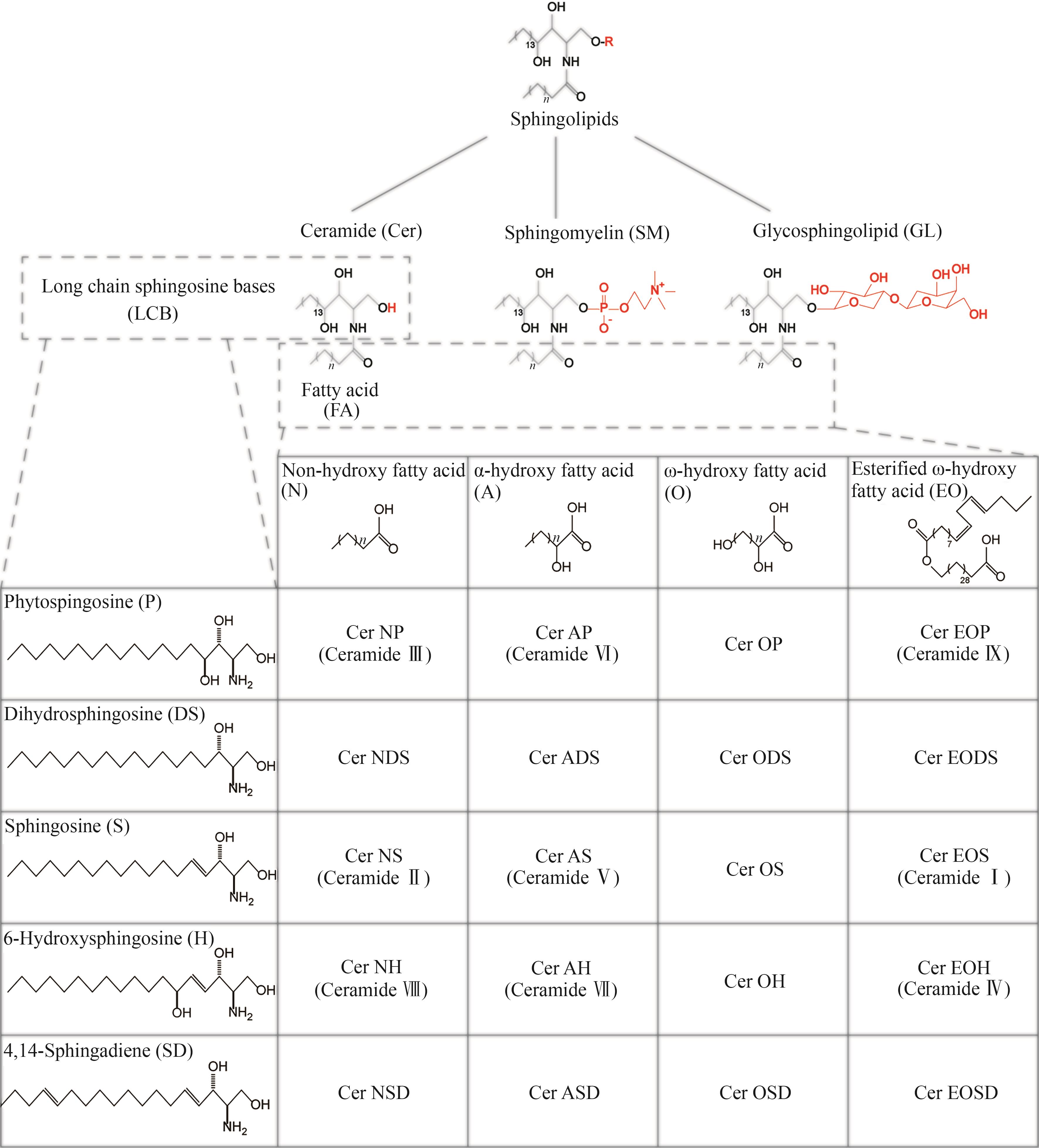
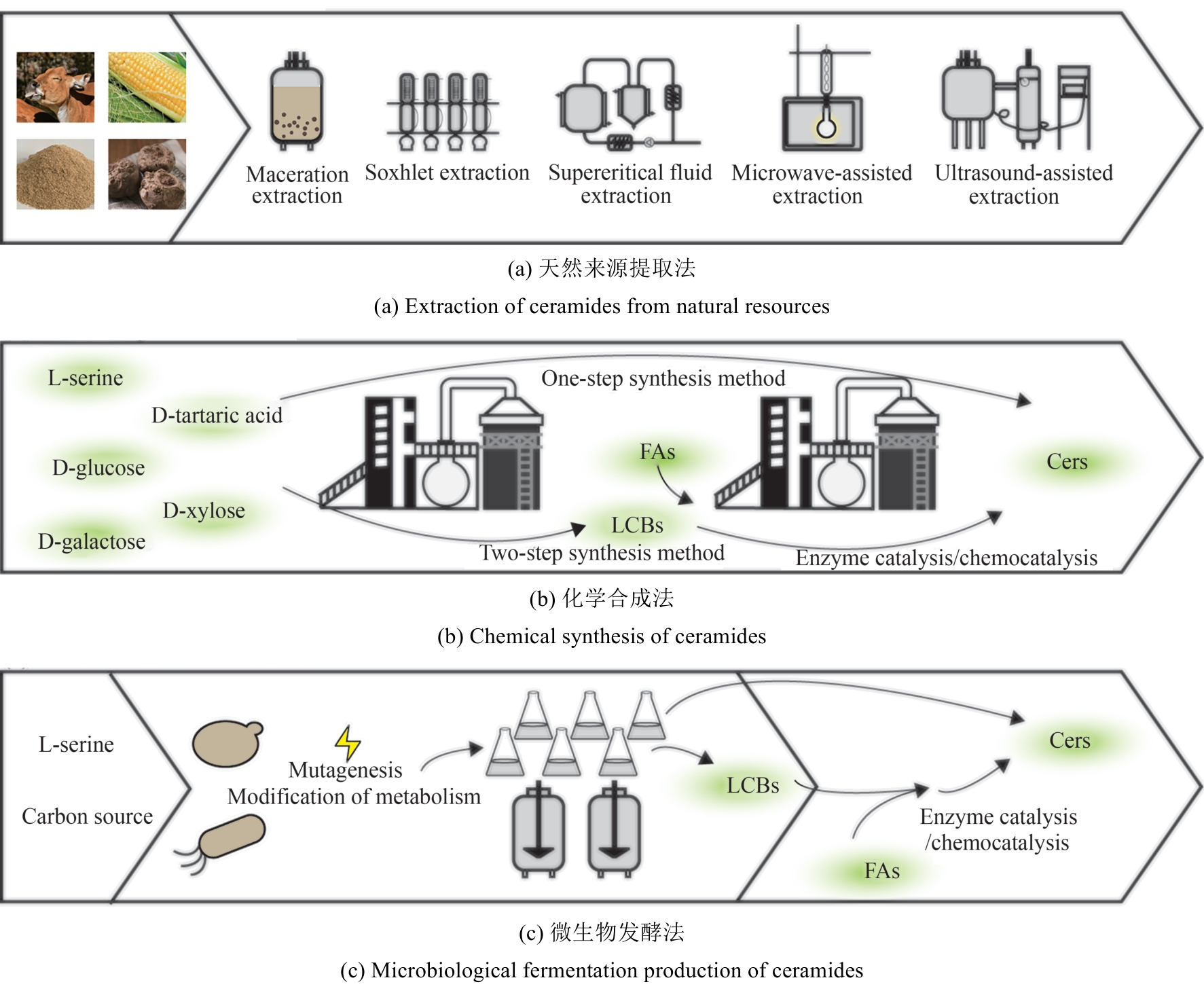
合成生物学 ›› 2025, Vol. 6 ›› Issue (2): 422-444.DOI: 10.12211/2096-8280.2024-059
神经酰胺类鞘脂的绿色生物制造
鲁锦畅1,2, 武耀康1,2, 吕雪芹1,2, 刘龙1,2, 陈坚1,2, 刘延峰1,2
- 1.江南大学生物工程学院,糖化学与生物技术教育部重点实验室,江苏 无锡 214112
2.江南大学未来食品科学中心,江苏 无锡 214112
-
收稿日期:2024-08-01修回日期:2024-10-11出版日期:2025-04-30发布日期:2025-05-20 -
通讯作者:刘延峰 -
作者简介:鲁锦畅 (1999—),女,博士研究生。研究方向为微生物代谢工程。E-mail:7230201010@stu.jiangnan.edu.cn刘延峰 (1987—),男,博士,研究员。研究方向为微生物代谢工程。E-mail:yanfengliu@jiangnan.edu.cn -
基金资助:国家自然科学基金(32222069)
Green biomanufacturing of ceramide sphingolipids
LU Jinchang1,2, WU Yaokang1,2, LV Xueqin1,2, LIU Long1,2, CHEN Jian1,2, LIU Yanfeng1,2
- 1.School of Biotechnology,Key Laboratory of Carbohydrate Chemistry and Biotechnology,Ministry of Education,Jiangnan University,Wuxi 214122,Jiangsu,China
2.Science Center for Future Foods,Jiangnan University,Wuxi 214122,Jiangsu,China
-
Received:2024-08-01Revised:2024-10-11Online:2025-04-30Published:2025-05-20 -
Contact:LIU Yanfeng
摘要:
神经酰胺是一种存在于所有真核生物中的多功能生物活性物质,在细胞信号转导、细胞增殖、分化、凋亡和免疫调节中发挥着重要作用。神经酰胺天然存在于皮肤角质层中,起着支持肌肤屏障、保持水分、抗氧化衰老、抗菌抗炎等作用。因此,神经酰胺及其衍生物在化妆品、生物医药、功能食品等领域具有广阔的市场前景。神经酰胺构型存在多个立体中心,化学从头合成难度大,已知市售的天然或类神经酰胺化合物主要是通过传统天然提取法及生物化学相结合的半合成法获得。近年来,利用微生物合成神经酰胺等鞘脂类化合物已有报道,但从头合成效率还处于较低水平,如何实现细胞工厂高效生产神经酰胺具有重大意义。本文从神经酰胺的生理功能和应用出发,系统地综述了神经酰胺类物质的生理效应及功能;阐述了神经酰胺的天然提取方法、神经酰胺及其前体化合物的化学合成方法;并从鞘脂合成途径及关键酶出发介绍,引出途径调控与优化、产物的运输储存与分泌、关键酶的挖掘与表达等改造策略;最后,从神经酰胺合成面临的聚集毒性、高效运输分泌、数字化改造催化元件、基因调控靶点的拓展等方面进行了展望。合成生物学和生物技术的持续进步有助于扩大微生物细胞工厂的生产能力,实现神经酰胺等鞘脂类化合物的可持续绿色生物制造。
中图分类号:
引用本文
鲁锦畅, 武耀康, 吕雪芹, 刘龙, 陈坚, 刘延峰. 神经酰胺类鞘脂的绿色生物制造[J]. 合成生物学, 2025, 6(2): 422-444.
