合成生物学 ›› 2025, Vol. 6 ›› Issue (4): 920-939.DOI: 10.12211/2096-8280.2025-056
体外多酶组装与生物级联催化:进展与展望
马牧青, 吴彦, 曲茂华, 卢夏锋, 曹敏, 杜峰, 季荣涛, 董磊迟, 罗志波
- 杭州微远生物科技有限公司,浙江 杭州 310024
-
收稿日期:2025-06-06修回日期:2025-06-30出版日期:2025-08-31发布日期:2025-09-03 -
通讯作者:罗志波 -
作者简介:马牧青 (1993—),女,博士,工程师。研究方向为天然产物合成生物学。E-mail:mqma@wybio.cc吴彦 (1982—),男,工程师。研究方向为合成生物学与药物化学。E-mail:ywu@wybio.cc罗志波 (1991—),男,博士,高级工程师,副研究员。研究方向为合成生物学、药物化学、酶分子工程与工业生物催化等,在合成生物学、新药创制及工程转化领域取得系列产业化成果。E-mail:zhiboluo@126.com
第一联系人:共同第一作者 -
基金资助:杭州市农业与社会发展领域公益性科研引导项目(20241029Y103)
Extracellular multi-enzyme assembly and biocatalytic cascade: advances and prospects
MA Muqing, WU Yan, QU Maohua, LU Xiafeng, CAO Min, DU Feng, JI Rongtao, DONG Leichi, LUO Zhibo
- Hangzhou Weiyuan Biotechnology Co. ,Ltd. ,Hangzhou 310024,Zhejiang,China
-
Received:2025-06-06Revised:2025-06-30Online:2025-08-31Published:2025-09-03 -
Contact:LUO Zhibo
摘要:
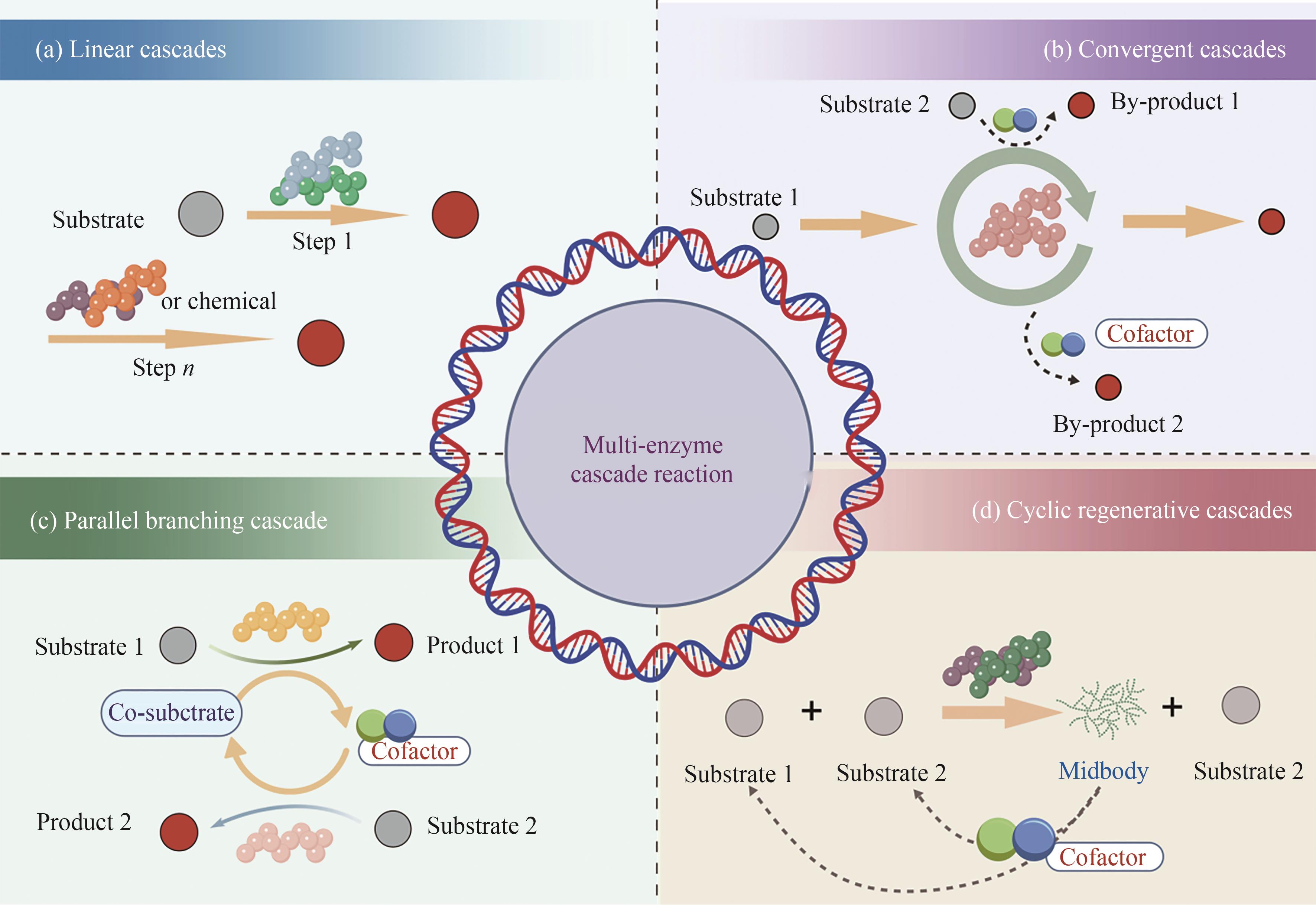
