7 receptor [J]. Bioorganic & Medicinal Chemistry Letters, 2008, 18(2): 571-575. |
| 56 | CHEN P W, LEE N C, CHIEN Y H, et al. Diagnosis of aromatic L-amino acid decarboxylase deficiency by measuring 3-O-methyldopa concentrations in dried blood spots [J]. Clinica Chimica Acta, International Journal of Clinical Chemistry, 2014, 431: 19-22. |
| 57 | DONG W F, LIU W, LIAO X W, et al. Asymmetric total synthesis of (-)-saframycin A from L-tyrosine [J]. The Journal of Organic Chemistry, 2011, 76(13): 5363-5368. |
| 58 | OHTAKE K, YAMAGUCHI A, MUKAI T, et al. Protein stabilization utilizing a redefined codon [J]. Scientific Reports, 2015, 5: 9762. |
| 59 | MCCUBBIN J A, MADDESS M L, LAUTENS M. Total synthesis of cryptophycin analogues via a scaffold approach[J]. Organic Letters, 2006, 8(14): 2993-2996. |
| 60 | CHEN X C, ZHU J. Total synthesis of the marine natural product (-)‐Cribrostatin 4 (Renieramycin H) [J]. Angewandte Chemie International Edition, 2007, 46(21): 3962-3965. |
| 61 | SEYEDSAYAMDOST M R, REECE S Y, NOCERA D G, et al. Mono-, di-, tri-, and tetra-substituted fluorotyrosines: new probes for enzymes that use tyrosyl radicals in catalysis [J]. Journal of the American Chemical Society, 2006, 128(5): 1569-1579. |
| 62 | NATARAJAN A, SCHWANS J P, HERSCHLAG D. Using unnatural amino acids to probe the energetics of oxyanion hole hydrogen bonds in the ketosteroid isomerase active site [J]. Journal of the American Chemical Society, 2014, 136(21): 7643-7654. |
| 63 | LI F H, SHI P, LI J S, et al. A genetically encoded 19F NMR probe for tyrosine phosphorylation [J]. Angewandte Chemie (International Ed in English), 2013, 52(14): 3958-3962. |
