合成生物学 ›› 2025, Vol. 6 ›› Issue (2): 373-390.DOI: 10.12211/2096-8280.2024-058
黄酮类化合物生物合成及其在化妆品中应用的研究
韦灵珍, 王佳, 孙新晓, 袁其朋, 申晓林
- 北京化工大学化工资源有效利用全国重点实验室,北京 100029
-
收稿日期:2024-07-31修回日期:2024-10-26出版日期:2025-04-30发布日期:2025-05-20 -
通讯作者:申晓林 -
作者简介:韦灵珍 (2001—),女,硕士研究生。研究方向为代谢工程及合成生物学。E-mail:2024201324@mail.buct.edu.cn申晓林 (1984—),女,博士,教授。研究方向为代谢工程及合成生物学。E-mail:shenxl@mail.buct.edu.cn -
基金资助:国家自然科学基金(22078011)
Biosynthesis of flavonoids and their applications in cosmetics
WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin
- State Key Laboratory of Chemical Resource Engineering,Beijing University of Chemical Technology,Beijing 100029,China
-
Received:2024-07-31Revised:2024-10-26Online:2025-04-30Published:2025-05-20 -
Contact:SHEN Xiaolin
摘要:
黄酮类化合物是一类广泛存在于自然界中的多酚类化合物,因其显著的抗氧化、抗炎、抗菌等生物活性在化妆品中广泛应用。然而,传统植物提取方法的局限性促使研究人员转向合成生物学以寻求更高效的生产途径。本文根据美白抗氧化、抗菌消炎、防晒抗衰老和增色增彩四个功能分类分别列举了几种常见黄酮类化合物在化妆品中的应用;介绍了黄酮类化合物的现有生物合成途径并总结了典型黄酮类化合物的最新研究进展;详细讨论了合成生物学及代谢工程策略。接着,针对黄酮类化合物在化妆品应用中的水溶性差和稳定性低的问题,总结了相应解决方案的研究进程。最后,总结并展望了人工智能辅助合成生物学的策略以应对黄酮类化合物合成过程中的挑战。同时,本文强调了黄酮类化合物的安全性和有效性评估的重要性,以推动其在化妆品行业的应用。
中图分类号:
引用本文
韦灵珍, 王佳, 孙新晓, 袁其朋, 申晓林. 黄酮类化合物生物合成及其在化妆品中应用的研究[J]. 合成生物学, 2025, 6(2): 373-390.
WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics[J]. Synthetic Biology Journal, 2025, 6(2): 373-390.
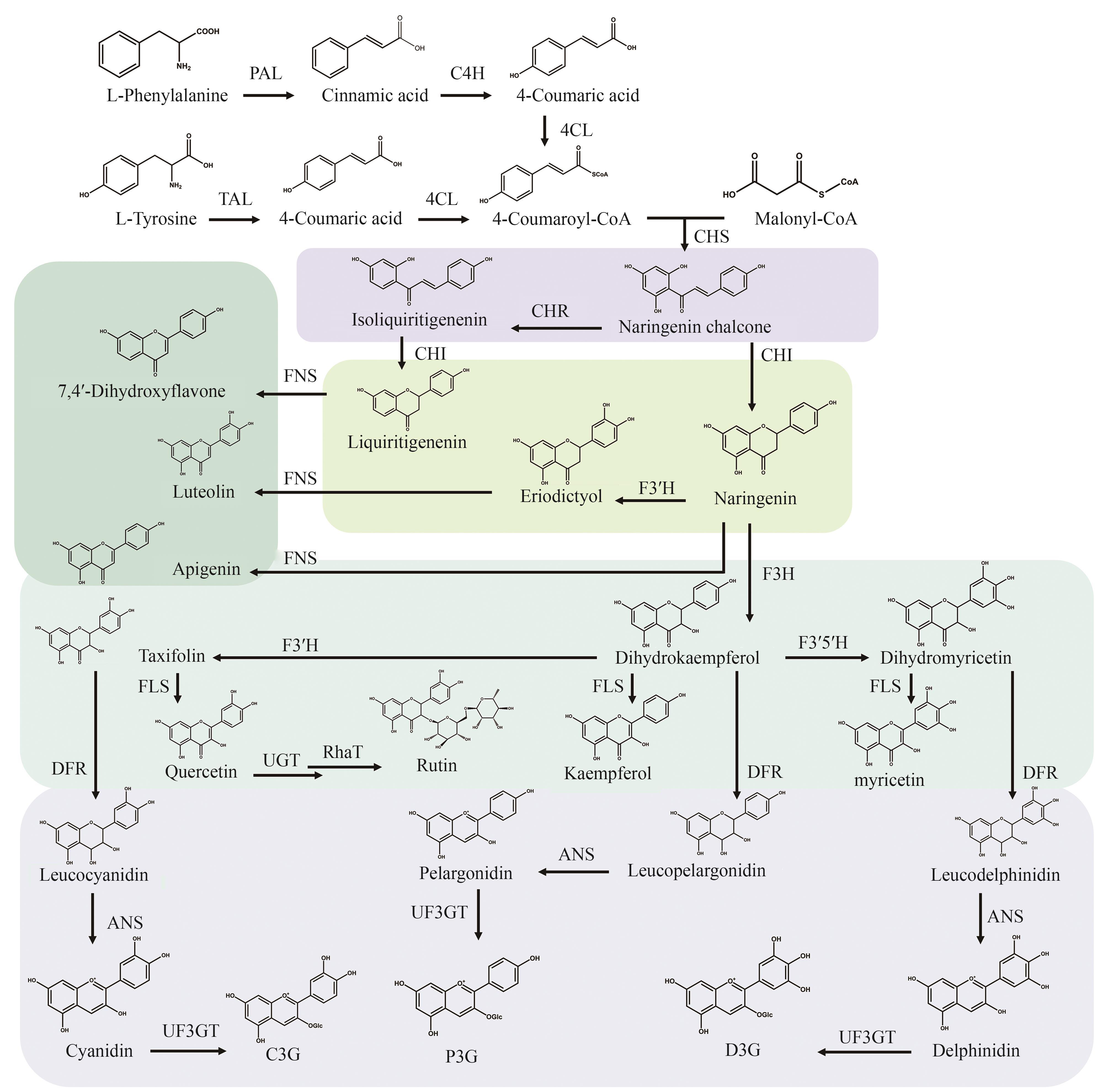
图1 黄酮类化合物的天然合成途径(PAL—苯丙氨酸解氨酶;C4H—肉桂酸4-羟化酶;TAL—酪氨酸解氨酶;4CL—4-香豆酸辅酶A连接酶;CHS—查尔酮合酶;CHR—查尔酮还原酶;CHI—查尔酮异构酶;FNS—黄酮合酶;F3′H—黄烷酮-3′-羟化酶;F3H—黄烷酮-3-羟化酶;F3′5′H—黄烷酮-3′,5′-羟化酶;FLS—黄酮醇合酶;UGT—UDP-葡萄糖基转移酶;RhaT—鼠李糖转移酶;DFR—二氢黄酮醇4-还原酶;ANS—花青素合酶;UF3GT—黄酮类化合物3-O-葡萄糖基转移酶;C3G—矢车菊素3-O-葡萄糖苷;P3G—天竺葵素-3-O-葡萄糖苷;D3G—飞燕草素-3-O-葡萄糖苷)
Fig. 1 Natural synthesis pathways of flavonoids(PAL—Phenylalaninammo-nialyase; C4H—Cinnamic acid-4-hydroxylase; TA—Tyrosine ammonialyase; 4CL—4-Coumarate coenzyme A ligase; CH—Chalcone synthase; CHR—Chalcone reductase; CHI—Chalcone isomerase; FNS—Flavone synthase; F3′H—Flavanone 3′-β-hydroxyalse; F3H—Flavanone 3-β-hydroxyalse; F3′5′H—Flavanone 3′,5′-β-hydroxyalse; FLS—Flavonol synthase; UGT—UDP-glycosyltransferase; RhaT—Rhamnose transferase; DFR—Dihydroflavonol 4-reductase; ANS—Anthocyanidin synthase; UF3GT—Flavonoid 3-O-glycosyltransferase; C3G—Cyanidin 3-O-glucoside chloride; P3G—Pelargonidin3-O-glucoside; D3G—Delphinidin-3-O-glucoside)
| 黄酮类化合物 | 底物 | 菌株 | 生产规模 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 根皮素 | 对羟基苯丙酸 | 酿酒酵母 | 摇瓶 | 83.2 mg/L | [ |
| 根皮素 | 对羟基苯丙酸 | 酿酒酵母 | 5 L发酵罐 | 619.5 mg/L | [ |
| 柚皮素 | 甘油 | 大肠杆菌 | 摇瓶 | 98.71 mg/L | [ |
| 柚皮素 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 2.5 g/L | [ |
| 柚皮素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 986.2 mg/L | [ |
| 柚皮素 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 3.4 g/L | [ |
| 柚皮素 | 葡萄糖和木糖 | 解脂耶氏酵母 | 摇瓶 | 715.3 mg/L | [ |
| 柚皮素 | 葡萄糖 | 解脂耶氏酵母 | 5 L发酵罐 | 8.3 g/L | [ |
| 圣草酚 | 葡萄糖 | 解脂耶氏酵母 | 5 L发酵罐 | 6.8 g/L | [ |
| 柚皮素 | 4-香豆酸 | 含油丝状真菌毛霉菌 | 摇瓶 | 2.2 mg/L | [ |
| 柚皮素 | 4-香豆酸 | 丝状真菌红青霉菌 | 摇瓶 | 0.88 mmol/L | [ |
| 柚皮素 | 葡萄糖 | 原核细菌链霉菌 | 摇瓶 | 184 mg/L | [ |
| 柚皮素 | 葡萄糖 | 大肠杆菌 | 摇瓶 | 523.7 mg/L | [ |
| 芹菜素 | 葡萄糖 | 解脂耶氏酵母和大肠杆菌 | 摇瓶 | 168 mg/L | [ |
| 白杨素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 41.9 mg/L | [ |
| 山柰酚 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 86 mg/L | [ |
| 槲皮素 | 葡萄糖 | 白链霉菌 | 摇瓶 | 0.1 mg/L | [ |
| 山柰酚 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 26.57 mg/L | [ |
| 槲皮素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 20.38 mg/L | [ |
| 山柰酚 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 956 mg/L | [ |
| 槲皮素 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 930 mg/L | [ |
| 芦丁 | 槲皮素 | 大肠杆菌 | 摇瓶 | 119.8 mg/L | [ |
| 矢车菊素3-O-葡萄糖苷 | 儿茶酚和葡萄糖 | 大肠杆菌 | 摇瓶 | 439 mg/L | [ |
| 天竺葵素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 33 mg/L | [ |
| 矢车菊素3-O-葡萄糖苷 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 8.0 mg/L | [ |
| 飞燕草素3-O-葡萄糖苷 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 3.5 mg/L | [ |
| 矢车菊素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 3.0 mg/L | [ |