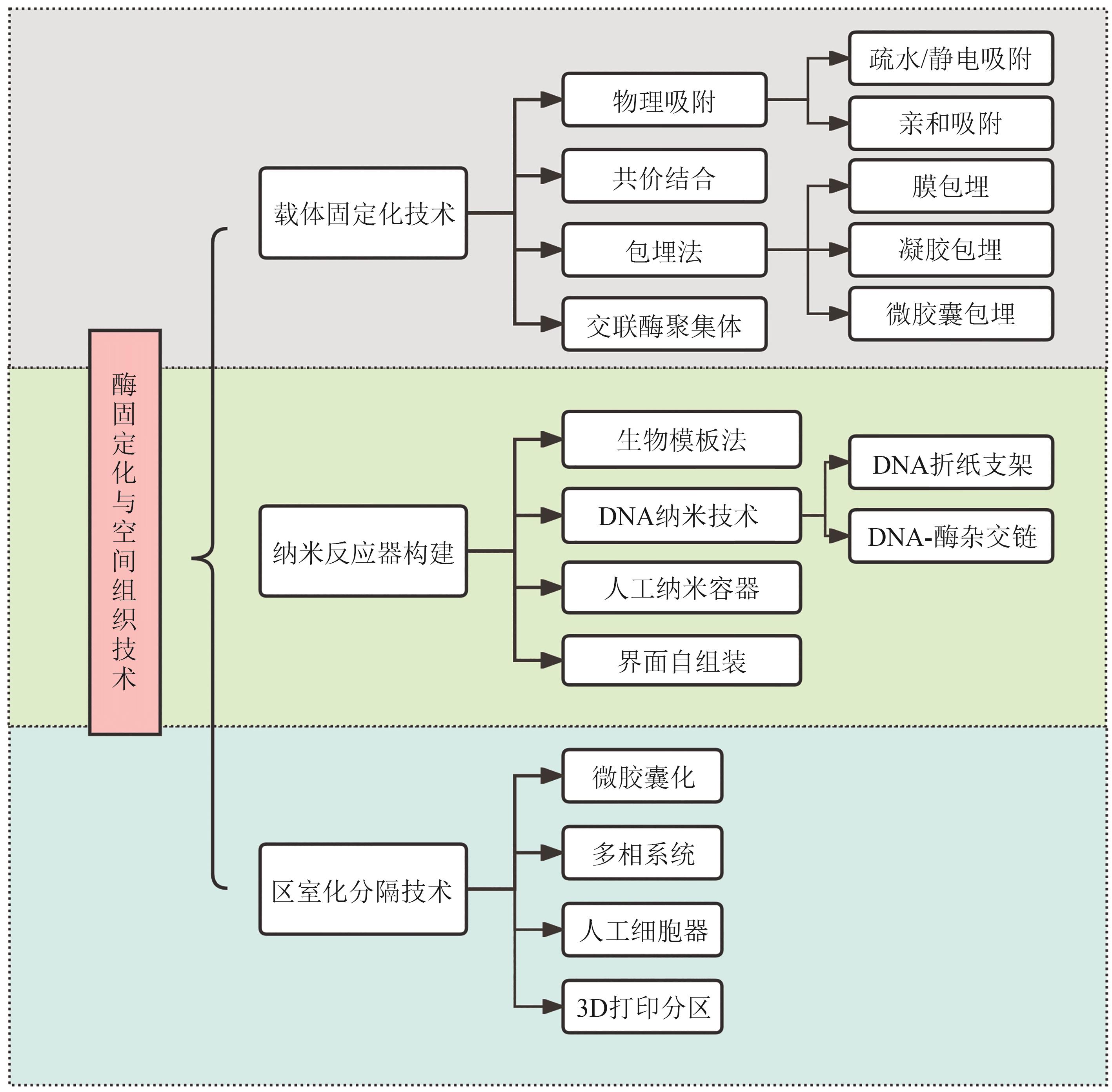
在全球碳中和战略背景下,绿色生物制造正加速取代传统高污染、高能耗的化工生产方式。多酶级联反应(multi-enzyme cascade reaction, MECR)作为新一代生物催化平台技术,通过模块化酶网络实现“一锅式”高效转化,展现出卓越的原子经济性、显著降低的能耗以及突出的环境友好性。本综述明确聚焦于体外多酶级联催化系统(in vitro multi-enzyme systems),涵盖以下研究范畴:①体外多酶级联体系的定义;②体外多酶级联体系的分类;③体外多酶级联体系的相关技术及应用。系统解析了体外多酶组装与生物级联催化的分子机制与技术体系:基于反应拓扑学特征,提出四类级联模型(线性/趋同/平行/循环),阐明其动力学优势;突破性技术涵盖AI驱动的酶理性设计、纳米限域空间组织及光/电辅因子再生系统。通过智能适配设计理念,本文深入解析了跨尺度酶模块的拓扑优化与催化耦合机制,整合了计算流体力学建模、载体界面分子工程及微环境传质调控等关键技术。产业化实践表明,该技术已成功实现手性药物中间体、高值天然产物等的高效绿色合成,推动医药、材料等领域的工艺革新。展望未来,动态微环境精准适配、人工智能辅助的酶网络设计及连续流规模化制备等方向将引领技术发展,为绿色生物制造的产业化升级提供重要理论支撑与技术路径。
中图分类号:
引用本文
马牧青, 吴彦, 曲茂华, 卢夏锋, 曹敏, 杜峰, 季荣涛, 董磊迟, 罗志波. 体外多酶组装与生物级联催化:进展与展望[J]. 合成生物学, 2025, 6(4): 920-939.
MA Muqing, WU Yan, QU Maohua, LU Xiafeng, CAO Min, DU Feng, JI Rongtao, DONG Leichi, LUO Zhibo. Extracellular multi-enzyme assembly and biocatalytic cascade: advances and prospects[J]. Synthetic Biology Journal, 2025, 6(4): 920-939.