| 64 | DI STEFANO A, SOZIO P, CERASA L S. Antiparkinson prodrugs [J]. Molecules, 2008, 13(1): 46-68. |
| 65 | SWOBODA K J, SAUL J P, MCKENNA C E, et al. Aromatic L‐amino acid decarboxylase deficiency: overview of clinical features and outcomes [J]. Annals of Neurology, 2003, 54(S6): S49-S55. |
| 66 | DENNIG A, BUSTO E, KROUTIL W, et al. Biocatalytic one-pot synthesis of L-tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia [J]. ACS Catalysis, 2015, 5(12): 7503-7506. |
| 67 | DAMARAJU V L, DAMARAJU S, YOUNG J D, et al. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy [J]. Oncogene, 2003, 22(47): 7524-7536. |
| 68 | ROBAK T, LECH-MARANDA E, KORYCKA A, et al. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity [J]. Current Medicinal Chemistry, 2006, 13(26): 3165-3189. |
| 69 | MESAROS C, ARORA J S, WHOLER A, et al. 8-Oxo-2'-deoxyguanosine as a biomarker of tobacco-smoking-induced oxidative stress [J]. Free Radical Biology & Medicine, 2012, 53(3): 610-617. |
| 70 | LI Y Y, DING Q B, OU L, et al. One-pot process of 2'-deoxyguanylic acid catalyzed by a multi-enzyme system [J]. Biotechnology and Bioprocess Engineering, 2015, 20(1): 37-43. |
| 71 | ENDO A. The origin of the statins [J]. International Congress Series, 2004, 1262: 3-8. |
| 72 | PATEL R N. Biocatalysis for synthesis of pharmaceuticals [J]. Bioorganic & Medicinal Chemistry, 2018, 26(7): 1252-1274. |
| 73 | SIERRA S, RAMOS M C, MOLINA P, et al. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death [J]. Journal of Alzheimer's Disease, 2011, 23(2): 307-318. |
| 74 | HOYOS P, PACE V, ALCÁNTARA A R. Biocatalyzed synthesis of statins: a sustainable strategy for the preparation of valuable drugs [J]. Catalysts, 2019, 9(3): 260. |