LU Jinchang, WU Yaokang, LV Xueqin, LIU Long, CHEN Jian, LIU Yanfeng. Green biomanufacturing of ceramide sphingolipids[J]. Synthetic Biology Journal, 2025, 6(2): 422-444.
| 出发底物 | 产物 | 总收率 | 参考文献 | |
|---|---|---|---|---|
| 鞘氨醇碱从头合成 | 2-叠氮-4-硝基苯基磺酸衍生物 | 鞘氨醇;植物鞘氨醇 | 58% | [ |
| 立体选择性的环氧化物 | 鞘氨醇 | 51% | [ | |
| D-葡萄糖衍生物 | D-赤式鞘氨醇; D-苏式鞘氨醇 | 52% | [ | |
| L-丝氨酸 | 鞘氨醇;鞘磷脂;1-磷酸鞘氨醇;鞘氨醇衍生物 | 37% | [ | |
| N-Boc-L-丝氨酸 | 鞘氨醇 | 71% | [ | |
| 神经酰胺从头合成 | 三羟甲基氨基甲烷;脂肪酸羟基取代物 | 神经酰胺类似物 | 33%~65% | [ |
| (2S)-2-氨基苯乙醇(苯甘氨醇);(1R,2R)2-氨基-1-苯基-1,3-丙二醇;(S)-2-氨基(-4-甲氧基)苯乙醇 | 神经酰胺类似物 | 63.5% | [ | |
| 羟化脂肪酸;环氧甘油基醚 | 神经酰胺类似物 | 60%~75% | [ | |
| N-十六烷基-2-氨基乙醇;环己烷;丙二酸二甲酯 | 神经酰胺类似物 | 69% | [ | |
| C16-烷基烯二聚体;二乙醇胺/N-甲基-2,3,4,5,6-五羟基己胺/D-氨基葡萄糖/3-氨基-1,2-丙二醇/N-(1,3-二羟基异丙基)胺/N-(2,3,4,5,6-五羟基己基)胺等 | 神经酰胺类似物 | 20%~90% | [ | |
| 脂肪酸与鞘碱化学法合成神经酰胺 | 神经鞘氨醇;不同碳链长度有机酸 | 神经酰胺类似物 | 51%~96% | [ |
| 共轭羧酸与N‑羟基琥珀酰亚胺;鞘氨醇 | 含共轭羧酸的神经酰胺 | 50%~70% | [ | |
| 羧酸;植物鞘氨醇 | 神经酰胺Ⅲ | 84% | [ | |
| 脂肪酸与鞘碱生物酶法法合成神经酰胺 | 二氢鞘氨醇;脂肪酸;Novozym 435 | 神经酰胺NG | 70%~98% | [ |
| 活化的羧酸衍生物;植物鞘氨醇/二氢鞘氨醇;Novozym 435 | 神经酰胺 | 98%~99.7% | [ | |
| 植物鞘氨酸;脂肪酸;Novozym 435 | 神经酰胺Ⅲ | 94% | [ |
表1 神经酰胺及前体鞘氨醇碱的化学合成法
Table 1 Chemical synthesis of ceramides and their precursor sphingosine base
| 出发底物 | 产物 | 总收率 | 参考文献 | |
|---|---|---|---|---|
| 鞘氨醇碱从头合成 | 2-叠氮-4-硝基苯基磺酸衍生物 | 鞘氨醇;植物鞘氨醇 | 58% | [ |
| 立体选择性的环氧化物 | 鞘氨醇 | 51% | [ | |
| D-葡萄糖衍生物 | D-赤式鞘氨醇; D-苏式鞘氨醇 | 52% | [ | |
| L-丝氨酸 | 鞘氨醇;鞘磷脂;1-磷酸鞘氨醇;鞘氨醇衍生物 | 37% | [ | |
| N-Boc-L-丝氨酸 | 鞘氨醇 | 71% | [ | |
| 神经酰胺从头合成 | 三羟甲基氨基甲烷;脂肪酸羟基取代物 | 神经酰胺类似物 | 33%~65% | [ |
| (2S)-2-氨基苯乙醇(苯甘氨醇);(1R,2R)2-氨基-1-苯基-1,3-丙二醇;(S)-2-氨基(-4-甲氧基)苯乙醇 | 神经酰胺类似物 | 63.5% | [ | |
| 羟化脂肪酸;环氧甘油基醚 | 神经酰胺类似物 | 60%~75% | [ | |
| N-十六烷基-2-氨基乙醇;环己烷;丙二酸二甲酯 | 神经酰胺类似物 | 69% | [ | |
| C16-烷基烯二聚体;二乙醇胺/N-甲基-2,3,4,5,6-五羟基己胺/D-氨基葡萄糖/3-氨基-1,2-丙二醇/N-(1,3-二羟基异丙基)胺/N-(2,3,4,5,6-五羟基己基)胺等 | 神经酰胺类似物 | 20%~90% | [ | |
| 脂肪酸与鞘碱化学法合成神经酰胺 | 神经鞘氨醇;不同碳链长度有机酸 | 神经酰胺类似物 | 51%~96% | [ |
| 共轭羧酸与N‑羟基琥珀酰亚胺;鞘氨醇 | 含共轭羧酸的神经酰胺 | 50%~70% | [ | |
| 羧酸;植物鞘氨醇 | 神经酰胺Ⅲ | 84% | [ | |
| 脂肪酸与鞘碱生物酶法法合成神经酰胺 | 二氢鞘氨醇;脂肪酸;Novozym 435 | 神经酰胺NG | 70%~98% | [ |
| 活化的羧酸衍生物;植物鞘氨醇/二氢鞘氨醇;Novozym 435 | 神经酰胺 | 98%~99.7% | [ | |
| 植物鞘氨酸;脂肪酸;Novozym 435 | 神经酰胺Ⅲ | 94% | [ |
| 宿主 | 产物 | 底物碳源 | 策略 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 威克汉姆西弗酵母 | 三乙酰鞘氨醇 | 葡萄糖 | 异源表达棉桃阿舒来源laf1和des1;突变SYR、DES | 33.45 mg/g DCW (500 mL挡板摇瓶) | [ |
| 威克汉姆西弗酵母(P.ciferrii lig4D strain CS.PCDPro2) | 四乙酰植物鞘氨醇 | 33 g/L葡萄糖 | 阻断shm1、shm2;缺失cha1;删除lcb4;过表达lcb1、lcb2;敲除orm1、orm2;过表达sur2 | 199 mg/g DCW;2 g/L (摇瓶培养) | [ |
| 威克汉姆西弗酵母(NRRL Y1031) | 四乙酰植物鞘氨醇 | 33 g/L葡萄糖 | — | (291.2±63.7) mg/L (120 mL/500 mL挡板摇瓶) | [ |
| 威克汉姆西弗酵母 NRRL Y-1031(M40) | 四乙酰植物鞘氨醇 | 30 g/L葡萄糖 30g /L糖蜜 | EMS诱变;BODIPY 505/515染色;荧光激活细胞分选(FACS) | 2.895 g/L (5.6 L生物反应器) | [ |
| 酿酒酵母K26 | 二乙酰植物鞘氨醇 | 33 g/L葡萄糖 | 质粒表达异源基因sli1、atf2 | (4.3±0.8) mg/L (120 mL/500 mL挡板摇瓶) | [ |
| 酿酒酵母K26 | 三乙酰植物鞘氨醇 | 33 g/L葡萄糖 | 质粒表达异源基因sli1、atf2 | (1.2±0.1) mg/L (120 mL/500 mL挡板摇瓶) | [ |
| 解脂耶氏酵母PO1g(MatA, leu2-270, ura3-302::URA3, xpr2-332. axp-2) | 四乙酰植物鞘氨醇 | 200 g/L甘油 橄榄油 | 异源表达sli1、atf2;删除lcb4基因;有性杂交;发酵优化 | (650±24) mg/L (5 L生物反应器) | [ |
威克汉姆西弗酵母 F-60-10A NRRL1031 诱变后菌株:Mutant736 | 四乙酰植物鞘氨醇 | 5 g/L丝氨酸; 50 g/L甘油; 补加甘油 | γ射线诱变 | 17.7 g/L (3 L生物反应器) | [ |
| 威克汉姆西弗酵母 | 四乙酰植物鞘氨醇 | 50 g/L甘油; 5 g/L L‑丝氨酸 | ARTP诱变 | 30.47 g/L (5 L生物反应器) | [ |
威克汉姆西弗酵母DSCC 7-25 (KCCM-10131) | 四乙酰植物鞘氨醇 | 25~35 g/L甘油 | 从NRRL Y-1031单倍体分离 | 14 g/L (500 L生物反应器) | [ |
| 威克汉姆西弗酵母CGMCC19562 | 四乙酰植物鞘氨醇 | 6.0 g/L L‑丝氨酸; 42.0 g/L甘油 | 单倍体分离 | 22.14 g/L (生物反应器) | [ |
酿酒酵母 CEN.PK2‑1D | 植物鞘氨醇 | 500 g/L葡萄糖 | 敲除lcb4、shm2、cha1;orm2:: tsc10;elo3::sur2;shm1::lcb1,lcb2; delta 22::hac1 | 2817 mg/L;150.54 mg/g干重 (5 L生物反应器) | [ |
| 酿酒酵母NCYC 3608(MATalpha gal2 ho::HygMX ura3::KanMX) | 植物鞘氨醇 | 20 g/L葡萄糖 | 缺失his3、leu2、ura3、cha1、cha2、lcb4、lcb5、orm2;质粒过表达ARS/CEN/URA/ScTSC10/ScSUR2、ARS/CEN/HIS/ScLCB1/ScLCB2、ARS/CEN/LEU | 2169 mg/L | [ |
| 酿酒酵母KCCM50515 | 神经酰胺 | 20 g/L葡萄糖 | 发酵优化 | 1.46 mg/L | [ |
酿酒酵母 KCCM 50515(Matα ura3-52 lys2-801 ade2-101 trp1-∆63 his3-∆200 leu2-∆1) | 神经酰胺 | 20 g/L葡萄糖 | 过表达tscl0 | 9.8 mg/g cell | [ |
| 酿酒酵母 | 神经酰胺 | 葡萄糖;半乳糖 | 过表达tsc10 | 10.52 mg/g cell | [ |
| 酿酒酵母 SCEL2,1 | 神经酰胺 | 葡萄糖;半乳糖 | 过表达lcb1、lcb2 | 10.08 mg /g cell | [ |
| 酿酒酵母 SCEG1C1 | 神经酰胺 | 葡萄糖;半乳糖 | 过表达lag1、lac1 | 9.88 mg/g cell | [ |
| 酿酒酵母 | 神经酰胺NS | 葡萄糖 | 敲除sur2和scs7;引入人类鞘脂去饱和酶基因des1;失活ydc1;过表达isc1;des1基因产物的内质网定位 | 未定量 | [ |
| 巴斯德毕赤酵母GS115 | 神经酰胺(d18:0) | 10 g/L甘油 | 敲除Ku70同源的基因 PAS_chr3_0329;敲除orm1、orm2 同系物同源基因 PAS_chr4_0427 | 90.22 mg/L | [ |
表2 神经酰胺及前体衍生物的生物合成法
Table 2 Biosynthesis of ceramides and their precursor derivatives
| 宿主 | 产物 | 底物碳源 | 策略 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 威克汉姆西弗酵母 | 三乙酰鞘氨醇 | 葡萄糖 | 异源表达棉桃阿舒来源laf1和des1;突变SYR、DES | 33.45 mg/g DCW (500 mL挡板摇瓶) | [ |
| 威克汉姆西弗酵母(P.ciferrii lig4D strain CS.PCDPro2) | 四乙酰植物鞘氨醇 | 33 g/L葡萄糖 | 阻断shm1、shm2;缺失cha1;删除lcb4;过表达lcb1、lcb2;敲除orm1、orm2;过表达sur2 | 199 mg/g DCW;2 g/L (摇瓶培养) | [ |
