Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (6): 1191-1222.DOI: 10.12211/2096-8280.2023-041
• Invited Review • Previous Articles Next Articles
Electro-assisted carbon dioxide biotransformation
LIU Weisong1,2, ZHANG Kuncheng1,2, CUI Huijuan1, ZHU Zhiguang1,2, ZHANG Yiheng1,2, ZHANG Lingling1,2
- 1.Key Laboratory of Engineering Biology for Low-Carbon Manufacturing,Tianjin Institute of Industrial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2023-06-25Revised:2023-08-28Online:2024-01-19Published:2023-12-31 -
Contact:ZHANG Lingling
电能辅助二氧化碳生物转化
刘伟松1,2, 张坤城1,2, 崔会娟1, 朱之光1,2, 张以恒1,2, 张玲玲1,2
- 1.中国科学院天津工业生物技术研究所,低碳合成工程生物学重点实验室,天津 300308
2.中国科学院大学,北京 100049
-
通讯作者:张玲玲 -
作者简介:刘伟松 (1997—),男,博士研究生。研究方向为酶电催化CO2还原。E-mail:liuws@tib.cas.cn张玲玲 (1988—),女,研究员,博士生导师。研究方向为酶工程、酶电合成和酶燃料电池等。E-mail:zhangll@tib.cas.cn -
基金资助:天津市合成生物技术创新能力提升行动项目(TSBICIP-CXRC-024);中国科学院稳定支持基础研究领域青年团队计划(YSBR-072-3)
CLC Number:
Cite this article
LIU Weisong, ZHANG Kuncheng, CUI Huijuan, ZHU Zhiguang, ZHANG Yiheng, ZHANG Lingling. Electro-assisted carbon dioxide biotransformation[J]. Synthetic Biology Journal, 2023, 4(6): 1191-1222.
刘伟松, 张坤城, 崔会娟, 朱之光, 张以恒, 张玲玲. 电能辅助二氧化碳生物转化[J]. 合成生物学, 2023, 4(6): 1191-1222.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-041
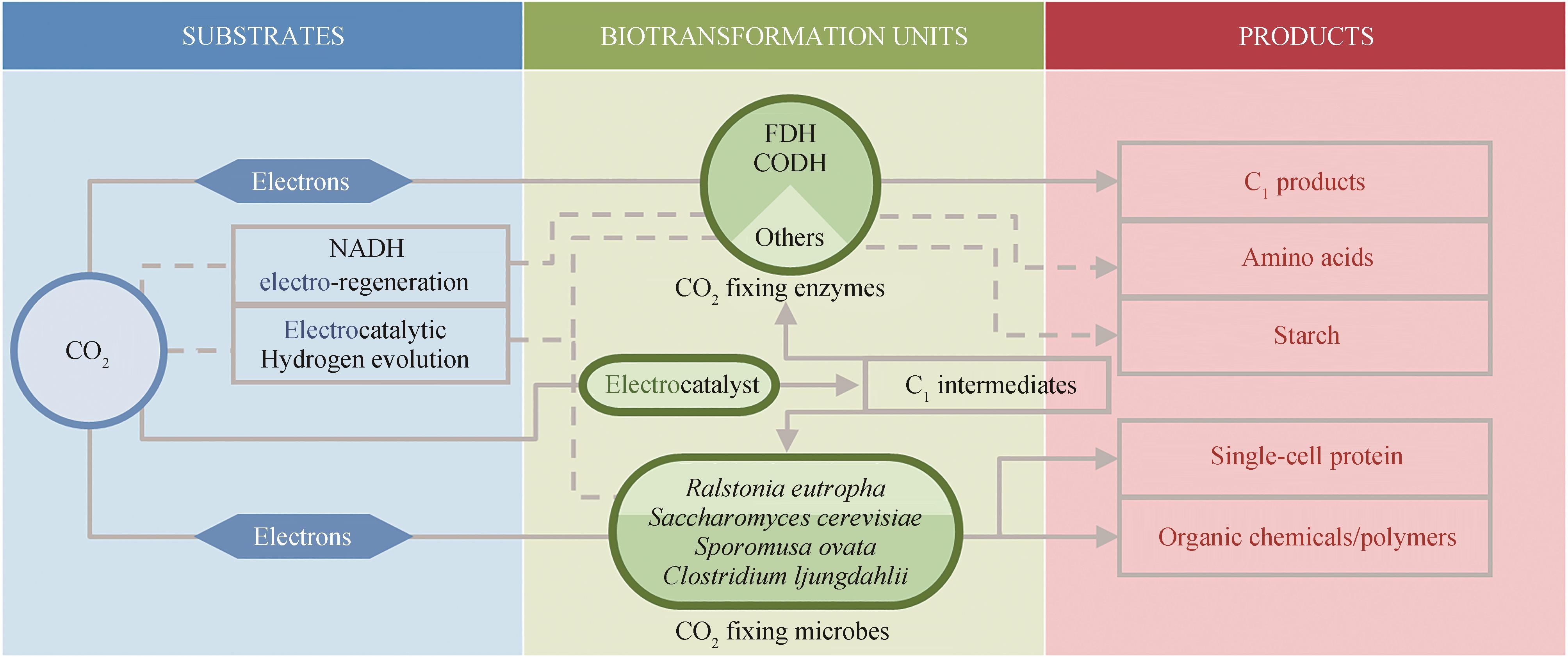
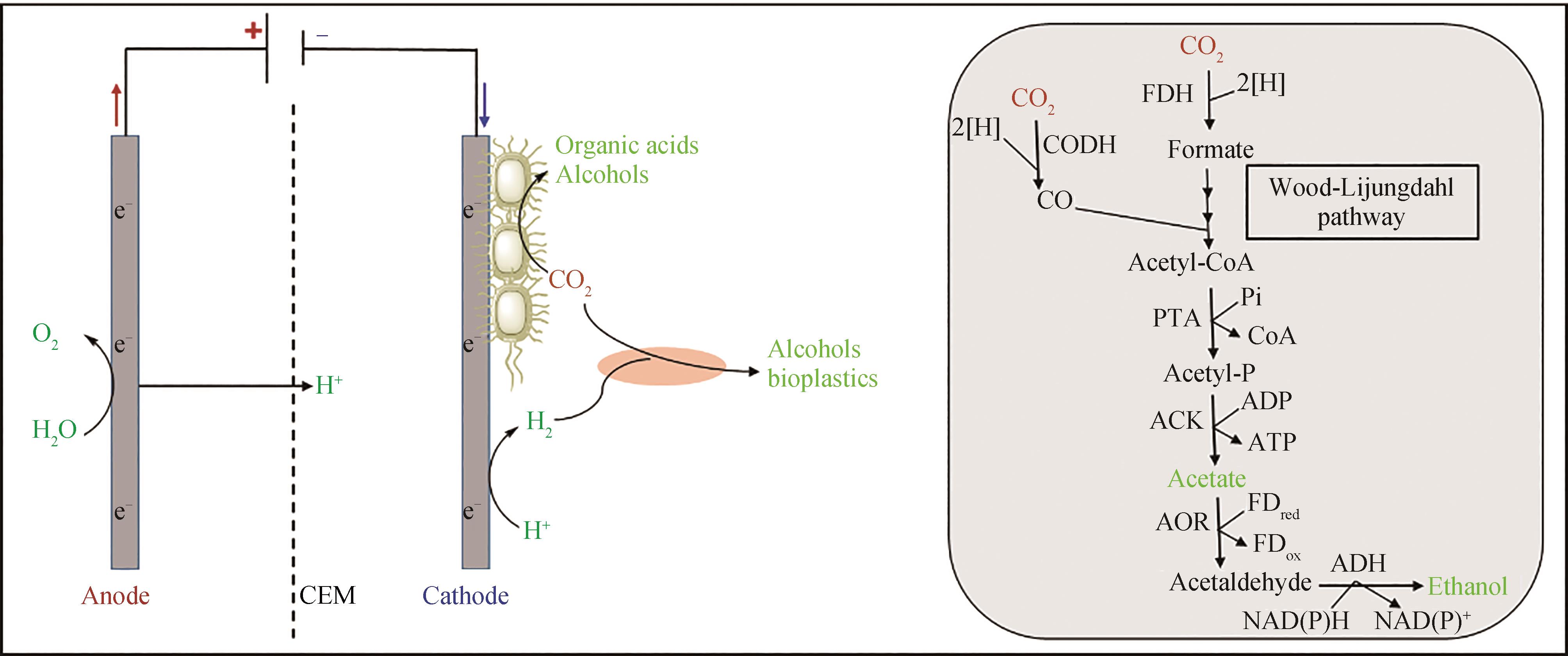