| 75 | ŠVARC A, FEKETE M, HERNANDEZ K, et al. An innovative route for the production of atorvastatin side-chain precursor by DERA-catalysed double aldol addition [J]. Chemical Engineering Science, 2021, 231: 116312. |
| 76 | LINDOR K D, KOWDLEY K V, HEATHCOTE E J, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial[J]. Hepatology, 2004, 39(3): 770-778. |
| 77 | ZHANG Y J, ZHENG X J, HUANG F J, et al. Ursodeoxycholic acid alters bile acid and fatty acid profiles in a mouse model of diet-induced obesity [J]. Frontiers in Pharmacology, 2019, 10: 842. |
| 78 | HANAFI N I, MOHAMED A S, SHEIKH A K S H, et al. Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart [J]. Biomolecules, 2018, 8(4): 159. |
| 79 | HIRSCHFIELD G M, MASON A, LUKETIC V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid [J]. Gastroenterology, 2015, 148(4): 751-61.e8. |
| 80 | HE H W, MENNONE A, BOYER J L, et al. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells [J]. Hepatology, 2011, 53(2): 548-557. |
| 81 | TONIN F, ARENDS I W. Latest development in the synthesis of ursodeoxycholic acid (UDCA): a critical review [J]. Beilstein Journal of Organic Chemistry, 2018, 14(1): 470-483. |
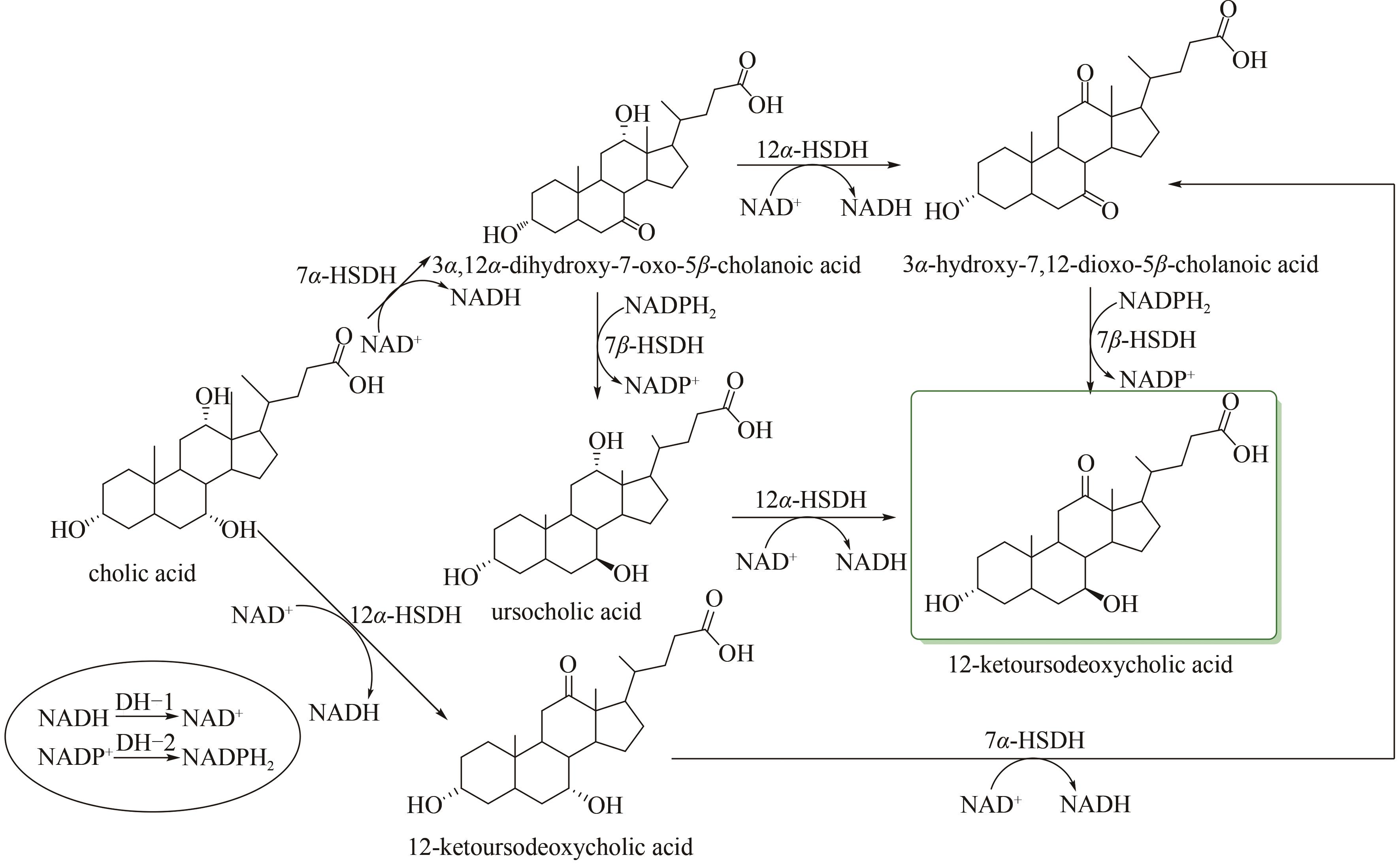
| 82 | POLYKETIDES N T S O E, MONTI D, FERRANDI E E, et al. One-Pot multienzymatic synthesis of 12-ketoursodeoxycholic acid: subtle cofactor specificities rule the reaction equilibria of five biocatalysts working in a row [J]. Advanced Synthesis & Catalysis, 2009, 351(9): 1303-1311. |
| 83 | PANDEY R K, FERNANDES R A, KUMAR P. An asymmetric dihydroxylation route to enantiomerically pure norfluoxetine and fluoxetine [J]. Tetrahedron Letters, 2002, 43(25): 4425-4426. |