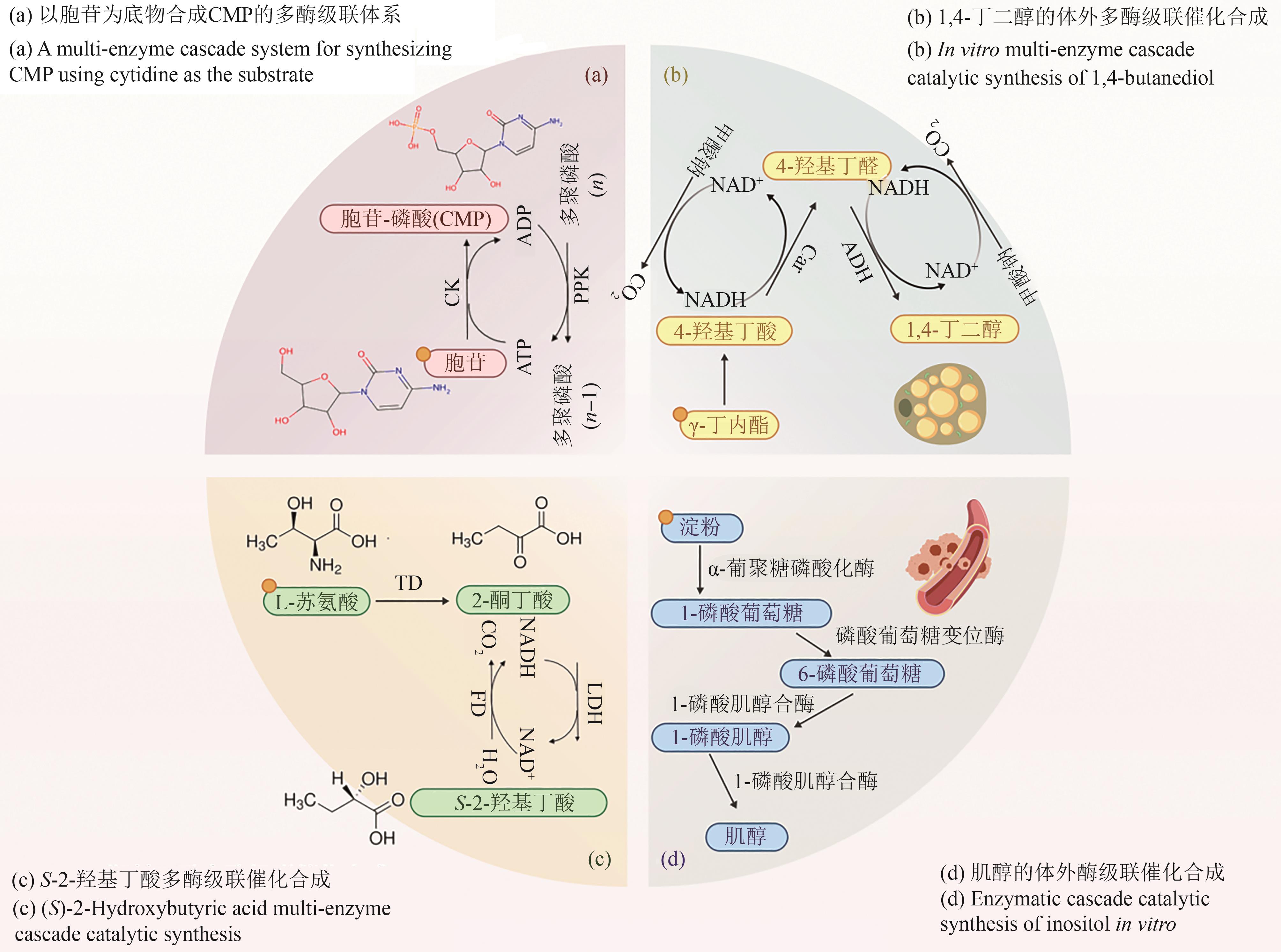
图3 体外多酶级联体系催化合成应用实例[19](图3使用 BioGDP.com制作)
Fig. 3 Application examples of in vitro multi-enzyme cascade system for catalytic synthesis[19](Fig. 3 was created with BioGDP.com)
| 应用领域 | 酶系统组成 | 设计思路 | 性能指标 | 优势 | 参考文献 |
|---|---|---|---|---|---|
| 1,4-丁二醇合成 | 己内酯水解酶+羧酸还原酶+醇脱氢酶 | 三酶级联简化化学路线 辅酶循环(FDH) | 替代高压化学法 减少有毒试剂使用 | 产量2.41 g/L 反应步骤从9步减至3步 | [ |
| S-2-羟基丁酸 | 苏氨酸脱氨酶+乳酸脱氢酶+甲酸脱氢酶 | 动态动力学拆分 NADH循环系统 | 高转化率(97%) 光学纯度>99% | 产量143 g/L 无需外源辅酶 | [ |
| 索磷布韦中间体 | 转氨酶+亚胺还原酶 | 适配NADPH再生系统 选择热稳定性突变体 | 产物e.e.值>99.5% 高转化率(>90%) | 产率提升至92% 半衰期延长至120 h | [ |
| 手性胺合成 | 转氨酶+甲酸脱氢酶 | 热力学耦合设计 辅酶NADH原位再生 | 原子经济性>98% 产物e.e.值>99.9% | 时空产率8.3 g/(L·h) 辅酶周转数12000 | [ |
| 肌醇合成 | 淀粉磷酸化酶+肌醇-1-磷酸合酶等四酶 | 以淀粉为廉价底物 仿生代谢路径重构 | 转化率84.6% 成本较化学法低70% | 产量42.3 g/L 4步反应“一锅法” | [ |
| D-甘露醇生产 | 甘露糖脱氢酶+葡萄糖脱氢酶 | 双酶辅因子循环 底物通道效应优化 | 摩尔转化率81.9% 避免化学还原步骤 | 产量81.9 g/L 反应条件温和 | [ |
表1 体外多酶催化应用案例比较分析表
Table 1 Comparative analysis table of application cases of in vitro multi-enzyme catalysis
| 应用领域 | 酶系统组成 | 设计思路 | 性能指标 | 优势 | 参考文献 |
|---|---|---|---|---|---|
| 1,4-丁二醇合成 | 己内酯水解酶+羧酸还原酶+醇脱氢酶 | 三酶级联简化化学路线 辅酶循环(FDH) | 替代高压化学法 减少有毒试剂使用 | 产量2.41 g/L 反应步骤从9步减至3步 | [ |
| S-2-羟基丁酸 | 苏氨酸脱氨酶+乳酸脱氢酶+甲酸脱氢酶 | 动态动力学拆分 NADH循环系统 | 高转化率(97%) 光学纯度>99% | 产量143 g/L 无需外源辅酶 | [ |
| 索磷布韦中间体 | 转氨酶+亚胺还原酶 | 适配NADPH再生系统 选择热稳定性突变体 | 产物e.e.值>99.5% 高转化率(>90%) | 产率提升至92% 半衰期延长至120 h | [ |
| 手性胺合成 | 转氨酶+甲酸脱氢酶 | 热力学耦合设计 辅酶NADH原位再生 | 原子经济性>98% 产物e.e.值>99.9% | 时空产率8.3 g/(L·h) 辅酶周转数12000 | [ |
| 肌醇合成 | 淀粉磷酸化酶+肌醇-1-磷酸合酶等四酶 | 以淀粉为廉价底物 仿生代谢路径重构 | 转化率84.6% 成本较化学法低70% | 产量42.3 g/L 4步反应“一锅法” | [ |
| D-甘露醇生产 | 甘露糖脱氢酶+葡萄糖脱氢酶 | 双酶辅因子循环 底物通道效应优化 | 摩尔转化率81.9% 避免化学还原步骤 | 产量81.9 g/L 反应条件温和 | [ |
| [1] | WATARI T, HATA S, NAKAJIMA K, et al. Limited quantity and quality of steel supply in a zero-emission future[J]. Nature Sustainability, 2023, 6(3): 336-343. |
| [2] | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2): 801-838. |
| [3] | SCHRITTWIESER J H, VELIKOGNE S, HALL M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules[J]. Chemical Reviews, 2018, 118(1): 270-348. |
| [4] | XIAO Y Q, FENG C, FU J, et al. Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation[J]. Nature Catalysis, 2020, 3(11): 932-940. |
| [5] | ROSA R, SPINELLI R, NERI P, et al. Life cycle assessment of chemical vs enzymatic-assisted extraction of proteins from black soldier fly prepupae for the preparation of biomaterials for potential agricultural use[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14752-14764. |
| [6] | 国家统计局社会科技和文化产业统计司. 中国高技术产业统计年鉴[M]. 北京: 中国统计出版社, 2023. |
| Department of Social Science, Technology and Cultural Industry Statistics, National Bureau of Statistics. Statistical yearbook of China’s high-tech industries[M]. Beijing: China Statistics Press, 2023. | |
| [7] | WU B, WANG Y W, DAI Y H, et al. Current status and future prospective of bio-ethanol industry in China[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111079. |
| [8] | DENG W P, YAN L F, WANG B J, et al. Efficient catalysts for the green synthesis of adipic acid from biomass[J]. Angewandte Chemie International Edition, 2021, 60(9): 4712-4719. |
| [9] | LI M, ZHANG Z J, KONG X D, et al. Engineering Streptomyces coelicolor carbonyl reductase for efficient atorvastatin precursor synthesis[J]. Applied and Environmental Microbiology, 2017, 83(12): e00603-17. |
| [10] | DARGÓ G, KIS D, RÁDULY A, et al. Furandicarboxylic acid (FDCA): electrosynthesis and its facile recovery from polyethylene furanoate (PEF) via depolymerization[J]. ChemSusChem, 2025, 18(3): e202401190. |
| [11] | MOKALE KOGNOU A L, SHRESTHA S, JIANG Z H, et al. High-fructose corn syrup production and its new applications for 5-hydroxymethylfurfural and value-added furan derivatives: promises and challenges[J]. Journal of Bioresources and Bioproducts, 2022, 7(3): 148-160. |
| [12] | 李举谋, 石焜, 张志钧, 等. 多酶级联反应的构建及其在双官能团功能化学品合成中的应用[J]. 生物工程学报, 2023, 39(6): 2158-2189. |
| LI J M, SHI K, ZHANG Z J, et al. Construction of multi-enzyme cascade reactions and its application in the synthesis of bifunctional chemicals[J]. Chinese Journal of Biotechnology, 2023, 39(6): 2158-2189. | |