Fig. 1 Schematic diagram of common strategies for electro-assisted carbon dioxide biotransformation(Solid line: direct use of electric energy transformation route; Dashed line: indirect use of electric energy transformation route)
分类 Classification | 酶 Enzymes | 物种 Organism | 类型 Classification | 电子供体 Electron donor | kcat/s | 参考文献 References |
|---|---|---|---|---|---|---|
| Metal-independent | CbFDH | Candida boidinii | NAD+-dependent | NADH | 0.1 | [ |
| TsFDH | Thiobacillus sp.KNK65MA | NAD+-dependent | NADH | 0.3 | [ | |
| MtFDH | Myceliophthora thermophila | NAD+-dependent | NADH | 0.1 | [ | |
| CmFDH | Candida methylica | NAD+-dependent | NADH | 0.01 | [ | |
| CtFDH | Chaetomium thermophilum | NAD+-dependent | NADH | 0.02 | [ | |
| PoFDH | Pseudornonas oxalaticus | NAD+-dependent | NADH | 3 | [ | |
| Metal-dependent | SfFDH-1 | Syntrophobacter fumaroxidans | W-containing | MV+ | 0.04 | [ |
| SfFDH-2 | Syntrophobacter fumaroxidans | W-containing | MV+ | 0.01 | [ | |
| DvFDH | Desulfovibrio vulgaris Hildenborough | W-containing | MV+ | 3 | [ | |
| TkFDH | Thermoanaerobacter kuvui | W-containing | H2 | 2654 | [ | |
| CcFDH | Clostridium carboxidivorans | W-containing | NADH | 0.08 | [ | |
| EcFDH | Escherichia coli | Mo-containing | MV+ | <1 | [ | |
| ClFDH | Clostridium ljungdahlii | Mo-containing | NADH | 0.01 | [ | |
| RcFDH | Rhodobacter capsulatus | Mo-containing | NADH | 1.48 | [ | |