| 84 | KUMAR P, UPADHYAY R K, PANDEY R K. Asymmetric dihydroxylation route to (R)-isoprenaline,(R)-norfluoxetine and (R)-fluoxetine [J]. Tetrahedron: Asymmetry, 2004, 15(24): 3955-3959. |
| 85 | CAO L, LEE J, CHEN W, et al. Enantioconvergent production of (R)-1-phenyl-1,2-ethanediol from styrene oxide by combining the Solanum tuberosum and an evolved Agrobacterium radiobacter AD1 epoxide hydrolases [J]. Biotechnology and Bioengineering, 2006, 94(3): 522-529. |
| 86 | HU Q S, XU Y, NIE Y. Highly enantioselective reduction of 2-hydroxy-1-phenylethanone to enantiopure (R)-phenyl-1,2-ethanediol using Saccharomyces cerevisiae of remarkable reaction stability [J]. Bioresource Technology, 2010, 101(22): 8502-8508. |
| 87 | LI B, NIE Y, MU X Q, et al. De novo construction of multi-enzyme system for one-pot deracemization of (R,S)-1-phenyl-1,2-ethanediol by stereoinversion of (S)-enantiomer to the corresponding counterpart [J]. Journal of Molecular Catalysis B: Enzymatic, 2016, 129: 21-28. |
| 88 | RAMACHANDRAN S, FONTANILLE P, PANDEY A, et al. Gluconic acid: properties, applications and microbial production [J]. Food Technology & Biotechnology, 2006, 44(2): 185-195. |
| 89 | ANASTASSIADIS S, MORGUNOV I G. Gluconic acid production [J]. Recent Patents on Biotechnology, 2007, 1(2): 167-180. |
| 90 | SU H H, GUO Z W, WU X L, et al. Efficient bioconversion of sucrose to high-value-added glucaric acid by in vitro metabolic engineering [J]. ChemSusChem, 2019, 12(10): 2278-2285. |