| 威克汉姆西弗酵母(NRRL Y1031) | 四乙酰植物鞘氨醇 | 33 g/L葡萄糖 | — | (291.2±63.7) mg/L (120 mL/500 mL挡板摇瓶) | [ |
| 威克汉姆西弗酵母 NRRL Y-1031(M40) | 四乙酰植物鞘氨醇 | 30 g/L葡萄糖 30g /L糖蜜 | EMS诱变;BODIPY 505/515染色;荧光激活细胞分选(FACS) | 2.895 g/L (5.6 L生物反应器) | [ |
| 酿酒酵母K26 | 二乙酰植物鞘氨醇 | 33 g/L葡萄糖 | 质粒表达异源基因sli1、atf2 | (4.3±0.8) mg/L (120 mL/500 mL挡板摇瓶) | [ |
| 酿酒酵母K26 | 三乙酰植物鞘氨醇 | 33 g/L葡萄糖 | 质粒表达异源基因sli1、atf2 | (1.2±0.1) mg/L (120 mL/500 mL挡板摇瓶) | [ |
| 解脂耶氏酵母PO1g(MatA, leu2-270, ura3-302::URA3, xpr2-332. axp-2) | 四乙酰植物鞘氨醇 | 200 g/L甘油 橄榄油 | 异源表达sli1、atf2;删除lcb4基因;有性杂交;发酵优化 | (650±24) mg/L (5 L生物反应器) | [ |
威克汉姆西弗酵母 F-60-10A NRRL1031 诱变后菌株:Mutant736 | 四乙酰植物鞘氨醇 | 5 g/L丝氨酸; 50 g/L甘油; 补加甘油 | γ射线诱变 | 17.7 g/L (3 L生物反应器) | [ |
| 威克汉姆西弗酵母 | 四乙酰植物鞘氨醇 | 50 g/L甘油; 5 g/L L‑丝氨酸 | ARTP诱变 | 30.47 g/L (5 L生物反应器) | [ |
威克汉姆西弗酵母DSCC 7-25 (KCCM-10131) | 四乙酰植物鞘氨醇 | 25~35 g/L甘油 | 从NRRL Y-1031单倍体分离 | 14 g/L (500 L生物反应器) | [ |
| 威克汉姆西弗酵母CGMCC19562 | 四乙酰植物鞘氨醇 | 6.0 g/L L‑丝氨酸; 42.0 g/L甘油 | 单倍体分离 | 22.14 g/L (生物反应器) | [ |
酿酒酵母 CEN.PK2‑1D | 植物鞘氨醇 | 500 g/L葡萄糖 | 敲除lcb4、shm2、cha1;orm2:: tsc10;elo3::sur2;shm1::lcb1,lcb2; delta 22::hac1 | 2817 mg/L;150.54 mg/g干重 (5 L生物反应器) | [ |
| 酿酒酵母NCYC 3608(MATalpha gal2 ho::HygMX ura3::KanMX) | 植物鞘氨醇 | 20 g/L葡萄糖 | 缺失his3、leu2、ura3、cha1、cha2、lcb4、lcb5、orm2;质粒过表达ARS/CEN/URA/ScTSC10/ScSUR2、ARS/CEN/HIS/ScLCB1/ScLCB2、ARS/CEN/LEU | 2169 mg/L | [ |
| 酿酒酵母KCCM50515 | 神经酰胺 | 20 g/L葡萄糖 | 发酵优化 | 1.46 mg/L | [ |
酿酒酵母 KCCM 50515(Matα ura3-52 lys2-801 ade2-101 trp1-∆63 his3-∆200 leu2-∆1) | 神经酰胺 | 20 g/L葡萄糖 | 过表达tscl0 | 9.8 mg/g cell | [ |
| 酿酒酵母 | 神经酰胺 | 葡萄糖;半乳糖 | 过表达tsc10 | 10.52 mg/g cell | [ |
| 酿酒酵母 SCEL2,1 | 神经酰胺 | 葡萄糖;半乳糖 | 过表达lcb1、lcb2 | 10.08 mg /g cell | [ |
| 酿酒酵母 SCEG1C1 | 神经酰胺 | 葡萄糖;半乳糖 | 过表达lag1、lac1 | 9.88 mg/g cell | [ |
| 酿酒酵母 | 神经酰胺NS | 葡萄糖 | 敲除sur2和scs7;引入人类鞘脂去饱和酶基因des1;失活ydc1;过表达isc1;des1基因产物的内质网定位 | 未定量 | [ |
| 巴斯德毕赤酵母GS115 | 神经酰胺(d18:0) | 10 g/L甘油 | 敲除Ku70同源的基因 PAS_chr3_0329;敲除orm1、orm2 同系物同源基因 PAS_chr4_0427 | 90.22 mg/L | [ |
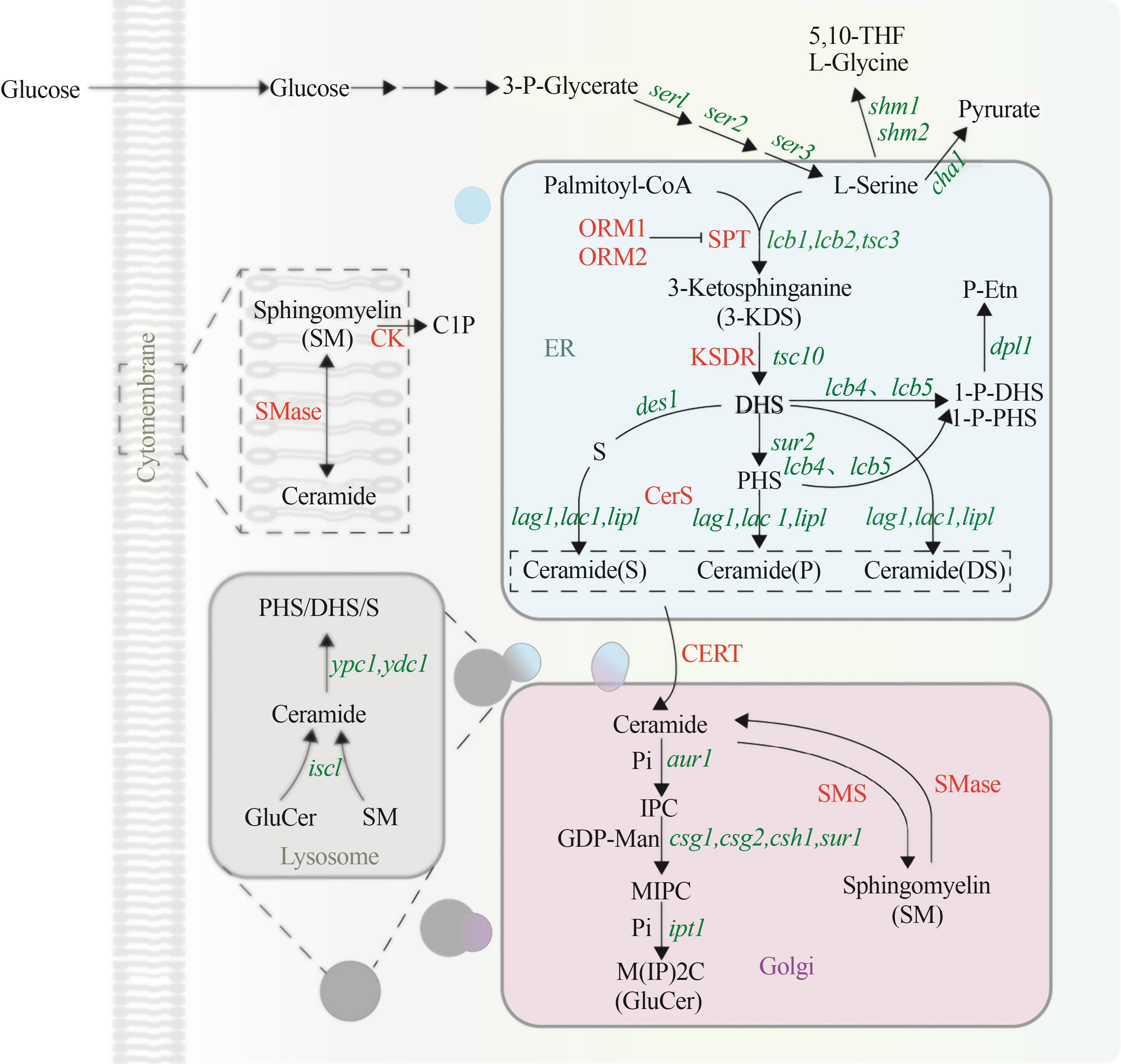
图3 酵母鞘脂代谢通路(绿色代表编码基因,红色代表酶。3-P-Glycerate—3磷酸甘油酸;Palmitoy CoA—棕榈酰辅酶A;5,10-THF—5,10-二甲基四氢叶酸;L-Glycine—L-甘氨酸;3-KDS—3-酮基-二氢鞘氨醇;DHS—二氢鞘氨醇;PHS—植物鞘氨醇;S—鞘氨醇;1-P-DHS—1-磷酸二氢鞘氨醇;1-P-PHS—1-磷酸植物鞘氨醇;P-Etn—磷酸乙醇胺;Pi—磷酸基团;IPC—肌醇磷酸神经酰胺;GDP-Man—鸟苷二磷酸甘露糖;MIPC—甘露糖肌醇磷酸神经酰胺;M(IP)2C—甘露糖-(肌醇-P)2-神经酰胺; GlcCer—葡萄糖糖神经酰胺; SM—鞘磷脂;C1P—神经酰胺1-磷酸盐;ser1—3-磷酸丝氨酸氨基转移酶编码基因;ser2—磷酸甘油酸途径的磷酸丝氨酸磷酸酶编码基因;ser3—3-磷酸甘油酸脱氢酶编码基因;shm1,shm2—L-丝氨酸羟甲基转移酶编码基因;cha1—L-丝氨酸脱氨酶编码基因;lcb1、lcb2、tsc3—丝氨酸棕榈酰转移酶编码基因;tsc10—3-酮基-二氢鞘氨醇还原酶编码基因;des1—鞘脂Δ4-去饱和酶编码基因;lcb4,lcb5—鞘氨醇激酶编码基因;dpl1—鞘碱磷酸裂解酶编码基因;sur2—C4 羟化酶编码基因;lag1,lac1,lip1—神经酰胺合成酶编码基因;aur1—神经酰胺磷酸肌醇转移酶编码基因;csg1,csg2,csh1,sur1—甘露糖基肌醇磷酸神经酰胺合酶催编码基因;ipt1—肌醇磷酸转移酶编码基因;isc1—复杂鞘脂头基水解酶编码基因;ypc1、ydc1—碱性神经酰胺酶编码基因;ORM1,ORM2—介导鞘脂稳态蛋白;SPT—丝氨酸棕榈酰转移酶;KDSR—3-酮基-二氢鞘氨醇还原酶;CerS—神经酰胺合酶;CERT—神经酰胺转运蛋白; SMS—鞘磷脂合成酶;SMase—鞘磷脂酶家族;CK—经酰胺激酶;ER—内质网)
Fig. 3 Metabolic pathway of sphingolipid in yeast(Green represents the coding gene, red represents the enzyme. 5,10-THF—5,10-dimethyltetrahydrofolate; 3-KDS—3-keto-dihydrosphingosine; DHS—dihydrosphingosine; PHS—phytosphingosine; S—S phingosine; 1-P-DHS—dihydrosphingosine-1-phosphate; 1-P-PHS—phytosphingosine-1-phosphate; P-Etn—phosphoryl ethanolamine; Pi—phosphate group; IPC—inositol phosphorylceramide; GDP-Man—GDP mannose; MIPC—mannosyl-inositol phosphorylceramide; M(IP)2C—mannosyl-diinositol phosphorylceramide; GlcCer—glucosylceramide; SM—sphingomyelin; C1P—ceramide-1phosphate; ser1—3-phosphoserine aminotransferase encoding gene; ser2—phosphoserine phosphatase in the phosphoglycerate pathway encoding gene; ser3—3-phosphoglycerate dehydrogenase encoding gene; shm1, shm2—L-serine hydroxymethyltransferase encoding gene; cha1—L-serine deaminase encoding gene; lcb1, lcb2, tsc3—serine palmitoyltransferase encoding gene; tsc10—3-keto-dihydrosphingosine reductase encoding gene; des1—sphingolipid Δ4-desaturase encoding gene; lcb4, lcb5—sphingosine kinase encoding gene; dpl1—sphingobase-1-phosphate lyase encoding gene; sur2—C4 hydroxylase encoding gene; lag1, lac1, lip1—ceramide synthase encoding gene; aur1—ceramide phosphoinositide transferase encoding gene; csg1, csg2, csh1, sur1—mannosylinositol phosphorylceramide synthase encoding gene; ipt1—inositol phosphotransferase encoding gene; isc1—complex sphingolipid headgroup hydrolase encoding gene; ypc1, ydc1—alkaline ceramidase encoding gene; ORM1, ORM2—mediate sphingolipid homeostasis protein; SPT—serine palmitoyl transferase; KDSR—3-ketodihydrosphingosine reductase; CerS—ceramide synthase; CERT—ceramide transfer protein; SMS—sphingomyelin synthase; SMase—sphingomyelinase; CK—ceramide kinase; ER—endoplasmic reticulum)
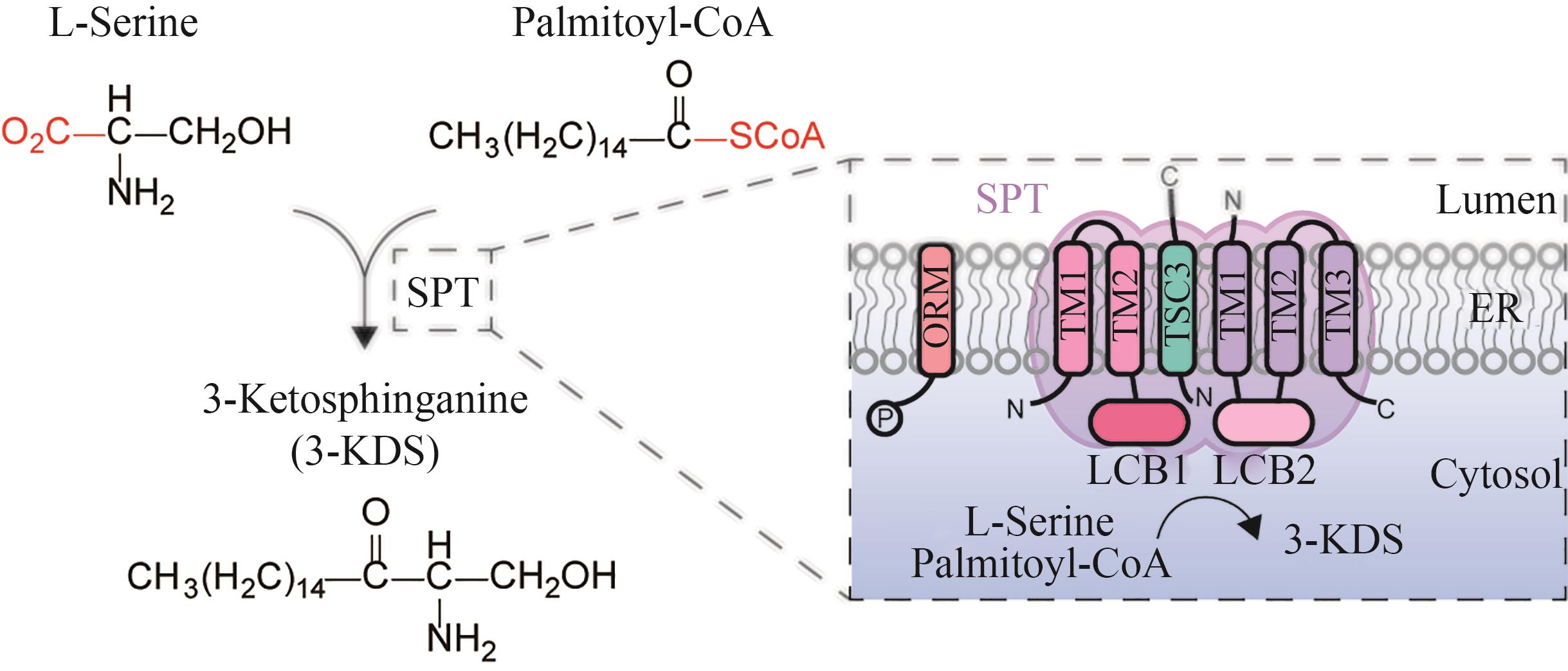
图4 丝氨酸棕榈酰转移酶催化合成3-KDS简图(SPT—丝氨酸棕榈酰转移酶;ORM—调节鞘脂稳态蛋白;3-KDS—3-酮基-二氢鞘氨醇;TM—跨膜区;LCB1,LCB2,TSC3—丝氨酸棕榈酰转移酶亚基;ER—内质网)