| 飞燕草素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 0.7 mg/L | [ |
表1 代表性天然黄酮类化合物的生物合成进展
Table 1 Advances in the biosynthesis of typical natural flavonoids
| 黄酮类化合物 | 底物 | 菌株 | 生产规模 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 根皮素 | 对羟基苯丙酸 | 酿酒酵母 | 摇瓶 | 83.2 mg/L | [ |
| 根皮素 | 对羟基苯丙酸 | 酿酒酵母 | 5 L发酵罐 | 619.5 mg/L | [ |
| 柚皮素 | 甘油 | 大肠杆菌 | 摇瓶 | 98.71 mg/L | [ |
| 柚皮素 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 2.5 g/L | [ |
| 柚皮素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 986.2 mg/L | [ |
| 柚皮素 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 3.4 g/L | [ |
| 柚皮素 | 葡萄糖和木糖 | 解脂耶氏酵母 | 摇瓶 | 715.3 mg/L | [ |
| 柚皮素 | 葡萄糖 | 解脂耶氏酵母 | 5 L发酵罐 | 8.3 g/L | [ |
| 圣草酚 | 葡萄糖 | 解脂耶氏酵母 | 5 L发酵罐 | 6.8 g/L | [ |
| 柚皮素 | 4-香豆酸 | 含油丝状真菌毛霉菌 | 摇瓶 | 2.2 mg/L | [ |
| 柚皮素 | 4-香豆酸 | 丝状真菌红青霉菌 | 摇瓶 | 0.88 mmol/L | [ |
| 柚皮素 | 葡萄糖 | 原核细菌链霉菌 | 摇瓶 | 184 mg/L | [ |
| 柚皮素 | 葡萄糖 | 大肠杆菌 | 摇瓶 | 523.7 mg/L | [ |
| 芹菜素 | 葡萄糖 | 解脂耶氏酵母和大肠杆菌 | 摇瓶 | 168 mg/L | [ |
| 白杨素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 41.9 mg/L | [ |
| 山柰酚 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 86 mg/L | [ |
| 槲皮素 | 葡萄糖 | 白链霉菌 | 摇瓶 | 0.1 mg/L | [ |
| 山柰酚 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 26.57 mg/L | [ |
| 槲皮素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 20.38 mg/L | [ |
| 山柰酚 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 956 mg/L | [ |
| 槲皮素 | 葡萄糖 | 酿酒酵母 | 5 L发酵罐 | 930 mg/L | [ |
| 芦丁 | 槲皮素 | 大肠杆菌 | 摇瓶 | 119.8 mg/L | [ |
| 矢车菊素3-O-葡萄糖苷 | 儿茶酚和葡萄糖 | 大肠杆菌 | 摇瓶 | 439 mg/L | [ |
| 天竺葵素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 33 mg/L | [ |
| 矢车菊素3-O-葡萄糖苷 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 8.0 mg/L | [ |
| 飞燕草素3-O-葡萄糖苷 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 3.5 mg/L | [ |
| 矢车菊素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 3.0 mg/L | [ |
| 飞燕草素 | 葡萄糖 | 酿酒酵母 | 摇瓶 | 0.7 mg/L | [ |
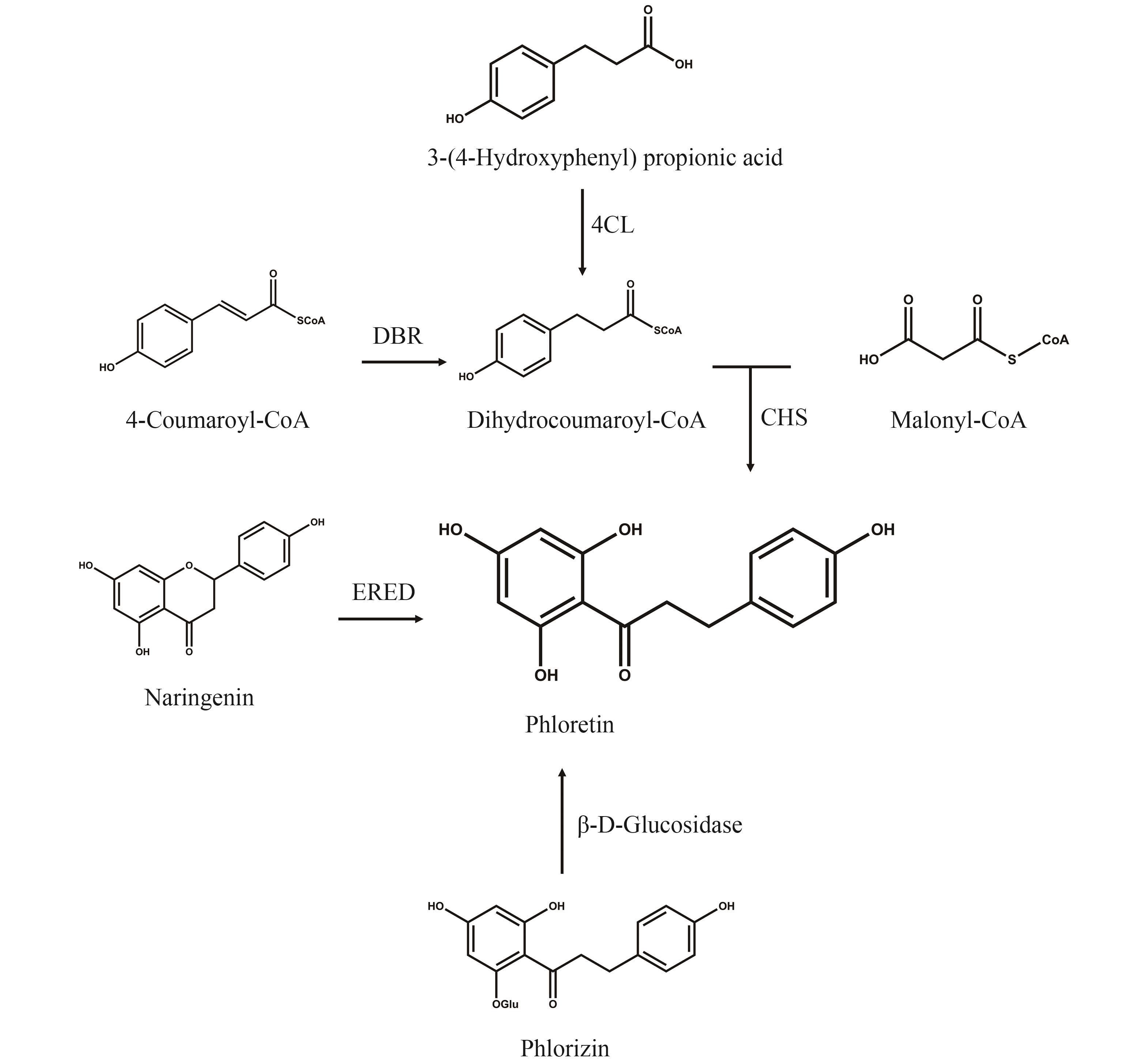
图2 根皮素的合成途径(4CL—4-香豆酸辅酶A连接酶;DBR—NADPH依赖型双键还原酶;CHS—查尔酮合酶;ERED—烯酸还原酶)
Fig. 2 Synthetic pathway for phloretin(4CL—4-Coumarate coenzyme A ligase; DBR—Double-bond reductase; CHS—Chalcone synthase; ERED—Enoate reductase)
| 1 | LIU J K. Natural products in cosmetics[J]. Natural Products and Bioprospecting, 2022, 12(1): 40. |
| 2 | KIM S J, PARK B G, JIN H B, et al. Efficient production of natural sunscreens shinorine, porphyra-334, and mycosporine-2-glycine in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2023, 78: 137-147. |
| 3 | WEN J W, WANG Y Y, LU X, et al. An integrated multi-omics approach reveals polymethoxylated flavonoid biosynthesis in Citrus reticulata cv. Chachiensis[J]. Nature Communications, 2024, 15(1): 3991. |
| 4 | ZUO A R, DONG H H, YU Y Y, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups[J]. Chinese Medicine, 2018, 13: 51. |
| 5 | BONDONNO N P, DALGAARD F, KYRØ C, et al. Flavonoid intake is associated with lower mortality in the Danish Diet Cancer and Health Cohort[J]. Nature Communications, 2019, 10(1): 3651. |
| 6 | FERREYRA M L F, SERRA P, CASATI P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure[J]. Physiologia Plantarum, 2021, 173(3): 736-749. |
| 7 | KHOO H E, AZLAN A, TANG S T, et al. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits[J]. Food & Nutrition Research, 2017, 61(1): 1361779. |
| 8 | WANG S, MENG D, FENG M L, et al. Efficient plant triterpenoids synthesis in Saccharomyces cerevisiae: from mechanisms to engineering strategies[J]. ACS Synthetic Biology, 2024, 13(4): 1059-1076. |
| 9 | AHMAD R, ALQATHAMA A, ALDHOLMI M, et al. Ultrasonic-assisted extraction of fenugreek flavonoids and its geographical-based comparative evaluation using green UHPLC-DAD analysis[J]. Ultrasonics Sonochemistry, 2023, 95: 106382. |
| 10 | SHENG H K, SUN X X, YAN Y J, et al. Metabolic engineering of microorganisms for the production of flavonoids[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 589069. |
| 11 | CHOI K R, LEE S Y. Systems metabolic engineering of microorganisms for food and cosmetics production[J]. Nature Reviews Bioengineering, 2023, 1: 832-857. |