| [13] | LOPEZ-GALLEGO F, SCHMIDT-DANNERT C. Multi-enzymatic synthesis[J]. Current Opinion in Chemical Biology, 2010, 14(2): 174-183. |
| [14] | KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux[J]. Nature Communications, 2019, 10: 4248. |
| [15] | ABIDIN M Z, SARAVANAN T, ZHANG J L, et al. Modular enzymatic cascade synthesis of vitamin B5 and its derivatives[J]. Chemistry-A European Journal, 2018, 24(66): 17434-17438. |
| [16] | DONG H R, GUO N X, HU D C, et al. Chemoenzymatic total synthesis of alchivemycin A[J]. Nature Synthesis, 2024, 3(9): 1124-1133. |
| [17] | SHI Q C, ZHANG B Y, WU Z H, et al. Cascade catalytic systems for converting CO2 into C2+ products[J]. ChemSusChem, 2025, 18(7): e202401916. |
| [18] | 郭华, 张蕾, 董旭, 等. 固定化多酶级联反应器[J]. 化学进展, 2020, 32(4): 392-405. |
| GUO H, ZHANG L, DONG X, et al. Immobilized multi-enzyme cascade reactor[J]. Progress in Chemistry, 2020, 32(4): 392-405. | |
| [19] | JIANG S, LI H Q, ZHANG L, et al. Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics[J]. Nucleic Acids Research, 2025, 53(D1): D1670-D1676. |
| [20] | GAO Y, LI F, LUO Z S, et al. Modular assembly of an artificially concise biocatalytic cascade for the manufacture of phenethylisoquinoline alkaloids[J]. Nature Communications, 2024, 15: 30. |
| [21] | KHOBRAGADE T P, SARAK S, PAGAR A D, et al. Synthesis of sitagliptin intermediate by a multi-enzymatic cascade system using lipase and transaminase with benzylamine as an amino donor[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 757062. |
| [22] | SIEGEL J B, ZANGHELLINI A, LOVICK H M, et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction[J]. Science, 2010, 329(5989): 309-313. |
| [23] | WANG C, ZHANG H Y, WANG Y, et al. A general strategy for the synthesis of hierarchically ordered metal-organic frameworks with tunable macro-, meso-, and micro-pores[J]. Small, 2023, 19(3): 2206116. |
| [24] | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| [25] | RAUTER M, NIETZ D, KUNZE G. Cutinase ACut2 from blastobotrysraffinosifermentans for the selective desymmetrization of the symmetric diester diethyl adipate to the monoester monoethyl adipate[J]. Microorganisms, 2022, 10(7): 1316. |
| [26] | WANG F H, QI H B, LI H M, et al. State-of-the-art strategies and research advances for the biosynthesis of D-amino acids[J]. Critical Reviews in Biotechnology, 2024, 44(4): 495-513. |
| [27] | SUN Y P, SHU T, MA J X, et al. Rational design of ZIF-8 for constructing luminescent biosensors with glucose oxidase and AIE-type gold nanoclusters[J]. Analytical Chemistry, 2022, 94(7): 3408-3417. |
| [28] | ENGEL J, BORNSCHEUER U T, KARA S. Kinetics modeling of a convergent cascade catalyzed by monooxygenase-alcohol dehydrogenase coupled enzymes[J]. Organic Process Research & Development, 2021, 25(3): 411-420. |
| [29] | ZHANG D P, JING X R, ZHANG W L, et al. Highly selective synthesis of D-amino acids from readily available L-amino acids by a one-pot biocatalytic stereoinversion cascade[J]. RSC Advances, 2019, 9(51): 29927-29935. |
| [30] | LI S F, ZHANG W, ZHANG W, et al. Recent advances in the synthesis and analysis of atorvastatin and its intermediates[J]. Current Medicinal Chemistry, 2024, 31(37): 6063-6083. |
| [31] | TIBREWAL N, TANG Y. Biocatalysts for natural product biosynthesis[J]. Annual Review of Chemical and Biomolecular Engineering, 2014, 5: 347-366. |
| [32] | MONTERREY D T, AZCONA L, REVUELTA J, et al. Polyphosphate kinase from Burkholderia cenocepacia, one enzyme catalyzing a two-step cascade reaction to synthesize ATP from AMP[J]. International Journal of Molecular Sciences, 2024, 25(23): 12995. |
| [33] | XIAO W L, HUANG T E, ZHOU J, et al. Inhibition of MAT2A impairs skeletal muscle repair function[J]. Biomolecules, 2024, 14(9): 1098. |
| [34] | RODRIGUEZ-ABETXUKO A, REIFS A, SÁNCHEZ-DEALCÁZAR D, et al. A versatile chemoenzymatic nanoreactor that mimics NAD(P)H oxidase for the in situ regeneration of cofactors[J]. Angewandte Chemie International Edition, 2022, 61(39): e202206926. |
| [35] | SHI J F, WU Y Z, ZHANG S H, et al. Bioinspired construction of multi-enzyme catalytic systems[J]. Chemical Society Reviews, 2018, 47(12): 4295-4313. |