| 91 | ZHAO F H, LI H, JIANG Y J, et al. Co-immobilization of multi-enzyme on control-reduced graphene oxide by non-covalent bonds: an artificial biocatalytic system for the one-pot production of gluconic acid from starch [J]. Green Chemistry, 2014, 16(5): 2558-2565. |
| 92 | PETROLL K, CARE A, BERGQUIST P L, et al. A novel framework for the cell-free enzymatic production of glucaric acid [J]. Metabolic Engineering, 2020, 57: 162-173. |
| 93 | PETROLL K, KOPP D, CARE A, et al. Tools and strategies for constructing cell-free enzyme pathways [J]. Biotechnology Advances, 2019, 37(1): 91-108. |
| 94 | OLDFIELD E, LIN F Y. Terpene biosynthesis: modularity rules [J]. Angewandte Chemie International Edition, 2012, 51(5): 1124-1137. |
| 95 | DIXON R A. Plant natural products: the molecular genetic basis of biosynthetic diversity [J]. Current Opinion in Biotechnology, 1999, 10(2): 192-197. |
| 96 | WITHERS S T, KEASLING J D. Biosynthesis and engineering of isoprenoid small molecules [J]. Applied Microbiology and Biotechnology, 2007, 73(5): 980-990. |
| 97 | KRINGS U, BERGER R G. Biotechnological production of flavours and fragrances [J]. Applied Microbiology and Biotechnology, 1998, 49(1): 1-8. |
| 98 | AJIKUMAR P K, TYO K, CARLSEN S, et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms [J]. Molecular Pharmaceutics, 2008, 5(2): 167-190. |
| 99 | ALONSO-GUTIERREZ J, CHAN R, BATTH T S, et al. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production [J]. Metabolic Engineering, 2013, 19: 33-41. |
| 100 | YANG J M, NIE Q J, REN M, et al. Metabolic engineering of Escherichia coli for the biosynthesis of α-pinene [J]. Biotechnology for Biofuels, 2013, 6(1): 60. |