Fig. 4 Schematic diagram for the catalytic synthesis of 3-KDS by serine palmitoyl transferase(SPT—serine palmitoyl transferase; ORM—mediate sphingolipid homeostasis protein;3-KDS—3-keto-dihydrosphingosine; TM—Transmembrane; LCB1, LCB2, TSC3—subunits of serine palmitoyltransferase; ER—endoplasmic reticulum)
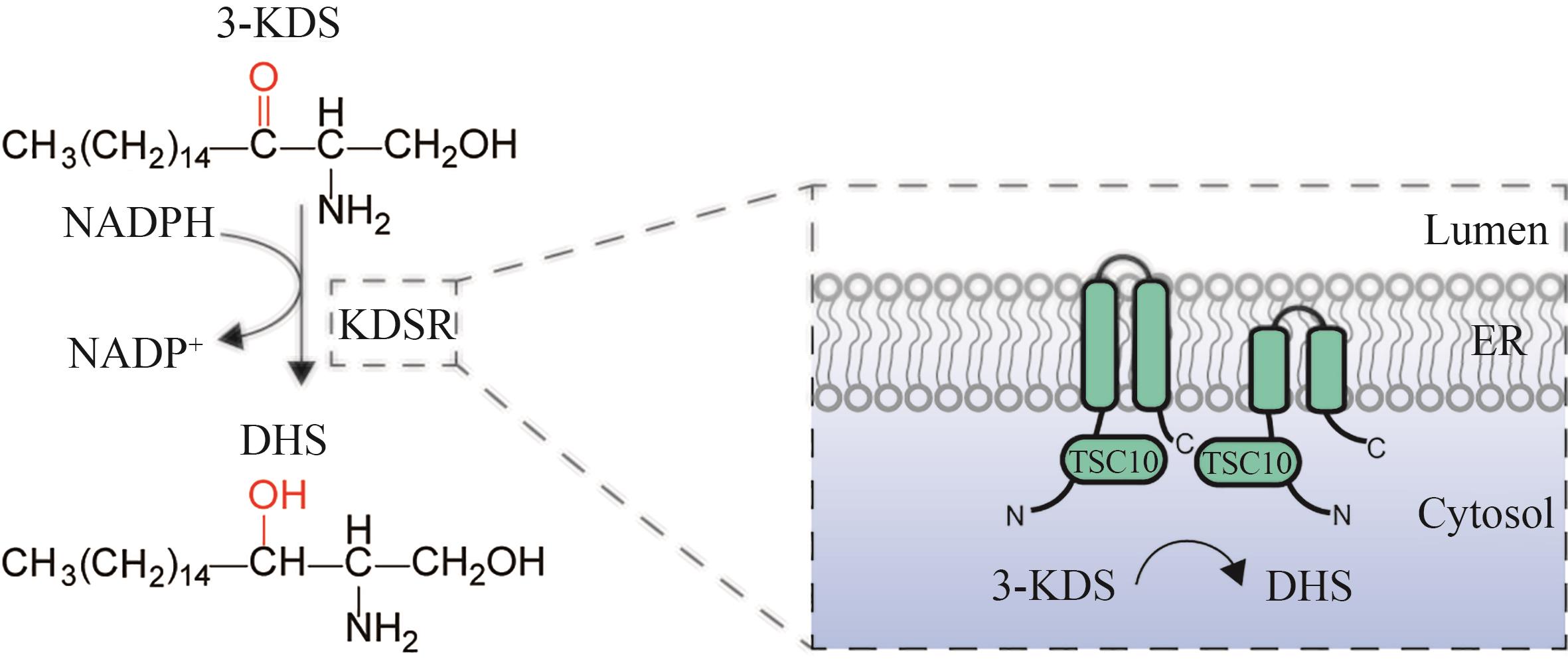
图5 3-酮二氢鞘氨醇还原酶催化合成DHS简图(3-KDS—3-酮基-二氢鞘氨醇;KDSR—3-酮基-二氢鞘氨醇还原酶;DHS—二氢鞘氨醇;ER—内质网)
Fig. 5 Schematic diagram for the catalytic synthesis of DHS by 3-ketodihydrosphingosine reductase(3-KDS—3-keto-dihydrosphingosine; KDSR—3-keto-dihydrosphingosine reductase; TSC10—3-keto-dihydrosphingosine reductase; DHS—dihydrosphingosine; ER—endoplasmic reticulum)
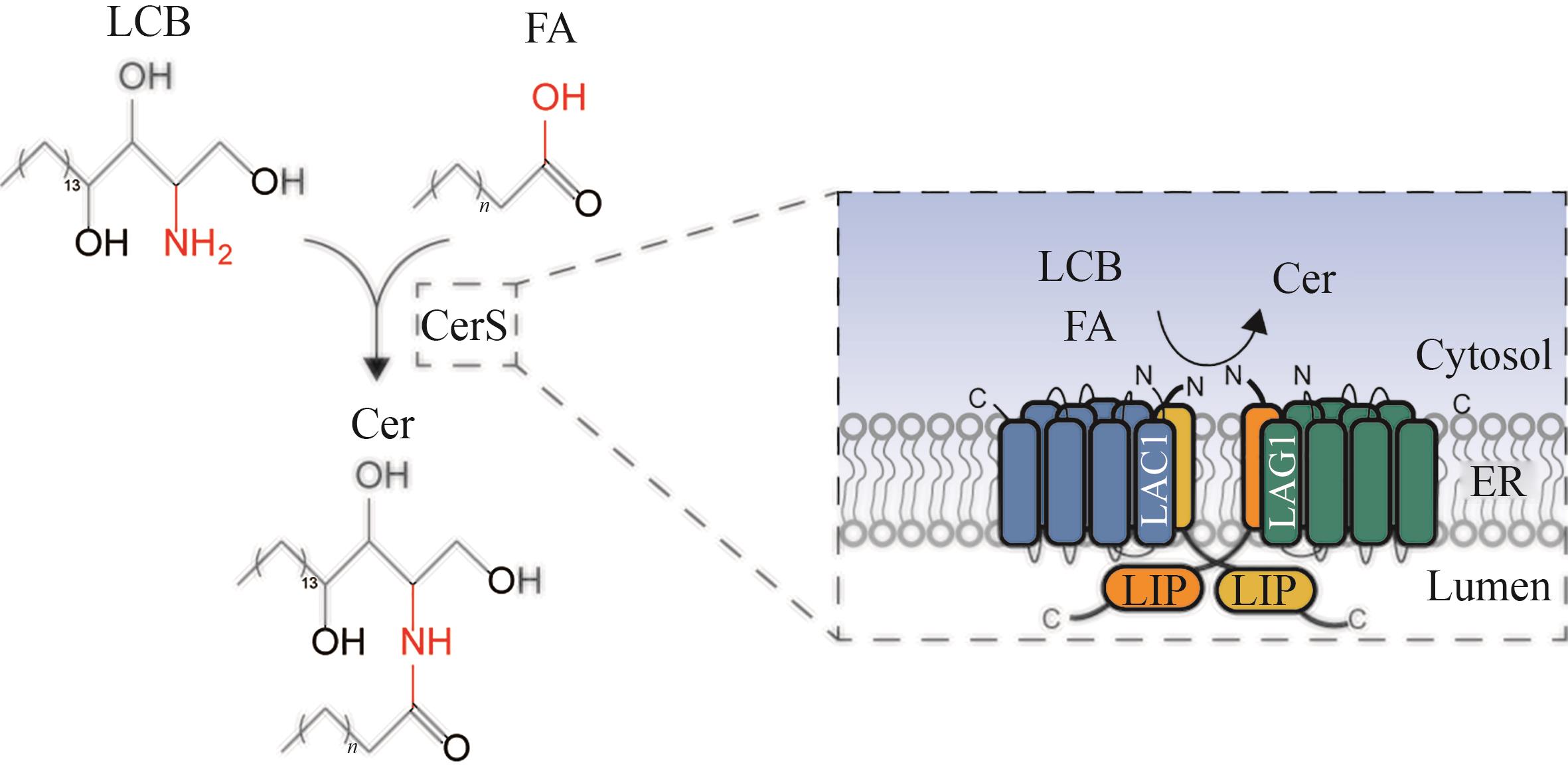
图6 神经酰胺合酶催化合成神经酰胺简图(LCB—长链鞘氨醇碱;FA—脂肪酸;CerS—神经酰胺合酶;Cer—神经酰胺;LAG1,LAC1,LIP1—神经酰胺合成酶亚基;ER—内质网)
Fig. 6 Schematic diagram for the catalytic synthesis of ceramide by ceramide synthase(LCB—long chain sphingosine bases; FA—fatty acid; CerS—ceramide synthase; Cer—ceramide; LAG1, LAC1, LIP1—subunits of ceramide synthase; ER—endoplasmic reticulum)
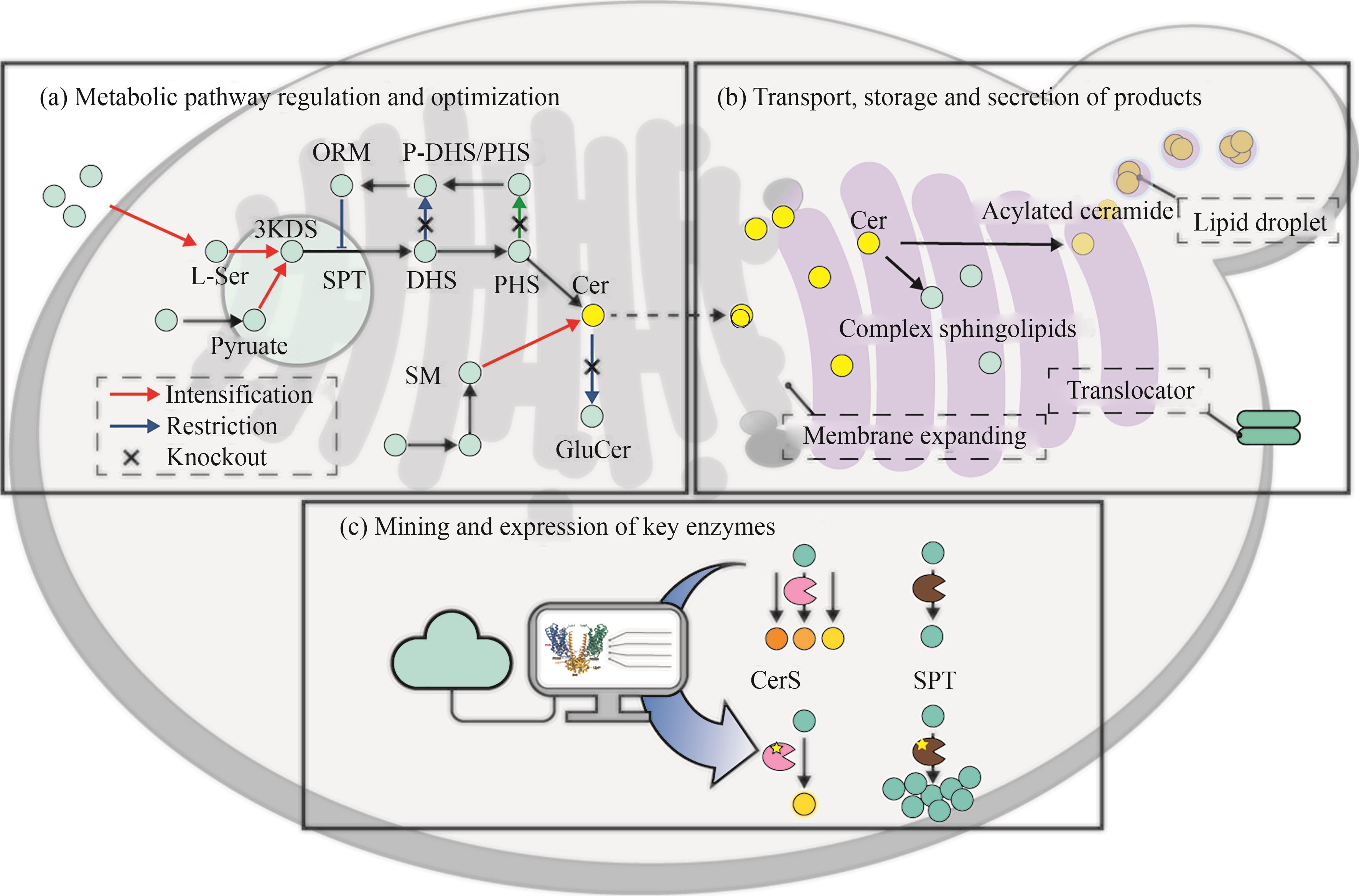
图7 神经酰胺生物合成途径改造策略(3-KDS—3-酮基-二氢鞘氨醇;ORM—介导鞘脂稳态蛋白; SPT—丝氨酸棕榈酰转移酶;DHS—二氢鞘氨醇;PHS—植物鞘氨醇;Cer—神经酰胺;GlcCer—葡萄糖糖神经酰胺;SM—鞘磷脂;CerS—神经酰胺合酶)
Fig. 7 Strategy for the modification of the ceramide biosynthetic pathway(3-KDS—3-keto-dihydrosphingosine; ORM—mediate sphingolipid homeostasis protein; SPT—serine palmitoyl transferase; DHS—dihydrosphingosine; PHS—phytosphingosine; Cer—ceramide; GlcCer—glucosylceramide; SM—sphingomyelin; CerS—ceramide synthase)
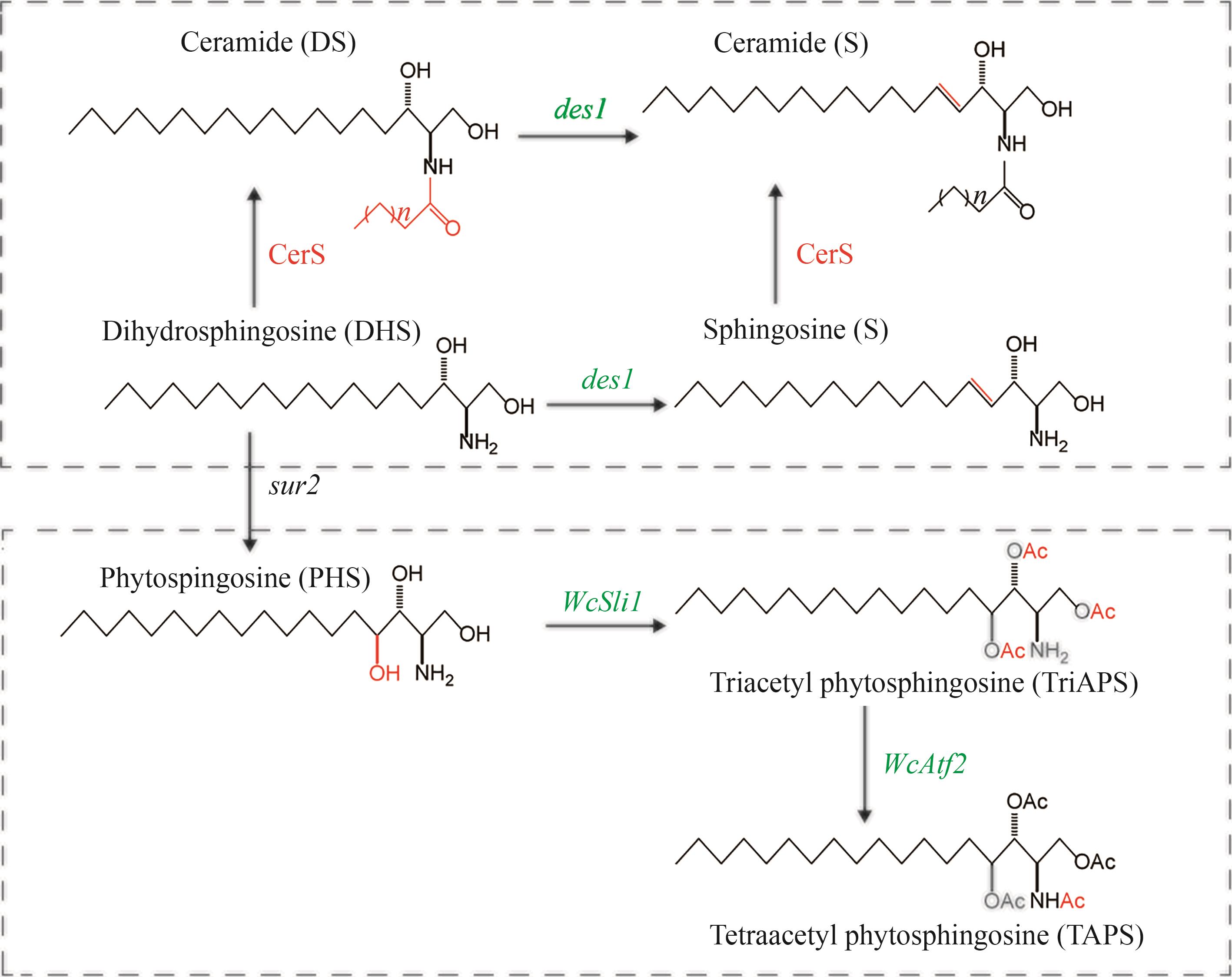
图8 DES1、SLI1、ATF2催化合成神经酰胺示意图(CerS—神经酰胺合酶;des1—人鞘脂去饱和酶编码基因;WcSli1,WcAtf2—威克汉姆西弗酵母鞘碱N/O-乙酰转移酶编码基因)
Fig. 8 Diagram for the catalytic synthesis of ceramides by DES1、SLI1 and ATF2(CerS—Ceramide synthase; des1—Human sphingolipid desaturase encoding gene; WcSli1, WcAtf2—Wickhamomyces ciferrii sphingobase N/O-acetyltransferase encoding genes)
| 1 | MURPHY B, GRIMSHAW S, HOPTROFF M, et al. Alteration of barrier properties, stratum corneum ceramides and microbiome composition in response to lotion application on cosmetic dry skin[J]. Scientific Reports, 2022, 12(1): 5223. |
| 2 | FUJII M. The pathogenic and therapeutic implications of ceramide abnormalities in atopic dermatitis[J]. Cells, 2021, 10(9): 2386. |
| 3 | SCHMITT T, NEUBERT R H H. State of the art in stratum corneum research: the biophysical properties of ceramides[J]. Chemistry and Physics of Lipids, 2018, 216: 91-103. |
| 4 | CHA H J, HE C F, ZHAO H, et al. Intercellular and intracellular functions of ceramides and their metabolites in skin (Review)[J]. International Journal of Molecular Medicine, 2016, 38(1): 16-22. |
| 5 | FEINGOLD K R. Thematic review series: Skin Lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis[J]. Journal of Lipid Research, 2007, 48(12): 2531-2546. |
| 6 | VAN SMEDEN J, HOPPEL L, VAN DER HEIJDEN R, et al. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery[S][J]. Journal of Lipid Research, 2011, 52(6): 1211-1221. |
| 7 | MASUKAWA Y, NARITA H, SHIMIZU E, et al. Characterization of overall ceramide species in human stratum corneums [J]. Journal of Lipid Research, 2008, 49(7): 1466-1476. |
| 8 | T’KINDT R, JORGE L, DUMONT E, et al. Profiling and characterizing skin ceramides using reversed-phase liquid chromatography-quadrupole time-of-flight mass spectrometry[J]. Analytical Chemistry, 2012, 84(1): 403-411. |
| 9 | LI X, YANG Q, ZHENG J, et al. Efficacy and safety of a topical moisturizer containing linoleic acid and ceramide for mild-to-moderate psoriasis vulgaris: a multicenter randomized controlled trial[J]. Dermatologic Therapy, 2020, 33(6): e14263. |
| 10 | SHIN K O, MIHARA H, ISHIDA K, et al. Exogenous ceramide serves as a precursor to endogenous ceramide synthesis and as a modulator of keratinocyte differentiation[J]. Cells, 2022, 11(11): 1742. |
| 11 | UCHIDA Y, PARK K. Ceramides in skin health and disease: an update[J]. American Journal of Clinical Dermatology, 2021, 22(6): 853-866. |
| 12 | SPADA F, HARRISON I P, BARNES T M, et al. A daily regimen of a ceramide-dominant moisturizing cream and cleanser restores the skin permeability barrier in adults with moderate eczema: a randomized trial[J]. Dermatologic Therapy, 2021, 34(4): e14970. |
| 13 | CAO Y, ZHANG X H, HE X F, et al. Efficacy of ceramide-containing sunscreen on skin barrier[J]. Journal of Cosmetic Dermatology, 2024, 23(2): 525-528. |
| 14 | ZHENG Y, HUNT R L, VILLARUZ A E, et al. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides[J]. Cell Host & Microbe, 2022, 30(3): 301-313.e9. |
| 15 | KIM D S, KIM S Y, CHUNG J H, et al. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes[J]. Cellular Signalling, 2002, 14(9): 779-785. |
| 16 | PHILIPS N, TUASON M, CHANG T, et al. Differential effects of ceramide on cell viability and extracellular matrix remodeling in keratinocytes and fibroblasts[J]. Skin Pharmacology and Physiology, 2009, 22(3): 151-157. |