| [36] | PENG T, TIAN J, ZHAO Y Y, et al. Multienzyme redox system with cofactor regeneration for cyclic deracemization of sulfoxides[J]. Angewandte Chemie International Edition, 2022, 61(37): e202209272. |
| [37] | GHIMIRE N, OH T J. Cell-free system for one-pot production of protocatechuate via a two-enzyme cascade with coenzyme regeneration[J]. International Journal of Biological Macromolecules, 2025, 306: 141269. |
| [38] | ZHOU M J, BOUAZZAOUI S, JONES L E, et al. Isolation and structural determination of non-racemic tertiary cathinone derivatives[J]. Organic & Biomolecular Chemistry, 2015, 13(37): 9629-9636. |
| [39] | DEL VECCHIO D. Modularity, context-dependence, and insulation in engineered biological circuits[J]. Trends in Biotechnology, 2015, 33(2): 111-119. |
| [40] | KIM Y C, YOO H W, PARK B G, et al. One-pot biocatalytic route from alkanes to α, ω-diamines by whole-cell consortia of engineered Yarrowia lipolytica and Escherichia coli [J]. ACS Synthetic Biology, 2024, 13(7): 2188-2198. |
| [41] | SAVILE C K, JANEY J M, MUNDORFF E C, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture[J]. Science, 2010, 329(5989): 305-309. |
| [42] | HUANG X Q, FENG J Q, CUI J W, et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition[J]. Nature Catalysis, 2022, 5(7): 586-593. |
| [43] | NGUYEN L T, YANG K L. Combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase for catalyzing cascade chemical reactions[J]. Enzyme and Microbial Technology, 2017, 100: 52-59. |
| [44] | FERNANDES C G, SAWANT S C, MULE T A, et al. Enhancing cellulases through synergistic β-glucosidases for intensifying cellulose hydrolysis[J]. Process Biochemistry, 2022, 120: 202-212. |
| [45] | YU X, CHEN X Y, YU H L, et al. Regio- and stereo-selective amination of fatty acids tod-amino acids by a three-step one-pot cascade[J]. Green Chemistry, 2023, 25(9): 3469-3474. |
| [46] | CUI J D, QIU J Q, FAN X W, et al. Biotechnological production and applications of microbial phenylalanine ammonia lyase: a recent review[J]. Critical Reviews in Biotechnology, 2014, 34(3): 258-268. |
| [47] | 任杰, 曾安平. 基于二氧化碳的生物制造: 从基础研究到工业应用的挑战[J]. 合成生物学, 2021, 2(6): 854-862. |
| REN J, ZENG A P. CO2 based biomanufacturing: from basic research to industrial application[J]. Synthetic Biology Journal, 2021, 2(6): 854-862. | |
| [48] | NAM K H. Glucose isomerase: functions, structures, and applications[J]. Applied Sciences, 2022, 12(1): 428. |
| [49] | 徐铮, 徐恺, 陈昱金, 等. 异构酶在生物制造中的研究进展[J]. 食品与发酵工业, 2021, 47(5): 244-251. |
| XU Z, XU K, CHEN Y J, et al. Recent advances on isomerases for bio-manufacturing[J]. Food and Fermentation Industries, 2021, 47(5): 244-251. | |
| [50] | HAMMER S C, KUBIK G, WATKINS E, et al. Anti-Markovnikov alkene oxidation by metal-oxo-mediated enzyme catalysis[J]. Science, 2017, 358(6360): 215-218. |
| [51] | GUO J M, XU C Y, LI J, et al. Dual role of gluconic acid in the cascading saccharification of hemicellulose and cellulose from various lignocellulosic stuff[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(22): 8325-8339. |
| [52] | XU J, CEN Y X, SINGH W, et al. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters[J]. Journal of the American Chemical Society, 2019, 141(19): 7934-7945. |
| [53] | SUN S Z, NICHOLLS B T, BAIN D, et al. Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis[J]. Nature Catalysis, 2023, 7(1): 35-42. |
| [54] | LAUKO A, PELLOCK S J, SUMIDA K H, et al. Computational design of serine hydrolases[J]. Science, 388(6744): 24-54. |
| [55] | PAN Y J, LI G B, LIU R X, et al. Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acids[J]. Nature Communications, 2023, 14: 1669. |
| [56] | UTHARALA R, GRAB A, VAFAIZADEH V, et al. A microfluidic Braille valve platform for on-demand production, combinatorial screening and sorting of chemically distinct droplets[J]. Nature Protocols, 2022, 17(12): 2920-2965. |
| [57] | HOMMA F, HUANG J, VAN DER HOORN R A L. AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface[J]. Nature Communications, 2023, 14: 6040. |