| 12 | YANG G H, HONG S, YANG P J, et al. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria[J]. Nature Communications, 2021, 12(1): 790. |
| 13 | SHEN N, WANG T F, GAN Q, et al. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity[J]. Food Chemistry, 2022, 383: 132531. |
| 14 | DONG N Q, LIN H X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions[J]. Journal of Integrative Plant Biology, 2021, 63(1): 180-209. |
| 15 | PANDEY R P, PARAJULI P, KOFFAS M A G, et al. Microbial production of natural and non-natural flavonoids: pathway engineering, directed evolution and systems/synthetic biology[J]. Biotechnology Advances, 2016, 34(5): 634-662. |
| 16 | JIANG C M, LIU X N, CHEN X Q, et al. Raising the production of phloretin by alleviation of by-product of chalcone synthase in the engineered yeast[J]. Science China Life Sciences, 2020, 63(11): 1734-1743. |
| 17 | HWANG H G, MILITO A, YANG J S, et al. Riboswitch-guided chalcone synthase engineering and metabolic flux optimization for enhanced production of flavonoids[J]. Metabolic Engineering, 2023, 75: 143-152. |
| 18 | TONG Y J, LI N, ZHOU S H, et al. Improvement of chalcone synthase activity and high-efficiency fermentative production of (2S)-naringenin via in vivo biosensor-guided directed evolution[J]. ACS Synthetic Biology, 2024, 13(5): 1454-1466. |
| 19 | LI H B, MA W J, WANG W G, et al. Synergetic engineering of multiple pathways for de novo (2S)-naringenin biosynthesis in Saccharomyces cerevisiae [J]. ACS Sustainable Chemistry & Engineering, 2024, 12(1): 59-71. |
| 20 | WEI W P, ZHANG P, SHANG Y Z, et al. Metabolically engineering of Yarrowia lipolytica for the biosynthesis of naringenin from a mixture of glucose and xylose[J]. Bioresource Technology, 2020, 314: 123726. |
| 21 | LIU M S, WU J J, YUE M Y, et al. YaliCMulti and YaliHMulti: stable, efficient multi-copy integration tools for engineering Yarrowia lipolytica [J]. Metabolic Engineering, 2024, 82: 29-40. |
| 22 | YUE M Y, LIU M S, GAO S, et al. High-level de novo production of (2S)-eriodictyol in Yarrowia lipolytica by metabolic pathway and NADPH regeneration engineering[J]. Journal of Agricultural and Food Chemistry, 2024, 72(8): 4292-4300. |
| 23 | ZAN X Y, XU Y T, CHEN G G, et al. Biosynthesis of naringenin from p-coumaric acid in engineering oleaginous filamentous fungus Mucor circinelloides [J]. ACS Food Science & Technology, 2024, 4(3): 747-756. |
| 24 | PENG B, DAI L, IACOVELLI R, et al. Heterologous naringenin production in the filamentous fungus Penicillium rubens [J]. Journal of Agricultural and Food Chemistry, 2023, 71(51): 20782-20792. |
| 25 | ÁLVAREZ-ÁLVAREZ R, BOTAS A, ALBILLOS S M, et al. Molecular genetics of naringenin biosynthesis, a typical plant secondary metabolite produced by Streptomyces clavuligerus [J]. Microbial Cell Factories, 2015, 14: 178. |
| 26 | ZHOU S H, YUAN S F, NAIR P H, et al. Development of a growth coupled and multi-layered dynamic regulation network balancing malonyl-CoA node to enhance (2S)-naringenin biosynthesis in Escherichia coli [J]. Metabolic Engineering, 2021, 67: 41-52. |
| 27 | MARSAN C B, LEE S G, NGUYEN A, et al. Leveraging a Y. lipolytica naringenin chassis for biosynthesis of apigenin and associated glucoside[J]. Metabolic Engineering, 2024, 83: 1-11. |
| 28 | XU W Q, LIU M S, LI H B, et al. De novo synthesis of chrysin in Saccharomyces cerevisiae [J]. Journal of Agricultural and Food Chemistry, 2024, 72(12): 6481-6490. |
| 29 | LYU X M, ZHAO G L, NG K R, et al. Metabolic engineering of Saccharomyces cerevisiae for de novo production of kaempferol[J]. Journal of Agricultural and Food Chemistry, 2019, 67(19): 5596-5606. |
| 30 | MARÍN L, GUTIÉRREZ-DEL-RÍO I, ENTRIALGO-CADIERNO R, et al. De novo biosynthesis of myricetin, kaempferol and quercetin in Streptomyces albus and Streptomyces coelicolor [J]. PLoS One, 2018, 13(11): e0207278. |
| 31 | RODRIGUEZ A, STRUCKO T, STAHLHUT S G, et al. Metabolic engineering of yeast for fermentative production of flavonoids[J]. Bioresource Technology, 2017, 245: 1645-1654. |