| 17 | FISCHER C L, DRAKE D R, DAWSON D V, et al. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria[J]. Antimicrobial Agents and Chemotherapy, 2012, 56(3): 1157-1161. |
| 18 | BIBEL D J, ALY R, SHINEFIELD H R. Antimicrobial activity of sphingosines[J]. Journal of Investigative Dermatology, 1992, 98(3): 269-273. |
| 19 | DONGFACK M D J, LALLEMAND M C, KUETE V, et al. A new sphingolipid and furanocoumarins with antimicrobial activity from Ficus exasperata [J]. Chemical & Pharmaceutical Bulletin, 2012, 60(8): 1072-1075. |
| 20 | BECAM J, WALTER T, BURGERT A, et al. Antibacterial activity of ceramide and ceramide analogs against pathogenic Neisseria [J]. Scientific Reports, 2017, 7(1): 17627. |
| 21 | 戚建华, 边凌林, 罗燕, 等. 一种神经酰胺类化合物和应用: CN106631871A[P]. 2017-05-10. |
| QI J H, BIAN L L, LUO Y, et al. A ceramide compound and application thereof: CN106631871A[P]. 2017-05-10. | |
| 22 | OHTA K, HIRAKI S, MIYANABE M, et al. Appearance of intact molecules of dietary ceramides prepared from soy sauce lees and rice glucosylceramides in mouse plasma[J]. Journal of Agricultural and Food Chemistry, 2021, 69(32): 9188-9198. |
| 23 | MORAD S A F, CABOT M C. Ceramide-orchestrated signalling in cancer cells[J]. Nature Reviews Cancer, 2013, 13(1): 51-65. |
| 24 | CASTRO B M, PRIETO M, SILVA L C. Ceramide: a simple sphingolipid with unique biophysical properties[J]. Progress in Lipid Research, 2014, 54: 53-67. |
| 25 | ZHANG Y H, VASKO M R, NICOL G D. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons[J]. The Journal of Physiology, 2002, 544(2): 385-402. |
| 26 | ASHLEY COWART L, OBEID L M. Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function[J]. Biochimica et Biophysica Acta, 2007, 1771(3): 421-431. |
| 27 | NICHOLSON R J, NORRIS M K, POSS A M, et al. The lard works in mysterious ways: ceramides in nutrition-linked chronic disease[J]. Annual Review of Nutrition, 2022, 42: 115-144. |
| 28 | OHTA K, HIRAKI S, MIYANABE M, et al. Dietary ceramide prepared from soy sauce lees improves skin barrier function in hairless mice[J]. Journal of Oleo Science, 2021, 70(9): 1325-1334. |
| 29 | ZHU F F, ZHAO B, HU B, et al. Review of available “extraction + purification” methods of natural ceramides and their feasibility for sewage sludge analysis[J]. Environmental Science and Pollution Research, 2023, 30(26): 68022-68053. |
| 30 | 张可青, 王建华, 于姝燕, 等. 植物中神经酰胺类化合物提取工艺研究进展[J]. 内蒙古中医药, 2022, 41(6): 131-133. |
| ZHANG K Q, WANG J H, YU S Y, et al. Research progress on extraction technology of ceramides from plants[J]. Inner Mongolia Journal of Traditional Chinese Medicine, 2022, 41(6): 131-133. | |
| 31 | YAHYA N A, ATTAN N, WAHAB R A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds[J]. Food and Bioproducts Processing, 2018, 112: 69-85. |
| 32 | OJHA K S, AZNAR R, O’DONNELL C, et al. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources[J]. TrAC Trends in Analytical Chemistry, 2020, 122: 115663. |
| 33 | WILD R, SCHMIDT R R. Sphingosine and phytosphingosine from D-threose synthesis of a 4-keto-ceramide[J]. Tetrahedron: Asymmetry, 1994, 5(11): 2195-2208. |
| 34 | TORSSELL S, SOMFAI P. A practical synthesis of D-erythro-sphingosine using a cross-metathesis approach[J]. Organic & Biomolecular Chemistry, 2004, 2(11): 1643-1646. |
| 35 | CHAUDHARI V D, AJISH KUMAR K S, DHAVALE D D. An efficient synthesis of D-erythro- and D-threo-sphingosine from D-glucose: olefin cross-metathesis approach[J]. Organic Letters, 2005, 7(26): 5805-5807. |
| 36 | YAMAMOTO T, HASEGAWA H, HAKOGI T, et al. Versatile synthetic method for sphingolipids and functionalized sphingosine derivatives via olefin cross metathesis[J]. Organic Letters, 2006, 8(24): 5569-5572. |
| 37 | YANG H, LIEBESKIND L S. A concise and scalable synthesis of high enantiopurity (–)-D-erythro-sphingosine using peptidyl thiol ester-boronic acid cross-coupling[J]. Organic Letters, 2007, 9(16): 2993-2995. |
| 38 | 禹柄英, 张元僖, 朱泳协, 等. 新型的类神经酰胺化合物其及其制备方法: CN104854081A[P]. 2015-08-19. |
| WOO B Y, JANG W H, JOO Y H, et al. The invention discloses a novel ceramide-like compound and a preparation method thereof: CN104854081A[P]. 2015-08-19. | |
| 39 | 刘希望. 神经酰胺类似化合物的合成及其生物活性研究[D]. 咸阳: 西北农林科技大学, 2010. |
| LIU X W. Synthesis and bioactivity of ceramide-like compounds [D]. Xianyang: Northwest Agriculture & Forestry University, 2010. | |
| 40 | CRITCHLEY P, RAWLINGS A V, SCOTT I R. Synthetic pseudoceramide and cosmetic compositions thereof: US5206020A[P]. 1993-04-27. |
| 41 | MOTION K R, JANOUSEK A, WATKINS S D. Hydroxy alkyl amides of dicarboxylic acids and their use in cosmetic compositions: US5656668A[P]. 1997-08-12. |
| 42 | BAIK I S, LEE J G, PARK B D, et al. Pseudoceramides, and dermatologic external preparations containing the same: US6221371B1 [P]. 2001-04-24. |
| 43 | PARK B D, LEE K M, PARK I J, et al. Novel pseudoceramides and their synthesis using alkyl ketene dimer[J]. Journal of the Society of Cosmetic Scientists of Korea, 1997, 23: 92-96. |