| [58] | JANG T, SHIN S J, LIM H K, et al. DFT-CES2: quantum mechanics based embedding for mean-field QM/MM of solid-liquid interfaces[J]. JACS Au, 2025, 5(4): 2047-2058. |
| [59] | WU X L, YANG C, GE J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents[J]. Bioresources and Bioprocessing, 2017, 4(1): 24. |
| [60] | 董玲玲, 李斐煊, 雷航彬, 等. 仿生分区室固定化多酶体系[J]. 合成生物学, 2024, 5(6): 1518-1529. |
| DONG L L, LI F X, LEI H B, et al. Biomimetic compartmentalization immobilization of multi-enzyme system[J]. Synthetic Biology Journal, 2024, 5(6): 1518-1529. | |
| [61] | 毕春元, 任婷月, 张金玲, 等. 离子交换树脂共固定葡萄糖氧化酶-过氧化氢酶[J]. 食品与发酵工业, 2015, 41(7): 13-18. |
| BI C Y, REN T Y, ZHANG J L, et al. Co-immobilization of glucose oxidase and catalase on ion exchange resin[J]. Food and Fermentation Industries, 2015, 41(7): 13-18. | |
| [62] | WILLIAMS V, CUI Y X, JIANG X J, et al. Co-immobilized multienzyme system for the cofactor-driven cascade synthesis of (R)-2-amino-3-(2-bromophenyl)propanoic acid: a model reaction[J]. Organic Process Research & Development, 2022, 26(11): 3024-3033. |
| [63] | KIM M, LEE C, JEON K, et al. Harnessing a paper-folding mechanism for reconfigurable DNA origami[J]. Nature, 2023, 619(7968): 78-86. |
| [64] | WANG Y, SELIVANOVITCH E, DOUGLAS T. Enhancing multistep reactions: biomimetic design of substrate channeling using P22 virus-like particles[J]. Advanced Science, 2023, 10(13): 2206906. |
| [65] | YANG L, YUAN Q Y, LI T T, et al. Recent developments and applications of pH-responsive polymers[J]. Textile Research Journal, 2025: 00405175241305543. |
| [66] | GODOY-GALLARDO M, LABAY C, TRIKALITIS V D, et al. Multicompartment artificial organelles conducting enzymatic cascade reactions inside cells[J]. ACS Applied Materials & Interfaces, 2017, 9(19): 15907-15921. |
| [67] | YE J J, CHU T S, CHU J L, et al. A versatile approach for enzyme immobilization using chemically modified 3D-printed scaffolds[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(21): 18048-18054. |
| [68] | 苏枫. 微生物脂肪酶的固定化新技术及在生物柴油制备中的应用[D]. 武汉: 华中科技大学, 2017. |
| SU F. Improving performance of microbial lipases via new immobilization technology and application in biodiesel preparation[D]. Wuhan: Huazhong University of Science and Technology, 2017. | |
| [69] | 陈坚, Omasa Takeshi, Katakura Yoshio, 等. 固定化酶-离子交换组合系统进行青霉素G水解生产6-APA的模型化研究[J]. 生物工程学报, 1995, 11(4): 343-349. |
| CHEN J, OMASA T, KATAKURA Y, et al. Modeling of penicillin G hydrolysis to 6-APA in an immobilized enzyme-ion exchange system[J]. Chinese Journal of Biotechnology, 1995, 11(4): 343-349. | |
| [70] | 万娟娟, 刘旭峰, 戎海波, 等. 酶固定化技术及固定化酶应用的研究进展[J]. 现代化工, 2024, 44(S1): 73-79. |
| WAN J J, LIU X F, RONG H B, et al. An overview of enzyme immobilizing technologies and application of immobilized enzymes[J]. Modern Chemical Industry, 2024, 44(S1): 73-79. | |
| [71] | 尚红岩, 翁绮纹, 练文妃, 等. 纤维素酶固定化工艺条件优化及在甘蔗渣酶解中的应用[J]. 甘蔗糖业, 2024, 53(5): 42-48. |
| SHANG H Y, WENG Q W, LIAN W F, et al. Optimization of cellulase immobilization process conditions and its application in sugarcane bagasse enzymatic hydrolysis[J]. Sugarcane and Canesugar, 2024, 53(5): 42-48. | |
| [72] | 徐惠东, 尤扬, 游颖欣, 等. 一种高耐热乳糖酶的异源表达、固定化及酶学性质研究[J]. 食品与发酵工业, 2024, 50(21): 1-8. |
| XU H D, YOU Y, YOU Y X, et al. Heterologous expression and enzymatic characterization of a highly thermostable lactase and its immobilized enzyme[J]. Food and Fermentation Industries, 2024, 50(21): 1-8. | |
| [73] | TANG C D, ZHANG Z H, SHI H L, et al. Directed evolution of formate dehydrogenase and its application in the biosynthesis of L-phenylglycine from phenylglyoxylic acid[J]. Molecular Catalysis, 2021, 513: 111666. |
| [74] | GORAN J M, FAVELA C A, STEVENSON K J. Investigating the electrocatalytic oxidation of dihydronicotinamide adenine dinucleotide at nitrogen-doped carbon nanotube electrodes: implications to electrochemically measuring dehydrogenase enzyme kinetics[J]. ACS Catalysis, 2014, 4(9): 2969-2976. |
| [75] | LIU T, YIN Y X, YANG Y, et al. Layer-by-layer engineered all-liquid microfluidic chips for enzyme immobilization[J]. Advanced Materials, 2022, 34(5): 2105386. |