| 32 | TARTIK M, LIU J, MOHEDANO M T, et al. Optimizing yeast for high-level production of kaempferol and quercetin[J]. Microbial Cell Factories, 2023, 22(1): 74. |
| 33 | AN D G, YANG S M, KIM B G, et al. Biosynthesis of two quercetin O-diglycosides in Escherichia coli [J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(6): 841-849. |
| 34 | SHRESTHA B, PANDEY R P, DARSANDHARI S, et al. Combinatorial approach for improved cyanidin 3-O-glucoside production in Escherichia coli [J]. Microbial Cell Factories, 2019, 18(1): 7. |
| 35 | DU Y, YANG B R, YI Z Q, et al. Engineering Saccharomyces cerevisiae coculture platform for the production of flavonoids[J]. Journal of Agricultural and Food Chemistry, 2020, 68(7): 2146-2154. |
| 36 | XU S, LI G J, ZHOU J W, et al. Efficient production of anthocyanins in Saccharomyces cerevisiae by introducing anthocyanin transporter and knocking out endogenous degrading enzymes[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 899182. |
| 37 | ZHANG S B, DUAN E K. Fighting against skin aging: the way from bench to bedside[J]. Cell Transplantation, 2018, 27(5): 729-738. |
| 38 | SARIAN M N, AHMED Q U, SO’AD S Z M, et al. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship based study[J]. BioMed Research International, 2017, 2017(1): 8386065. |
| 39 | NITHIYA T, UDAYAKUMAR R. In vitro antioxidant properties of phloretin: an important phytocompound[J]. Journal of Biosciences and Medicines, 2016, 4(1): 85-94. |
| 40 | YANG Q, HAN L, LI J, et al. Activation of Nrf2 by phloretin attenuates palmitic acid-induced endothelial cell oxidative stress via AMPK-dependent signaling[J]. Journal of Agricultural and Food Chemistry, 2019, 67(1): 120-131. |
| 41 | ZHANG Y, WU Y L, LI B, et al. Phloretin prolongs lifespan of Caenorhabditis elegans via inhibition of NDUFS1 and NDUFS6 at mitochondrial complex Ⅰ[J]. Free Radical Biology and Medicine, 2024, 221: 283-295. |
| 42 | SÜNTAR I, ÇETINKAYA S, PANIERI E, et al. Regulatory role of Nrf2 signaling pathway in wound healing process[J]. Molecules, 2021, 26(9): 2424. |
| 43 | RUAN Q Q, WEN C M, JIN G H, et al. Phloretin-induced STAT3 inhibition suppresses pancreatic cancer growth and progression via enhancing Nrf2 activity[J]. Phytomedicine, 2023, 118: 154990. |
| 44 | VIDEIRA I F, MOURA D F L, MAGINA S. Mechanisms regulating melanogenesis[J]. Anais Brasileiros de Dermatologia, 2013, 88(1): 76-83. |
| 45 | CHEN J M, LI Q L, YE Y L, et al. Phloretin as both a substrate and inhibitor of tyrosinase: inhibitory activity and mechanism[J]. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy, 2020, 226: 117642. |
| 46 | MINSAT L, PEYROT C, BRUNISSEN F, et al. Synthesis of biobased phloretin analogues: an access to antioxidant and anti-tyrosinase compounds for cosmetic applications[J]. Antioxidants, 2021, 10(4): 512. |
| 47 | LI Y X, XIANG H, XUE X Y, et al. Dual antimelanogenic effect of nicotinamide-stabilized phloretin nanocrystals in larval zebrafish[J]. Pharmaceutics, 2022, 14(9): 1825. |
| 48 | ANUNCIATO CASARINI T P, FRANK L A, POHLMANN A R, et al. Dermatological applications of the flavonoid phloretin[J]. European Journal of Pharmacology, 2020, 889: 173593. |
| 49 | ELKANZI N A A, HRICHI H, ALOLAYAN R A, et al. Synthesis of chalcones derivatives and their biological activities: a review[J]. ACS Omega, 2022, 7(32): 27769-27786. |
| 50 | WANG Y K, HU S Y, XIAO F Y, et al. Dihydrochalcones in sweet tea: biosynthesis, distribution and neuroprotection function[J]. Molecules, 2022, 27(24): 8794. |
| 51 | GOSCH C, HALBWIRTH H, STICH K. Phloridzin: biosynthesis, distribution and physiological relevance in plants[J]. Phytochemistry, 2010, 71(8-9): 838-843. |
| 52 | NGUYEN N A, CAO N T, NGUYEN T H H, et al. Regioselective hydroxylation of phloretin, a bioactive compound from apples, by human cytochrome P450 enzymes[J]. Pharmaceuticals, 2020, 13(11): 330. |