| 44 | 杨超文, 叶柳. 含共轭羧酸的神经酰胺类化合物及其制备方法和应用: CN115433100A[P]. 2022-12-06. |
| YANG C W, YE L. Ceramide compounds containing conjugated carboxylic acids, preparation method and application thereof: CN115433100A[P]. 2022-12-06. | |
| 45 | 吴江, 张伟, 朱纯银. 一种酰胺的合成方法及其在神经酰胺3制备上的应用: CN 110078635 A[P]. 2019-08-02. |
| WU J, ZHANG W, ZHU C Y. An amide synthesis method and its application in the preparation of ceramide 3: CN 110078635 A[P]. 2019-08-02. | |
| 46 | M.F.埃克施泰因, M.D.范洛格切姆, H.H.文克, 等. 制备鞘脂的方法: CN110317840A[P]. 2019-10-11. |
| ECKSTEIN M F, VAN LOGCHEM M D, WENK H H, et al. Method for preparing sphingolipid: CN110317840A[P]. 2019-10-11. | |
| 47 | F.霍尔曼, O.图姆, P.格热比克, 等. 使用脂肪酶和脂肪酸甘油酯通过溶性鞘脂的酶促N-酰化反应制备鞘脂的方法: CN102057050B[P]. 2014-06-11. |
| HOLMANN F, TUM O, GRZEBYK P, et al. Method of preparing sphingolipid by enzymatic N-acylation of soluble sphingolipid using lipase and fatty acid glyceride: CN102057050B[P]. 2014-06-11. | |
| 48 | ZHANG X Y, MA Y J, OUYANG B, et al. Efficient lipase-catalyzed synthesis of ceramide Ⅲ series compounds in an eco-friendly solvent[J]. Molecular Catalysis, 2024, 558: 114006. |
| 49 | HE L, BYUN H S, BITTMAN R. Stereoselective preparation of ceramide and its skeleton backbone modified analogues via cyclic thionocarbonate intermediates derived by catalytic asymmetric dihydroxylation of α,β-unsaturated ester precursors[J]. The Journal of Organic Chemistry, 2000, 65(22): 7627-7633. |
| 50 | RAI A N, BASU A. Synthesis of the glycosphingolipid β-galactosyl ceramide and analogues via olefin cross metathesis[J]. The Journal of Organic Chemistry, 2005, 70(20): 8228-8230. |
| 51 | NAKAMURA T, SHIOZAKI M. Stereoselective synthesis of D-erythro-sphingosine and L-lyxo-phytosphingosine[J]. Tetrahedron, 2001, 57(44): 9087-9092. |
| 52 | LU X Q, BITTMAN R. Efficient and versatile synthesis of (2S,3R)-sphingosine and its 2-azido-3-O-benzylsphingosine analogue[J]. Tetrahedron Letters, 2005, 46(11): 1873-1875. |
| 53 | DUCLOS R I. The total syntheses of D-erythro-sphingosine, N-palmitoylsphingosine (ceramide), and glucosylceramide (cerebroside) via an azidosphingosine analog[J]. Chemistry and Physics of Lipids, 2001, 111(2): 111-138. |
| 54 | 朴尽五, 李知愿, 全圣贤, 等. 新型类神经酰胺化合物及其用途: CN111201216A[P]. 2020-05-26. |
| PARK J O, LEE J W, JEON S H, et al. Novel ceramide-like compounds and their applications: CN111201216A[P]. 2020-05-26. | |
| 55 | F.霍尔曼, O.图姆, C.特勒, 等. 使用真菌脂肪酶和脂肪酸烷基酯通过溶性鞘脂的酶促N-酰化反应制备鞘脂的方法: CN102057049B[P]. 2014-10-01. |
| HOLMANN F, TUM O, TELLERET C, et al. Sphingolipid is prepared by enzymatic N-acylation of soluble sphingolipid using fungal lipase and fatty acid alkyl ester: CN102057049B[P]. 2014-10-01. | |
| 56 | WICKERHAM L J, STODOLA F H. Formation of extracellular sphingolipides by microorganisms.Ⅰ. Tetraacetylphyto-sphingosine from Hansenula ciferri [J]. Journal of Bacteriology, 1960, 80(4): 484-491. |
| 57 | SCHORSCH C, KÖHLER T, ANDREA H, et al. High-level production of tetraacetyl phytosphingosine (TAPS) by combined genetic engineering of sphingoid base biosynthesis and L-serine availability in the non-conventional yeast Pichia ciferrii [J]. Metabolic Engineering, 2012, 14(2): 172-184. |
| 58 | VELD F TER, WOLFF D, SCHORSCH C, et al. Production of tetraacetyl phytosphingosine (TAPS) in Wickerhamomyces ciferrii is catalyzed by acetyltransferases Sli1p and Atf2p[J]. Applied Microbiology and Biotechnology, 2013, 97(19): 8537-8546. |
| 59 | PARK S B, TRAN Q G, RYU A J, et al. Fluorescence-activated cell sorting-mediated directed evolution of Wickerhamomyces ciferrii for enhanced production of tetraacetyl phytosphingosine[J]. Korean Journal of Chemical Engineering, 2022, 39(4): 1004-1010. |
| 60 | HAN C, JANG M, KIM M J, et al. Engineering Yarrowia lipolytica for de novo production of tetraacetyl phytosphingosine[J]. Journal of Applied Microbiology, 2021, 130(6): 1981-1992. |
| 61 | CHOI J Y, HWANG H J, CHO W Y, et al. Differences in the fatty acid profile, morphology, and tetraacetylphytosphingosine-forming capability between wild-type and mutant Wickerhamomyces ciferrii [J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 662979. |
| 62 | 纪晓俊, 崔柳伟, 王凯峰, 等. 一株高产四乙酰基植物鞘氨醇的菌株及其应用: CN117070382A[P]. 2023-11-17. |
| JI X J, CUI L W, WANG K F, et al. A strain with high yield of tetraacetyl phytosphingosine and its application: CN117070382A[P]. 2023-11-17. | |
| 63 | PARK C S, JEONG J H, HONG S Y, et al. Yeast Pichia ciferrii : US6194196B1[P]. 2001-02-27. |
| 64 | 张天萌, 刘金钊, 朱倩倩, 等. 一种高产鞘脂类微生物菌株、它的筛选方法和它的用途: CN114736815A[P]. 2022-07-12. |
| ZHANG T M, LIU J Z, ZHU Q Q, et al. A high-yielding sphingolipid microbial strain, its screening method and its use: CN114736815A[P]. 2022-07-12. | |
| 65 | 孙杰, 谢朋天, 魏春, 等. 一种高产植物鞘氨醇的酿酒酵母菌株: CN116103176A[P]. 2023-05-12. |