| [76] | LI G R, MA W D, YANG Y X, et al. Nanoscale covalent organic frameworks with donor-acceptor structures as highly efficient light-responsive oxidase-like mimics for colorimetric detection of glutathione[J]. ACS Applied Materials & Interfaces, 2021, 13(41): 49482-49489. |
| [77] | CHEN Y, TAO K, JI W, et al. Histidine as a key modulator of molecular self-assembly: peptide-based supramolecular materials inspired by biological systems[J]. Materials Today, 2022, 60: 106-127. |
| [78] | KIGHTLINGER W, DUNCKER K E, RAMESH A, et al. A cell-free biosynthesis platform for modular construction of protein glycosylation pathways[J]. Nature Communications, 2019, 10: 5404. |
| [79] | SU H H, GUO Z W, WU X L, et al. Efficient bioconversion of sucrose to high-value-added glucaric acid by in vitro metabolic engineering[J]. ChemSusChem, 2019, 12(10): 2278-2285. |
| [80] | SUN Z B, XU J L, LU X, et al. Directed mutation of β-glucanases from probiotics to enhance enzymatic activity, thermal and pH stability[J]. Archives of Microbiology, 2020, 202(7): 1749-1756. |
| [81] | BACHOSZ K, ZDARTA J, BILAL M, et al. Enzymatic cofactor regeneration systems: a new perspective on efficiency assessment[J]. Science of the Total Environment, 2023, 868: 161630. |
| [82] | HOLZMAN D C. The carbon footprint of biofuels: can we shrink it down to size in time?[J]. Environmental Health Perspectives, 2008, 116(6): A246-A252. |
| [83] | KHOBRAGADE T P, PAGAR A D, GIRI P, et al. Biocatalytic cascade for synthesis of sitagliptin intermediate employing coupled transaminase[J]. Biotechnology and Bioprocess Engineering, 2023, 28(2): 300-309. |
| [84] | 曹熙. 一种固定化脂肪酶的方法及其在生物柴油反应中的应用[D]. 北京: 北京化工大学, 2015. |
| CAO X. A method for fixing lipase and its application in bio-diesel reactions[D]. Beijing: Beijing University of Chemical Technology, 2015. | |
| [85] | TING W-W, NISHIKAWA S, YU W-C, et al. Chemo-enzymatic synthesis of coenzyme a using copurified enzymes from probiotic Escherichia coli Nissle[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(27): 10068-10074. |
| [86] | PAN Y J, LIU Y F, PHAN T L, et al. Biomanufacturing of inositol from corn stover with biological pretreatment by an in vitro synthetic biology platform[J]. ACS Sustainable Chemistry & Engineering, 2025, 13(1): 436-446. |
| [87] | LI Z L, NING X, ZHAO Y R, et al. Efficient one-pot synthesis of cytidine 5'-monophosphate using an extremophilic enzyme cascade system[J]. Journal of Agricultural and Food Chemistry, 2020, 68(34): 9188-9194. |
| [88] | FERREIRA S, BALOLA A, SVESHNIKOVA A, et al. Computer-aided design and implementation of efficient biosynthetic pathways to produce high added-value products derived from tyrosine in Escherichia coli [J]. Frontiers in Bioengineering and Biotechnology, 2024, 12: 1360740. |
| [89] | IMAM H T, MARR P C, MARR A C. Enzyme entrapment, biocatalyst immobilization without covalent attachment[J]. Green Chemistry, 2021, 23(14): 4980-5005. |
| [90] | YU T H, CUI H Y, LI J C, et al. Enzyme function prediction using contrastive learning[J]. Science, 2023, 379(6639): 1358-1363. |
| [91] | LI R F, WIJMA H J, SONG L, et al. Computational redesign of enzymes for regio- and enantioselective hydroamination[J]. Nature Chemical Biology, 2018, 14(7): 664-670. |
| [92] | JIAO Y F, WANG H Y, WANG H, et al. A DNA origami-based enzymatic cascade nanoreactor for chemodynamic cancer therapy and activation of antitumor immunity[J]. Science Advances, 2025, 11(2): eadr9196. |
| [93] | XU K Q, CHATZITAKIS A, BACKE P H, et al. In situ cofactor regeneration enables selective CO2 reduction in a stable and efficient enzymatic photoelectrochemical cell[J]. Applied Catalysis B: Environmental, 2021, 296: 120349. |
| [94] | 支睿, 李国辉, 毛银, 等. 己二酸生物合成的途径改造以及发酵条件优化[J]. 食品与发酵工业, 2024, 50(3): 38-44. |
| ZHI R, LI G H, MAO Y, et al. Metabolic pathway and fermentation optimization of the biosynthesis of adipic acid[J]. Food and Fermentation Industries, 2024, 50(3): 38-44. | |
| [95] | WANG F, ZHAO J, LI Q, et al. One-pot biocatalytic route from cycloalkanes to α, ω‐dicarboxylic acids by designed Escherichia coli consortia[J]. Nature Communications, 2020, 11: 5035. |