| 53 | JI J H, NGUYEN N A, NGUYEN T H H, et al. One-pot production of 3-hydroxyphloretin from phlorizin by Bacillus subtilis whole-cell system expressing CYP102A1[J]. ACS Food Science & Technology, 2024, 4(6): 1436-1443. |
| 54 | CHAMOLI S, KUMAR P, NAVANI N K, et al. Secretory expression, characterization and docking study of glucose-tolerant β-glucosidase from B. subtilis [J]. International Journal of Biological Macromolecules, 2016, 85: 425-433. |
| 55 | GALL D M, THOMSEN D M, PETERS D C, et al. Enzymatic conversion of flavonoids using bacterial chalcone isomerase and enoate reductase[J]. Angewandte Chemie International Edition, 2014, 53(5): 1439-1442. |
| 56 | BRAUNE A, GÜTSCHOW M, BLAUT M. An NADH-dependent reductase from Eubacterium ramulus catalyzes the stereospecific heteroring cleavage of flavanones and flavanonols[J]. Applied and Environmental Microbiology, 2019, 85(19): e01233-19. |
| 57 | LIU X, LIU J C, LEI D W, et al. Modular metabolic engineering for production of phloretic acid, phloretin and phlorizin in Escherichia coli [J]. Chemical Engineering Science, 2022, 247: 116931. |
| 58 | AGRAWAL R, HU A, BOLLAG W B. The skin and inflamm-aging[J]. Biology, 2023, 12(11): 1396. |
| 59 | YUAN D, WANG Z L, LI B, et al. Complexation of apigenin and oxymatrine leading to enhanced anti-inflammatory activity[J]. Journal of Natural Products, 2023, 86(5): 1179-1188. |
| 60 | CAI J, WEN H L, ZHOU H, et al. Naringenin: a flavanone with anti-inflammatory and anti-infective properties[J]. Biomedicine & Pharmacotherapy, 2023, 164: 114990. |
| 61 | BARRECA D, BELLOCCO E, LAGANÀ G, et al. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat[J]. Food Chemistry, 2014, 160: 292-297. |
| 62 | LI H, LI C Z, SHI C, et al. Transcriptome analysis reveals the inhibitory mechanism of phloretin on virulence expression of Staphylococcus aureus and its application in cooked chicken[J]. International Journal of Food Microbiology, 2024, 415: 110647. |
| 63 | CHEN Z Y, LI M, GUO Y J, et al. Discovery of natural agents against Staphylococcus aureus based on EIIC by protein modeling, virtual screening and molecular dynamics[J]. LWT, 2024, 198: 115914. |
| 64 | ESCRIBANO-FERRER E, QUERALT REGUÉ J, GARCIA-SALA X, et al. In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice[J]. Journal of Natural Products, 2019, 82(2): 177-182. |
| 65 | ZHANG W, ZHANG Y, ZHANG J G, et al. Naringenin ameliorates collagen-induced arthritis through activating AMPK-mediated autophagy in macrophages[J]. Immunity, Inflammation and Disease, 2023, 11(10): e983. |
| 66 | KUMAR S, TIKU A B. Biochemical and molecular mechanisms of radioprotective effects of naringenin, a phytochemical from citrus fruits[J]. Journal of Agricultural and Food Chemistry, 2016, 64(8): 1676-1685. |
| 67 | MARTINEZ R M, PINHO-RIBEIRO F A, STEFFEN V S, et al. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice[J]. Journal of Natural Products, 2015, 78(7): 1647-1655. |
| 68 | KURE A, NAKAGAWA K, KONDO M, et al. Metabolic fate of luteolin in rats: its relationship to anti-inflammatory effect[J]. Journal of Agricultural and Food Chemistry, 2016, 64(21): 4246-4254. |
| 69 | YANO S, UMEDA D, MAEDA N, et al. Dietary apigenin suppresses IgE and inflammatory cytokines production in C57BL/6N mice[J]. Journal of Agricultural and Food Chemistry, 2006, 54(14): 5203-5207. |
| 70 | MUHAMMAD A, FENG X D, RASOOL A, et al. Production of plant natural products through engineered Yarrowia lipolytica [J]. Biotechnology Advances, 2020, 43: 107555. |
| 71 | ZHANG H J, LI Z X, ZHOU S, et al. A fungal NRPS-PKS enzyme catalyses the formation of the flavonoid naringenin[J]. Nature Communications, 2022, 13(1): 6361. |
| 72 | LI H, LI D, YANG Z, et al. Flavones produced by mulberry flavone synthase type Ⅰ constitute a defense line against the ultraviolet-B stress[J]. Plants, 2020, 9(2): 215. |
| 73 | LIU W X, FENG Y, YU S H, et al. The flavonoid biosynthesis network in plants[J]. International Journal of Molecular Sciences, 2021, 22(23): 12824. |