| DdFDH | Desulfovibrio desulfuricans | Mo-containing | MV+ | 46.6 | [ | |
| CnFDH | Cupriavidus necator | Mo-containing | NADH | 11 | [ | |
| RaFDH | Rhodobacter aestuarii | Mo-containing | NADH | 0.8 | [ | |
| AwFDH | Acetobacterium woodii | Mo-containing | H2 | 28 | [ |
Table 1 Metal-independent and -dependent formate dehydrogenases for CO2 reduction
分类 Classification | 酶 Enzymes | 物种 Organism | 类型 Classification | 电子供体 Electron donor | kcat/s | 参考文献 References |
|---|---|---|---|---|---|---|
| Metal-independent | CbFDH | Candida boidinii | NAD+-dependent | NADH | 0.1 | [ |
| TsFDH | Thiobacillus sp.KNK65MA | NAD+-dependent | NADH | 0.3 | [ | |
| MtFDH | Myceliophthora thermophila | NAD+-dependent | NADH | 0.1 | [ | |
| CmFDH | Candida methylica | NAD+-dependent | NADH | 0.01 | [ | |
| CtFDH | Chaetomium thermophilum | NAD+-dependent | NADH | 0.02 | [ | |
| PoFDH | Pseudornonas oxalaticus | NAD+-dependent | NADH | 3 | [ | |
| Metal-dependent | SfFDH-1 | Syntrophobacter fumaroxidans | W-containing | MV+ | 0.04 | [ |
| SfFDH-2 | Syntrophobacter fumaroxidans | W-containing | MV+ | 0.01 | [ | |
| DvFDH | Desulfovibrio vulgaris Hildenborough | W-containing | MV+ | 3 | [ | |
| TkFDH | Thermoanaerobacter kuvui | W-containing | H2 | 2654 | [ | |
| CcFDH | Clostridium carboxidivorans | W-containing | NADH | 0.08 | [ | |
| EcFDH | Escherichia coli | Mo-containing | MV+ | <1 | [ | |