| [96] | LI X Y, CAO Y F, LUO K, et al. Highly active enzyme-metal nanohybrids synthesized in protein-polymer conjugates[J]. Nature Catalysis, 2019, 2(8): 718-725. |
| [97] | 郭艺鸣, 姜君逸, 潘学玮, 等. 多酶级联反应催化γ-丁内酯生成1,4-丁二醇[J]. 应用与环境生物学报, 2024, 30(1): 167-175. |
| GUO Y M, JIANG J Y, PAN X W, et al. Multi-enzyme cascade reaction catalyzed γ-butyrolactone to 1,4-butanediol[J]. Chinese Journal of Applied and Environmental Biology, 2024, 30(1): 167-175. | |
| [98] | 姜君逸, 郭艺鸣, 杨套伟, 等. 代谢工程改造大肠杆菌从头合成1,4-丁二醇[J]. 生物工程学报, 2024, 40(9): 3142-3157. |
| JIANG J Y, GUO Y M, YANG T W, et al. Metabolic engineering of Escherichia coli for de novo synthesis of 1,4-butanediol[J]. Chinese Journal of Biotechnology, 2024, 40(9): 3142-3157. | |
| [99] | 田灵芝, 周俊平, 杨套伟, 等. 基于多酶级联协调表达策略高效催化合成(S)-2-羟基丁酸[J]. 生物工程学报, 2021, 37(12): 4231-4242. |
| TIAN L Z, ZHOU J P, YANG T W, et al. Efficient cascade biosynthesis of (S)-2-hydroxybutyric acid[J]. Chinese Journal of Biotechnology, 2021, 37(12): 4231-4242. | |
| [100] | BENÍTEZ-MATEOS A I, ROURA PADROSA D, PARADISI F. Multistep enzyme cascades as a route towards green and sustainable pharmaceutical syntheses[J]. Nature Chemistry, 2022, 14(5): 489-499. |
| [101] | LIU L, WANG D H, CHEN F F, et al. Development of an engineered thermostable amine dehydrogenase for the synthesis of structurally diverse chiral amines[J]. Catalysis Science & Technology, 2020, 10(8): 2353-2358. |
| [102] | 王高杨. 肌醇-1-磷酸合成酶的固定化及其在肌醇合成中应用[D]. 天津: 天津科技大学, 2021. |
| WANG G Y. Immobilization of inositol-1-phosphate synthase and its application in inositol synthesis[D]. Tianjin: Tianjin University of Science & Technology, 2021. | |
| [103] | 魏梓佳, 樊宇成, 张槿博, 等. 肌醇-1-磷酸合酶的重组表达及在多酶级联催化合成肌醇中的应用[J]. 食品与发酵工业, 2025, 51(4): 280-287. |
| WEI Z J, FAN Y C, ZHANG J B, et al. Recombinant expression of inositol-1-phosphate synthase and its application in multienzyme cascade catalytic synthesis of inositol[J]. Food and Fermentation Industries, 2025, 51(4): 280-287. | |
| [104] | 潘珊, 胡孟凯, 潘学玮, 等. 基于双酶级联协调表达策略高效催化合成D-甘露醇[J]. 生物工程学报, 2022, 38(7): 2549-2565. |
| PAN S, HU M K, PAN X W, et al. Efficient biosynthesis of D-mannitol by coordinated expression of a two-enzyme cascade[J]. Chinese Journal of Biotechnology, 2022, 38(7): 2549-2565. | |
| [105] | 张建志, 付立豪, 唐婷, 等. 基于合成生物学策略的酶蛋白元件规模化挖掘[J]. 合成生物学, 2020, 1(3): 319-336. |
| ZHANG J Z, FU L H, TANG T, et al. Scalable mining of proteins for biocatalysis via synthetic biology[J]. Synthetic Biology Journal, 2020, 1(3): 319-336. | |
| [106] | 曲戈, 朱彤, 蒋迎迎, 等. 蛋白质工程: 从定向进化到计算设计[J]. 生物工程学报, 2019, 35(10): 1843-1856. |
| QU G, ZHU T, JIANG Y Y, et al. Protein engineering: from directed evolution to computational design[J]. Chinese Journal of Biotechnology, 2019, 35(10): 1843-1856. | |
| [107] | KASHINATH K P, SANJAY L R, ASHOKBHAI M K, et al. Continuous manufacturing based paradigm shift in pharmaceuticals production and current regulatory framework[J]. Chemical Engineering Research and Design, 2025, 215: 1-22. |
| [108] | 石婷, 宋展, 宋世怡, 等. 体外生物转化(ivBT): 生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| SHI T, SONG Z, SONG S Y, et al. In vitro bio transformation(ivBT): a new frontier of industrial biomanufacturing[J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [1] | 王明鹏, 陈蕾, 赵一冉, 张祎慜, 郑琪帆, 刘馨阳, 王毅学, 王钦宏. 卤化酶在生物催化中的应用:机制解析、定向进化和绿色制造的进展[J]. 合成生物学, 2025, 6(4): 728-763. |
| [2] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [3] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [4] | 康里奇, 谈攀, 洪亮. 人工智能时代下的酶工程[J]. 合成生物学, 2023, 4(3): 524-534. |
| [5] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [6] | 崔馨予, 吴冉冉, 王园明, 朱之光. 酶促生物电催化系统的设计构建与强化[J]. 合成生物学, 2022, 3(5): 1006-1030. |
| [7] | 杨璐, 瞿旭东. 亚胺还原酶在手性胺合成中的应用[J]. 合成生物学, 2022, 3(3): 516-529. |
| [8] | 熊亮斌, 宋璐, 赵云秋, 刘坤, 刘勇军, 王风清, 魏东芝. 甾体化合物绿色生物制造:从生物转化到微生物从头合成[J]. 合成生物学, 2021, 2(6): 942-963. |
| [9] | 吴淑可, 周颐, 王文, 张巍, 高鹏飞, 李智. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558. |
| [10] | 张以恒. 忆王义翘教授对生物炼制的贡献和我对此领域未来发展的观点[J]. 合成生物学, 2021, 2(4): 497-508. |
| [11] | 史然, 江正强. 2'-岩藻糖基乳糖的酶法合成研究进展和展望[J]. 合成生物学, 2020, 1(4): 481-494. |
| [12] | 许可, 王靖楠, 李春. 智能抗逆微生物细胞工厂与绿色生物制造[J]. 合成生物学, 2020, 1(4): 427-439. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||