| 74 | MARÍN L, GUTIÉRREZ-DEL-RÍO I, YAGÜE P, et al. De novo biosynthesis of apigenin, luteolin, and eriodictyol in the actinomycete Streptomyces albus and production improvement by feeding and spore conditioning[J]. Frontiers in Microbiology, 2017, 8: 921. |
| 75 | WU J, WANG X C, LIU Y, et al. Flavone synthases from Lonicera japonica and L. macranthoides reveal differential flavone accumulation[J]. Scientific Reports, 2016, 6: 19245. |
| 76 | YAN J, YU L, HE L Z, et al. Comparative transcriptome analysis of celery leaf blades identified an R2R3-MYB transcription factor that regulates apigenin metabolism[J]. Journal of Agricultural and Food Chemistry, 2019, 67(18): 5265-5277. |
| 77 | PRAVIN B, NANAWARE V, ASHWINI B, et al. Assessing the antioxidant properties of naringin and rutin and investigating their oxidative DNA damage effects in breast cancer[J]. Scientific Reports, 2024, 14(1): 15314. |
| 78 | SAEWAN N, JIMTAISONG A. Natural products as photoprotection[J]. Journal of Cosmetic Dermatology, 2015, 14(1): 47-63. |
| 79 | GAN Y W, YU B W, LIU R J, et al. Systematic analysis of the UDP-glucosyltransferase family: discovery of a member involved in rutin biosynthesis in Solanum melongena [J]. Frontiers in Plant Science, 2023, 14: 1310080. |
| 80 | TOMAZELLI L C, DE ASSIS RAMOS M M, SAUCE R, et al. SPF enhancement provided by rutin in a multifunctional sunscreen[J]. International Journal of Pharmaceutics, 2018, 552(1-2): 401-406. |
| 81 | TABOLACCI E, TRINGALI G, NOBILE V, et al. Rutin protects fibroblasts from UVA radiation through stimulation of Nrf2 pathway[J]. Antioxidants, 2023, 12(4): 820. |
| 82 | CHEN L, LI S W, LI W J, et al. Rutin prevents EqHV-8 induced infection and oxidative stress via Nrf2/HO-1 signaling pathway[J]. Frontiers in Cellular and Infection Microbiology, 2024, 14: 1386462. |
| 83 | GĘGOTEK A, JASTRZĄB A, DOBRZYŃSKA M, et al. Exogenous antioxidants impact on UV-induced changes in membrane phospholipids and the effectiveness of the endocannabinoid system in human skin cells[J]. Antioxidants, 2021, 10(8): 1260. |
| 84 | CHOI S J, LEE S N, KIM K R, et al. Biological effects of rutin on skin aging[J]. International Journal of Molecular Medicine, 2016, 38(1): 357-363. |
| 85 | MICEK I, NAWROT J, SERASZEK-JAROS A, et al. Taxifolin as a promising ingredient of cosmetics for adult skin[J]. Antioxidants, 2021, 10(10): 1625. |
| 86 | SINGH P, ARIF Y, BAJGUZ A, et al. The role of quercetin in plants[J]. Plant Physiology and Biochemistry, 2021, 166: 10-19. |
| 87 | CHAPMAN J M, MUDAY G K. Flavonols modulate lateral root emergence by scavenging reactive oxygen species in Arabidopsis thaliana [J]. The Journal of Biological Chemistry, 2021, 296: 100222. |
| 88 | REN J, TANG W Z, BARTON C D, et al. A highly versatile fungal glucosyltransferase for specific production of quercetin-7-O-β-D-glucoside and quercetin-3-O-β-D-glucoside in different hosts[J]. Applied Microbiology and Biotechnology, 2022, 106(1): 227-245. |
| 89 | XU H T, JIANG Z Q, LIN Z M, et al. FtUGT79A15 is responsible for rutinosylation in flavonoid diglycoside biosynthesis in Fagopyrum tataricum [J]. Plant Physiology and Biochemistry, 2022, 181: 33-41. |
| 90 | WANG L, ZHAO J L, MAO Y B, et al. Tartary buckwheat rutin: accumulation, metabolic pathways, regulation mechanisms, and biofortification strategies[J]. Plant Physiology and Biochemistry, 2024, 208: 108503. |
| 91 | NODA N, YOSHIOKA S, KISHIMOTO S, et al. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism[J]. Science Advances, 2017, 3(7): e1602785. |
| 92 | CASTAÑEDA-OVANDO A, DE LOURDES PACHECO-HERNÁNDEZ M, PÁEZ-HERNÁNDEZ M E, et al. Chemical studies of anthocyanins: a review[J]. Food Chemistry, 2009, 113(4): 859-871. |
| 93 | ROSE P M, CANTRILL V, BENOHOUD M, et al. Application of anthocyanins from blackcurrant (Ribes nigrum L.) fruit waste as renewable hair dyes[J]. Journal of Agricultural and Food Chemistry, 2018, 66(26): 6790-6798. |
| 94 | CÂMARA J S, LOCATELLI M, PEREIRA J A M, et al. Behind the scenes of anthocyanins-from the health benefits to potential applications in food, pharmaceutical and cosmetic fields[J]. Nutrients, 2022, 14(23): 5133. |