| SUN J, XIE P T, WEI C, et al. A strain of Saccharomyces cerevisiae with high yield of sphingosine: CN116103176A[P]. 2023-05-12. | |
| 66 | M. 施瓦布, M. 巴鲍, D. 费希尔, 等. 生产植物鞘氨醇或二氢神经鞘氨醇的方法: CN108473968A[P]. 2018-08-31. |
| SCHWAB M, BABAU M, FISCHER D, et al. Method for producing phytosphingosine or dihydrosphingosine: CN108473968A[P]. 2018-08-31. | |
| 67 | KWUN K H, LEE J H, RHO K H, et al. Production of ceramide with Saccharomyces cerevisiae [J]. Applied Biochemistry and Biotechnology, 2006, 133(3): 203-210. |
| 68 | KIM S K, NOH Y H, KOO J R, et al. Effect of expression of genes in the sphingolipid synthesis pathway on the biosynthesis of ceramide in Saccharomyces cerevisiae [J]. Journal of Microbiology and Biotechnology, 2010, 20(2): 356-362. |
| 69 | KIM S K, NOH Y H, KOO J R, et al. Effects of expression of lcb1/lcb2 and lac1/lag1 genes on the biosynthesis of ceramides[J]. Biotechnology and Bioprocess Engineering, 2011, 16(1): 1-6. |
| 70 | MURAKAMI S, SHIMAMOTO T, NAGANO H, et al. Producing human ceramide-NS by metabolic engineering using yeast Saccharomyces cerevisiae [J]. Scientific Reports, 2015, 5: 16319. |
| 71 | 黄铭, 吴佳欣, 张目, 等. 毕赤酵母PAS_chr4_0427基因敲除促进神经酰胺合成[J]. 食品与发酵工业, 2024, 50(17): 17-22. |
| HUANG M, WU J X, ZHANG M, et al. Knockout of PAS_chr4_0427 promotes ceramide synthesis in Pichia pastoris [J]. Food and Fermentation Industries, 2024, 50(17): 17-22. | |
| 72 | KURTZMAN C P. Phylogeny of the ascomycetous yeasts and the renaming of Pichia anomala to Wickerhamomyces anomalus [J]. Antonie Van Leeuwenhoek, 2011, 99(1): 13-23. |
| 73 | MAISTER H G, ROGOVIN S P, STODOLA F H, et al. Formation of extracellular sphingolipids by microorganisms: Ⅳ. Pilot-plant production of tetraacetylphytosphingosine by Hansenula ciferrii [J]. Applied Microbiology, 1962, 10(5): 401-406. |
| 74 | S.舍费尔, M.A.范登贝尔赫, D.博尔格尔, 等. 使用遗传工程化的微生物菌株的改进的鞘氨醇类碱的生产: CN101490260A[P]. 2009-07-22. |
| SCHAFFER S, VAN DEN BERGH M A, BOERGEL D, et al. Improved production of sphingoid bases using genetically engineered microbial strains: CN101490260A[P]. 2009-07-22. | |
| 75 | OLEA-OZUNA R J, POGGIO S, EDBERGSTRÖM, et al. Five structural genes required for ceramide synthesis in Caulobacter and for bacterial survival[J]. Environmental Microbiology, 2021, 23(1): 143-159. |
| 76 | FURUYA H, IDE Y, HAMAMOTO M, et al. Isolation of a novel bacterium, Blautia glucerasei sp. nov., hydrolyzing plant glucosylceramide to ceramide[J]. Archives of Microbiology, 2010, 192(5): 365-372. |
| 77 | HALAMKA T A, GARCIA A, EVANS T W, et al. Occurrence of ceramides in the Acidobacterium solibacter usitatus: implications for bacterial physiology and sphingolipids in soils[J]. Frontiers in Geochemistry, 2024, 2: 1400278. |
| 78 | STANKEVICIUTE G, TANG P J, ASHLEY B, et al. Convergent evolution of bacterial ceramide synthesis[J]. Nature Chemical Biology, 2022, 18(3): 305-312. |
| 79 | BROWN E M, KE X B, HITCHCOCK D, et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis[J]. Cell Host & Microbe, 2019, 25(5): 668-680.e7. |
| 80 | YAMAJI T, HANADA K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins[J]. Traffic, 2015, 16(2): 101-122. |
| 81 | CINGOLANI F, FUTERMAN A H, CASAS J. Ceramide synthases in biomedical research[J]. Chemistry and Physics of Lipids, 2016, 197: 25-32. |
| 82 | SAWAI H, OKAMOTO Y, LUBERTO C, et al. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae [J]. The Journal of Biological Chemistry, 2000, 275(50): 39793-39798. |
| 83 | DICKSON R C. Roles for sphingolipids in Saccharomyces cerevisiae [J]. Advances in Experimental Medicine and Biology, 2010, 688: 217-231. |
| 84 | ANDO H, KOMURA N. Recent progress in the synthesis of glycosphingolipids[J]. Current Opinion in Chemical Biology, 2024, 78: 102423. |
| 85 | WENNEKES T, VAN DEN BERG R J B H N, BOOT R G, et al. Glycosphingolipids-nature, function, and pharmacological modulation[J]. Angewandte Chemie International Edition, 2009, 48(47): 8848-8869. |
| 86 | HARRISON P J, DUNN T M, CAMPOPIANO D J. Sphingolipid biosynthesis in man and microbes[J]. Natural Product Reports, 2018, 35(9): 921-954. |
| 87 | YARD B A, CARTER L G, JOHNSON K A, et al. The structure of serine palmitoyltransferase; gateway to sphingolipid biosynthesis[J]. Journal of Molecular Biology, 2007, 370(5): 870-886. |
| 88 | HANADA K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism[J]. Biochimica et Biophysica Acta, 2003, 1632(1-3): 16-30. |
| 89 | GABLE K, HAN G S, MONAGHAN E, et al. Mutations in the yeast LCB1 and LCB2 genes, including those corresponding to the hereditary sensory neuropathy typeⅠmutations, dominantly inactivate serine palmitoyltransferase*[J]. Journal of Biological Chemistry, 2002, 277(12): 10194-10200. |
| 90 | GABLE K, SLIFE H, BACIKOVA D, et al. Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity[J]. Journal of Biological Chemistry, 2000, 275(11): 7597-7603. |