| 101 | ZHANG H, LIU Q, CAO Y, et al. Microbial production of sabinene—a new terpene-based precursor of advanced biofuel [J]. Microbial Cell Factories, 2014, 13: 20. |
| 102 | KORMAN T P, OPGENORTH P H, BOWIE J U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose [J]. Nature Communications, 2017, 8: 15526. |
| 103 | YANG M-L, KUN Y, GUO Y-P, et al. A photosensitivity insecticide, 5-aminolevulinic acid, exerts effectivetoxicity to Oxya chinensis (Orthoptera: Acridoidea) [J]. Agricultural Sciences in China, 2011, 10(7): 1056-1063. |
| 104 | ZHANG J L, KANG Z, CHEN J, et al. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli [J]. Scientific Reports, 2015, 5: 8584. |
| 105 | SASAKI K, WATANABE M, TANAKA T, et al. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid [J]. Applied Microbiology and Biotechnology, 2002, 58(1): 23-29. |
| 106 | LI T, GUO Y Y, QIAO G Q, et al. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate [J]. ACS Synthetic Biology, 2016, 5(11): 1264-1274. |
| 107 | MENG Q L, ZHANG Y F, JU X Z, et al. Production of 5-aminolevulinic acid by cell free multi-enzyme catalysis [J]. Journal of Biotechnology, 2016, 226: 8-13. |
| 108 | WANG S Z, ZHANG Y H, REN H, et al. Strategies and perspectives of assembling multi-enzyme systems [J]. Critical Reviews in Biotechnology, 2017, 37(8): 1024-1037. |
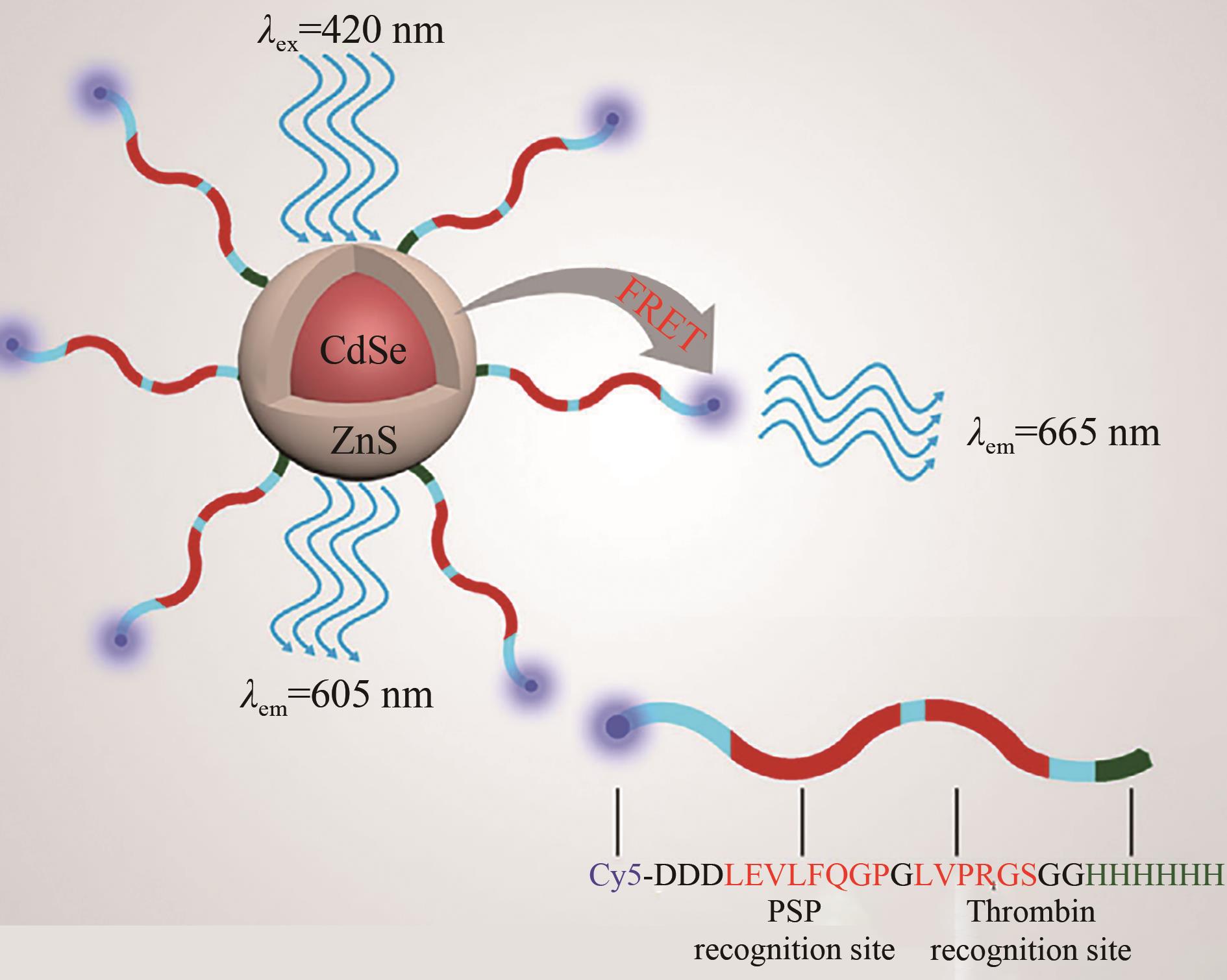
| 109 | QIU L, CUI P F, ZHU Z L, et al. Multienzyme detection and in-situ monitoring of enzyme activity by bending CE using quantum dots-based polypeptide substrate [J]. Electrophoresis. 2020, 41(12): 1103-1108. |
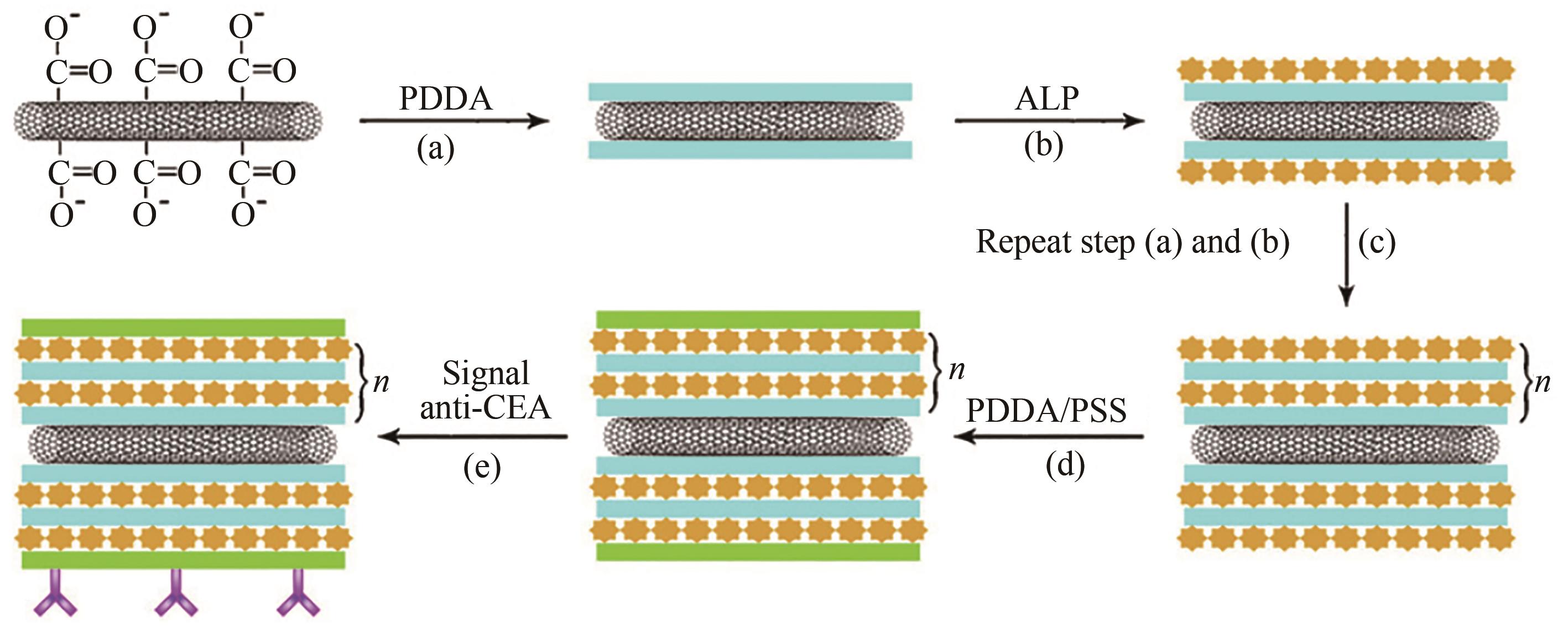