| 95 | XIE L H, LU Y, ZHOU Y Y, et al. Functional analysis of a methyltransferase involved in anthocyanin biosynthesis from blueberries (Vaccinium corymbosum)[J]. Journal of Agricultural and Food Chemistry, 2022, 70(51): 16253-16262. |
| 96 | AI Y, ZHU Z Q. Melatonin antagonizes jasmonate-triggered anthocyanin biosynthesis in Arabidopsis thaliana [J]. Journal of Agricultural and Food Chemistry, 2018, 66(21): 5392-5400. |
| 97 | MEI Y, XIE H, LIU S R, et al. Metabolites and transcriptional profiling analysis reveal the molecular mechanisms of the anthocyanin metabolism in the “Zijuan” tea plant (Camellia sinensis var. assamica)[J]. Journal of Agricultural and Food Chemistry, 2021, 69(1): 414-427. |
| 98 | WANG F, JI G S, XU Z B, et al. Metabolomics and transcriptomics provide insights into anthocyanin biosynthesis in the developing grains of purple wheat (Triticum aestivum L.)[J]. Journal of Agricultural and Food Chemistry, 2021, 69(38): 11171-11184. |
| 99 | EICHENBERGER M, HANSSON A, FISCHER D, et al. De novo biosynthesis of anthocyanins in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2018, 18(4): foy046. |
| 100 | SUNIL L, SHETTY N P. Biosynthesis and regulation of anthocyanin pathway genes[J]. Applied Microbiology and Biotechnology, 2022, 106(5-6): 1783-1798. |
| 101 | LEVISSON M, PATINIOS C, HEIN S, et al. Engineering de novo anthocyanin production in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2018, 17(1): 103. |
| 102 | ZHA J, ZANG Y, MATTOZZI M, et al. Metabolic engineering of Corynebacterium glutamicum for anthocyanin production[J]. Microbial Cell Factories, 2018, 17(1): 143. |
| 103 | DESHPANDE R D, SHAH D S, GURRAM S, et al. Formulation, characterization, pharmacokinetics and antioxidant activity of phloretin oral granules[J]. International Journal of Pharmaceutics, 2023, 645: 123386. |
| 104 | ARAFAH A, REHMAN M U, MIR T M, et al. Multi-therapeutic potential of naringenin (4',5,7-trihydroxyflavonone): experimental evidence and mechanisms[J]. Plants, 2020, 9(12): 1784. |
| 105 | MARIADOSS A V A, VINAYAGAM R, SENTHILKUMAR V, et al. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells[J]. International Journal of Biological Macromolecules, 2019, 130: 997-1008. |
| 106 | GU L Y, SUN R, WANG W J, et al. Nanostructured lipid carriers for the encapsulation of phloretin: preparation and in vitro characterization studies[J]. Chemistry and Physics of Lipids, 2022, 242: 105150. |
| 107 | CASARINI T P A, FRANK L A, BENIN T, et al. Innovative hydrogel containing polymeric nanocapsules loaded with phloretin: enhanced skin penetration and adhesion[J]. Materials Science and Engineering: C, 2021, 120: 111681. |
| 108 | QUNI S Z, ZHANG Y D, LIU L J, et al. NF-κB-signaling-targeted immunomodulatory nanoparticle with photothermal and quorum-sensing inhibition effects for efficient healing of biofilm-infected wounds[J]. ACS Applied Materials & Interfaces, 2024, 16(20): 25757-25772. |
| 109 | ALAM M S, SULTANA N, RASHID M A, et al. Quality by design-optimized glycerosome-enabled nanosunscreen gel of rutin hydrate[J]. Gels, 2023, 9(9): 752. |
| 110 | HASSAN A S, SOLIMAN G M. Rutin nanocrystals with enhanced anti-inflammatory activity: preparation and ex vivo/in vivo evaluation in an inflammatory rat model[J]. Pharmaceutics, 2022, 14(12): 2727. |
| 111 | BAŞARAN E, ÖZTÜRK A A, ŞENEL B, et al. Quercetin, rutin and quercetin-rutin incorporated hydroxypropyl β-cyclodextrin inclusion complexes[J]. European Journal of Pharmaceutical Sciences, 2022, 172: 106153. |
| 112 | CRISTIANO M C, BARONE A, MANCUSO A, et al. Rutin-loaded nanovesicles for improved stability and enhanced topical efficacy of natural compound[J]. Journal of Functional Biomaterials, 2021, 12(4): 74. |
| 113 | WEI Y Q, ZHANG J, MEMON A H, et al. Molecular model and in vitro antioxidant activity of a water-soluble and stable phloretin/hydroxypropyl-β-cyclodextrin inclusion complex[J]. Journal of Molecular Liquids, 2017, 236: 68-75. |