| 91 | REN J H, SAIED E M, ZHONG A, et al. Tsc3 regulates SPT amino acid choice in Saccharomyces cerevisiae by promoting alanine in the sphingolipid pathway[J]. Journal of Lipid Research, 2018, 59(11): 2126-2139. |
| 92 | SCHÄFER J H, KÖRNER C, ESCH B M, et al. Structure of the ceramide-bound SPOTS complex[J]. Nature Communications, 2023, 14(1): 6196. |
| 93 | BEELER T, BACIKOVA D, GABLE K, et al. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Δ mutant[J]. Journal of Biological Chemistry, 1998, 273(46): 30688-30694. |
| 94 | BRESLOW D K, COLLINS S R, BODENMILLER B, et al. Orm family proteins mediate sphingolipid homeostasis[J]. Nature, 2010, 463(7284): 1048-1053. |
| 95 | LIU Q, CHAN A K N, CHANG W H, et al. 3-Ketodihydrosphingosine reductase maintains ER homeostasis and unfolded protein response in leukemia[J]. Leukemia, 2022, 36(1): 100-110. |
| 96 | ZHAO P Q, ZHUANG Z W, GUAN X Y, et al. Crystal structure of the 3-ketodihydrosphingosine reductase TSC10 from Cryptococcus neoformans [J]. Biochemical and Biophysical Research Communications, 2023, 670: 73-78. |
| 97 | SCHORLING S, VALLÉE B, BARZ W P, et al. Lag1p and Lac1p are essential for the acyl-CoA-dependent ceramide synthase reaction in Saccharomyces cerevisae [J]. Molecular Biology of the Cell, 2001, 12(11): 3417-3427. |
| 98 | GUILLAS I, KIRCHMAN P A, CHUARD R, et al. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p[J]. The EMBO Journal, 2001, 20(11): 2655-2665. |
| 99 | SCHÄFER J H, CLAUSMEYER L, KÖRNER C, et al. Structure of the yeast ceramide synthase[J/OL]. Nature Structural & Molecular Biology. (2024-11-11)[2024-11-11]. . |
| 100 | ZHANG M M, LI Z Y, LIU Y W, et al. The ceramide synthase (CERS/LASS) family: functions involved in cancer progression[J]. Cellular Oncology, 2023, 46(4): 825-845. |
| 101 | XIE T, FANG Q, ZHANG Z K, et al. Structure and mechanism of a eukaryotic ceramide synthase complex[J]. The EMBO Journal, 2023, 42(24): e114889. |
| 102 | COWART L A, HANNUN Y A. Selective substrate supply in the regulation of yeast de novo sphingolipid synthesis[J]. The Journal of Biological Chemistry, 2007, 282(16): 12330-12340. |
| 103 | HANADA K, HARA T, NISHIJIMA M. Purification of the serine palmitoyltransferase complex responsible for sphingoid base synthesis by using affinity peptide chromatography techniques[J]. The Journal of Biological Chemistry, 2000, 275(12): 8409-8415. |
| 104 | SCHWAB M, BABAU M, FISCHER D, et al. Method for producing phytosphingosine or sphinganine: US20180179562[P]. 2021-09-07. |
| 105 | HAN S M, LONE M A, SCHNEITER R, et al. Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(13): 5851-5856. |
| 106 | LIU M, HUANG C J, POLU S R, et al. Regulation of sphingolipid synthesis through Orm1 and Orm2 in yeast[J]. Journal of Cell Science, 2012, 125(Pt 10): 2428-2435. |
| 107 | RODRIGUEZ-GALLARDO S, KUROKAWA K, SABIDO-BOZO S, et al. Ceramide chain length-dependent protein sorting into selective endoplasmic reticulum exit sites[J]. Science Advances, 2020, 6(50): eaba8237. |
| 108 | LIU L K, CHOUDHARY V, TOULMAY A, et al. An inducible ER-Golgi tether facilitates ceramide transport to alleviate lipotoxicity[J]. The Journal of Cell Biology, 2017, 216(1): 131-147. |
| 109 | IKEDA A, SCHLARMANN P, KUROKAWA K, et al. Tricalbins are required for non-vesicular ceramide transport at ER-Golgi contacts and modulate lipid droplet biogenesis[J]. iScience, 2020, 23(10): 101603. |
| 110 | ZELNIK I D, VENTURA A E, KIM J L, et al. The role of ceramide in regulating endoplasmic reticulum function[J]. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 2020, 1865(1): 158489. |
| 111 | LIU P T, SUN L, SUN Y X, et al. Decreased fluidity of cell membranes causes a metal ion deficiency in recombinant Saccharomyces cerevisiae producing carotenoids[J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(4): 525-535. |
| 112 | BU X, LIN J Y, CHENG J, et al. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2020, 13: 168. |
| [1] | 盛周煌, 陈智仙, 张彦. 酵母甘露糖蛋白的研究进展[J]. 合成生物学, 2025, 6(2): 408-421. |
| [2] | 韦灵珍, 王佳, 孙新晓, 袁其朋, 申晓林. 黄酮类化合物生物合成及其在化妆品中应用的研究[J]. 合成生物学, 2025, 6(2): 373-390. |
| [3] | 肖森, 胡立涛, 石智诚, 王发银, 余思婷, 堵国成, 陈坚, 康振. 可控分子量透明质酸的生物合成研究进展[J]. 合成生物学, 2025, 6(2): 445-460. |
| [4] | 汤传根, 王璟, 张烁, 张昊宁, 康振. 功能肽合成和挖掘策略研究进展[J]. 合成生物学, 2025, 6(2): 461-478. |
| [5] | 黄姝涵, 马赫, 罗云孜. 生物合成红景天苷的研究进展[J]. 合成生物学, 2025, 6(2): 391-407. |
| [6] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [7] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [8] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [9] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [10] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [11] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [12] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [13] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [14] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [15] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||