| 110 | XIANG Y, ZHANG Y Y, JIANG B Y, et al. Multi-enzyme layer-by-layer assembly for dual amplified ultrasensitive electronic detection of cancer biomarkers [J]. Sensors and Actuators B: Chemical, 2011, 155(1): 317-322. |
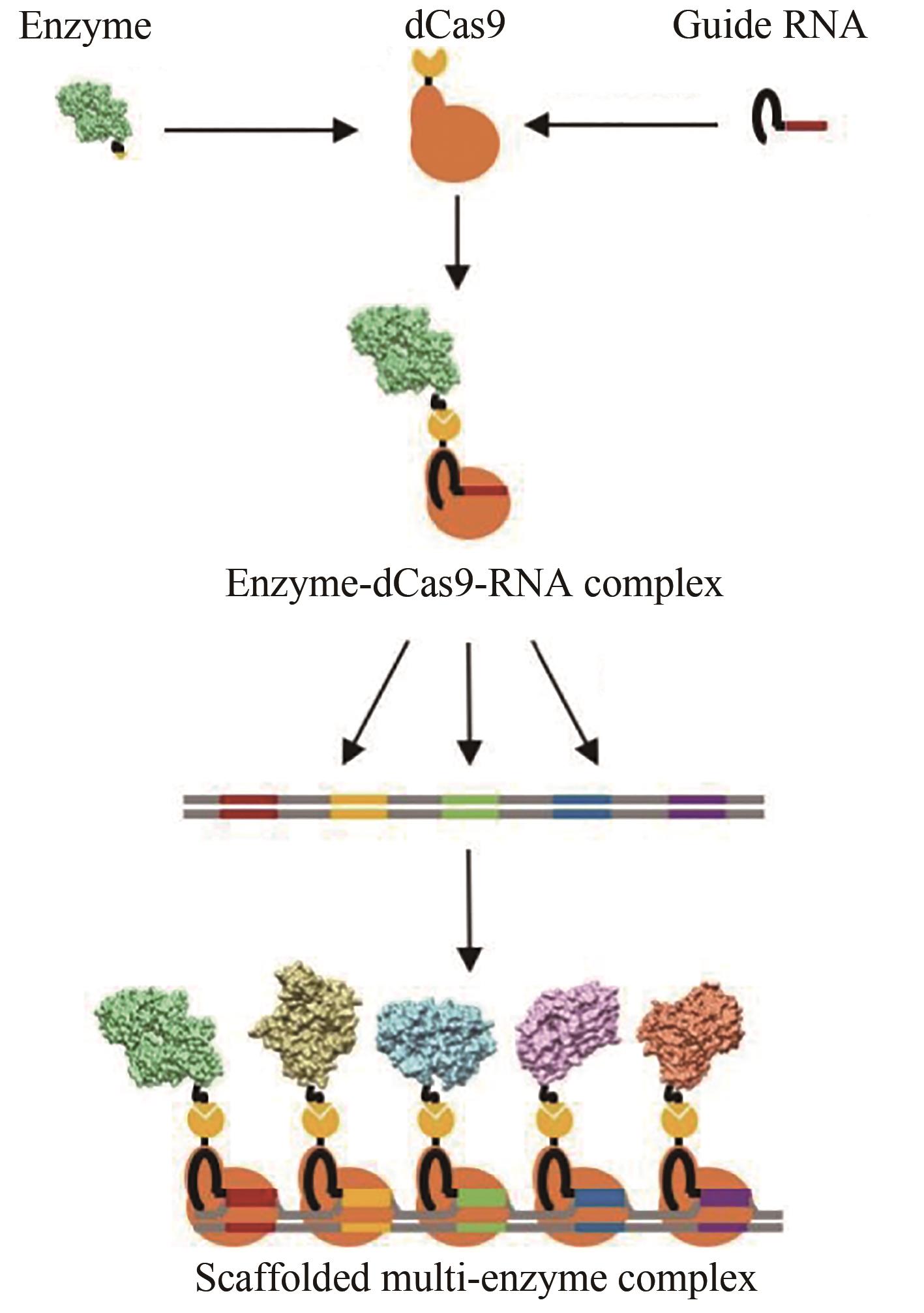
| 111 | LIM S, KIM J, KIM Y, et al. CRISPR/Cas-directed programmable assembly of multi-enzyme complexes [J]. Chemical Communications, 2020, 56(36): 4950-4953. |
| 112 | DUDLEY Q M, KARIM A S, JEWETT M C. Cell-free metabolic engineering: biomanufacturing beyond the cell [J]. Biotechnology Journal, 2015, 10(1): 69-82. |
| [1] | 温艳华, 刘合栋, 曹春来, 巫瑞波. 蛋白质工程在医药产业中的应用[J]. 合成生物学, 2025, 6(1): 65-86. |
| [2] | 董玲玲, 李斐煊, 雷航彬, 宋启迪, 王世珍. 仿生分区室固定化多酶体系[J]. 合成生物学, 2024, 5(6): 1518-1529. |
| [3] | 雷航彬, 何宁, 李斐煊, 董玲玲, 王世珍. 氢化酶固定化研究进展[J]. 合成生物学, 2024, 5(6): 1485-1497. |
| [4] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [5] | 付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049. |
| [6] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [7] | 刘晚秋, 季向阳, 许慧玲, 卢屹聪, 李健. 限制性内切酶的无细胞快速制备研究[J]. 合成生物学, 2023, 4(4): 840-851. |
| [8] | 唐士茗, 胡纪元, 郑穗平, 韩双艳, 林影. 基于无细胞体系的生物合成代谢模块设计、构建与快速途径原型[J]. 合成生物学, 2022, 3(6): 1250-1261. |
| [9] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [10] | 刘建明, 曾安平. 无细胞多酶分子机器赋能二氧化碳的高值利用及其挑战[J]. 合成生物学, 2022, 3(5): 825-832. |
| [11] | 吉博涛, 钱志刚, 夏小霞. 无细胞合成策略在生物材料研究中的应用[J]. 合成生物学, 2022, 3(4): 658-675. |
| [12] | 王汇滨, 车昌丽, 游松. Fe/α-酮戊二酸依赖型卤化酶在绿色卤化反应中的研究进展[J]. 合成生物学, 2022, 3(3): 545-566. |
| [13] | 楼玉姣, 徐鉴, 吴起. 生物催化惰性碳氢键的氘代反应研究进展[J]. 合成生物学, 2022, 3(3): 530-544. |
| [14] | 后佳琦, 姜楠, 马莲菊, 卢元. 无细胞蛋白质合成:从基础研究到工程应用[J]. 合成生物学, 2022, 3(3): 465-486. |
| [15] | 杨璐, 瞿旭东. 亚胺还原酶在手性胺合成中的应用[J]. 合成生物学, 2022, 3(3): 516-529. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||