| 114 | AREE T. How cyclodextrin encapsulation improves molecular stability of apple polyphenols phloretin, phlorizin, and ferulic acid: atomistic insights through structural chemistry[J]. Food Chemistry, 2023, 409: 135326. |
| 115 | KHAN N, SLATHIA G, SANEJA A. Unveiling the complexation mechanism of phloretin with sulfobutylether-β- cyclodextrin (Captisol®) and its impact on anticancer activity[J]. Journal of Molecular Liquids, 2023, 391: 123348. |
| 116 | RAINA N, HAQUE S, TULI H S, et al. Optimization and characterization of a novel antioxidant naringenin-loaded hydrogel for encouraging re-epithelization in chronic diabetic wounds: a preclinical study[J]. ACS Omega, 2023, 8(38): 34995-35011. |
| 117 | BIAN D H, PILEHVAR Y, KOUSHA S, et al. Bioactive wound healing 3D structure based on chitosan hydrogel loaded with naringin/cyclodextrin inclusion nano complex[J]. ACS Omega, 2024, 9(9): 10566-10576. |
| 118 | LV Y K, MARSAFARI M, KOFFAS M, et al. Optimizing oleaginous yeast cell factories for flavonoids and hydroxylated flavonoids biosynthesis[J]. ACS Synthetic Biology, 2019, 8(11): 2514-2523. |
| 119 | WU J Y, WANG T Y, DING H Y, et al. Enzymatic synthesis of novel vitexin glucosides[J]. Molecules, 2021, 26(20): 6274. |
| 120 | QIN Z J, WANG X L, GAO S, et al. Production of natural pigments using microorganisms[J]. Journal of Agricultural and Food Chemistry, 2023, 71(24): 9243-9254. |
| 121 | CHEN J, FANG W J, LIU W, et al. Microcapsules and nanoliposomes based strategies to improve the stability of blueberry anthocyanins[J]. Molecules, 2023, 28(21): 7344. |
| 122 | ZHANG L, YAO L, ZHAO F, et al. Protein and peptide-based nanotechnology for enhancing stability, bioactivity, and delivery of anthocyanins[J]. Advanced Healthcare Materials, 2023, 12(25): 2300473. |
| 123 | SUN J C, SUN W T, ZHANG G L, et al. High efficient production of plant flavonoids by microbial cell factories: challenges and opportunities[J]. Metabolic Engineering, 2022, 70: 143-154. |
| 124 | ABRAMSON J, ADLER J, DUNGER J, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3[J]. Nature, 2024, 630(8016): 493-500. |
| 125 | MA S N, HU R C, MA J Y, et al. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of anthocyanins and proanthocyanidins biosynthesis in Trifolium repens [J]. Industrial Crops and Products, 2022, 187: 115529. |
| 126 | ZHANG L, ZHANG Z J, FANG S Z, et al. Integrative analysis of metabolome and transcriptome reveals molecular regulatory mechanism of flavonoid biosynthesis in Cyclocarya paliurus under salt stress[J]. Industrial Crops and Products, 2021, 170: 113823. |
| 127 | LIANG L F, SUN F, WANG H B, et al. Metabolomics, metabolic flux analysis and cancer pharmacology[J]. Pharmacology & Therapeutics, 2021, 224: 107827. |
| [1] | 吴柯, 罗家豪, 李斐然. 机器学习驱动的基因组规模代谢模型构建与优化[J]. 合成生物学, 2025, 6(3): 566-584. |
| [2] | 田晓军, 张日新. 合成基因回路面临的细胞“经济学窘境”[J]. 合成生物学, 2025, 6(3): 532-546. |
| [3] | 章益蜻, 刘高雯. 合成生物学视角下的基因功能探索与酵母工程菌株文库构建[J]. 合成生物学, 2025, 6(3): 685-700. |
| [4] | 黄怡, 司同, 陆安静. 生物制造标准体系建设的现状、问题与建议[J]. 合成生物学, 2025, 6(3): 701-714. |
| [5] | 宋成治, 林一瀚. AI+定向进化赋能蛋白改造及优化[J]. 合成生物学, 2025, 6(3): 617-635. |
| [6] | 盛周煌, 陈智仙, 张彦. 酵母甘露糖蛋白的研究进展[J]. 合成生物学, 2025, 6(2): 408-421. |
| [7] | 张梦瑶, 蔡鹏, 周雍进. 合成生物学助力萜类香精香料可持续生产[J]. 合成生物学, 2025, 6(2): 334-356. |
| [8] | 张璐鸥, 徐丽, 胡晓旭, 杨滢. 合成生物学助力化妆品走进生物制造新时代[J]. 合成生物学, 2025, 6(2): 479-491. |
| [9] | 鲁锦畅, 武耀康, 吕雪芹, 刘龙, 陈坚, 刘延峰. 神经酰胺类鞘脂的绿色生物制造[J]. 合成生物学, 2025, 6(2): 422-444. |
| [10] | 伊进行, 唐宇琳, 李春雨, 吴鹤云, 马倩, 谢希贤. 氨基酸衍生物在化妆品中的应用及其生物合成研究进展[J]. 合成生物学, 2025, 6(2): 254-289. |
| [11] | 肖森, 胡立涛, 石智诚, 王发银, 余思婷, 堵国成, 陈坚, 康振. 可控分子量透明质酸的生物合成研究进展[J]. 合成生物学, 2025, 6(2): 445-460. |
| [12] | 王倩, 果士婷, 辛波, 钟成, 王钰. L-精氨酸的微生物合成研究进展[J]. 合成生物学, 2025, 6(2): 290-305. |
| [13] | 左一萌, 张姣姣, 连佳长. 酿酒酵母使能技术在化妆品原料合成中的应用[J]. 合成生物学, 2025, 6(2): 233-253. |
| [14] | 汤传根, 王璟, 张烁, 张昊宁, 康振. 功能肽合成和挖掘策略研究进展[J]. 合成生物学, 2025, 6(2): 461-478. |
| [15] | 郭婷婷, 韩湘凝, 黄熙婷, 张婷婷, 孔健. 乳酸菌的合成生物学工具及在合成益肤因子中的应用[J]. 合成生物学, 2025, 6(2): 320-333. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||