| ClFDH | Clostridium ljungdahlii | Mo-containing | NADH | 0.01 | [ | |
| RcFDH | Rhodobacter capsulatus | Mo-containing | NADH | 1.48 | [ | |
| DdFDH | Desulfovibrio desulfuricans | Mo-containing | MV+ | 46.6 | [ | |
| CnFDH | Cupriavidus necator | Mo-containing | NADH | 11 | [ | |
| RaFDH | Rhodobacter aestuarii | Mo-containing | NADH | 0.8 | [ | |
| AwFDH | Acetobacterium woodii | Mo-containing | H2 | 28 | [ |
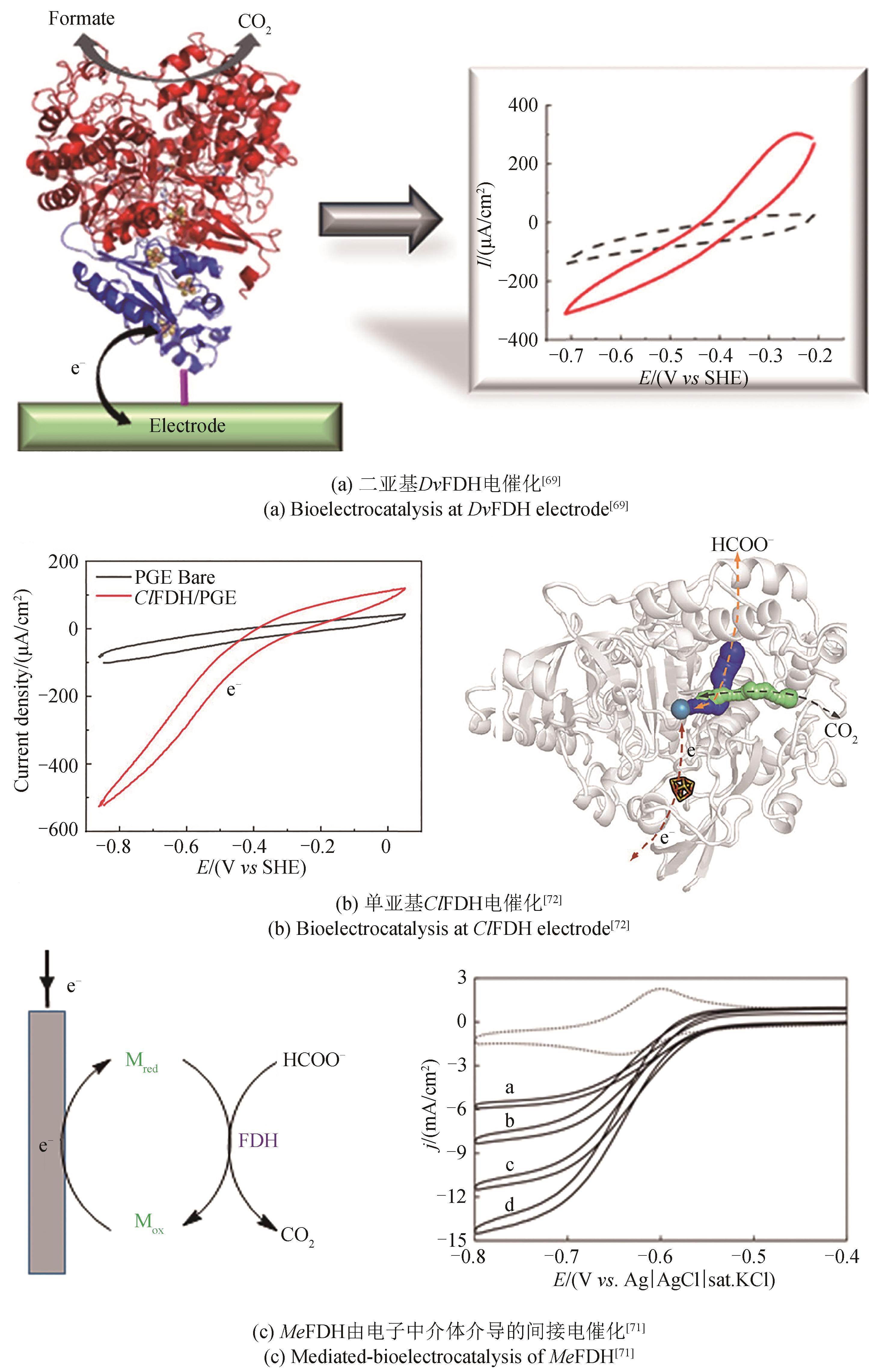
酶 Enzymes | 阴极材料 Cathode material | 电势 Potential /(V vs SHE) | 电流密度Current density /(μA/cm2) | 产率 Productivity | 产物 Product | 参考文献 References |
|---|---|---|---|---|---|---|
| PoFDH | p-type indium phosphide | -0.51 | n.d. | 1.5① | Formate | [ |
| CbFDH | Cu foil | -0.8 | n.d. | 2.1×10-3② | Formate | [ |
| CbFDH | Cu nanocrystals | -0.8 | n.d. | 6.8×10-3② | Formate | [ |
| CbFDH | Plain graphite rod | -0.8 | -3.09 | 0.15② | Formate | [ |
| CbFDH | Graphite | -0.8 | -0.20 | 28.9② | Formate | [ |
| CbFDH | Copper foam | -0.9 | n.d. | 3.6① | Formate | [ |
| CbFDH | PEI@SBA-15 | -0.51 | n.d. | 88.7② | Formate | [ |
| CbFDH | UiO-66-NH2 | -0.51 | n.d. | 101② | Formate | [ |
| CbFDH | ZIF-8 | -0.51 | n.d. | 76② | Formate | [ |
| TsFDH | Cu nanoparticles | -0.81 | n.d. | 3.6② | Formate | [ |
| CmFDH | Screen-printed gold | -0.81 | -35 | n.d.③ | Formate | [ |
| CbFDH | Carbon felt | -0.81 | n.d. | 0.02① | Formate | [ |
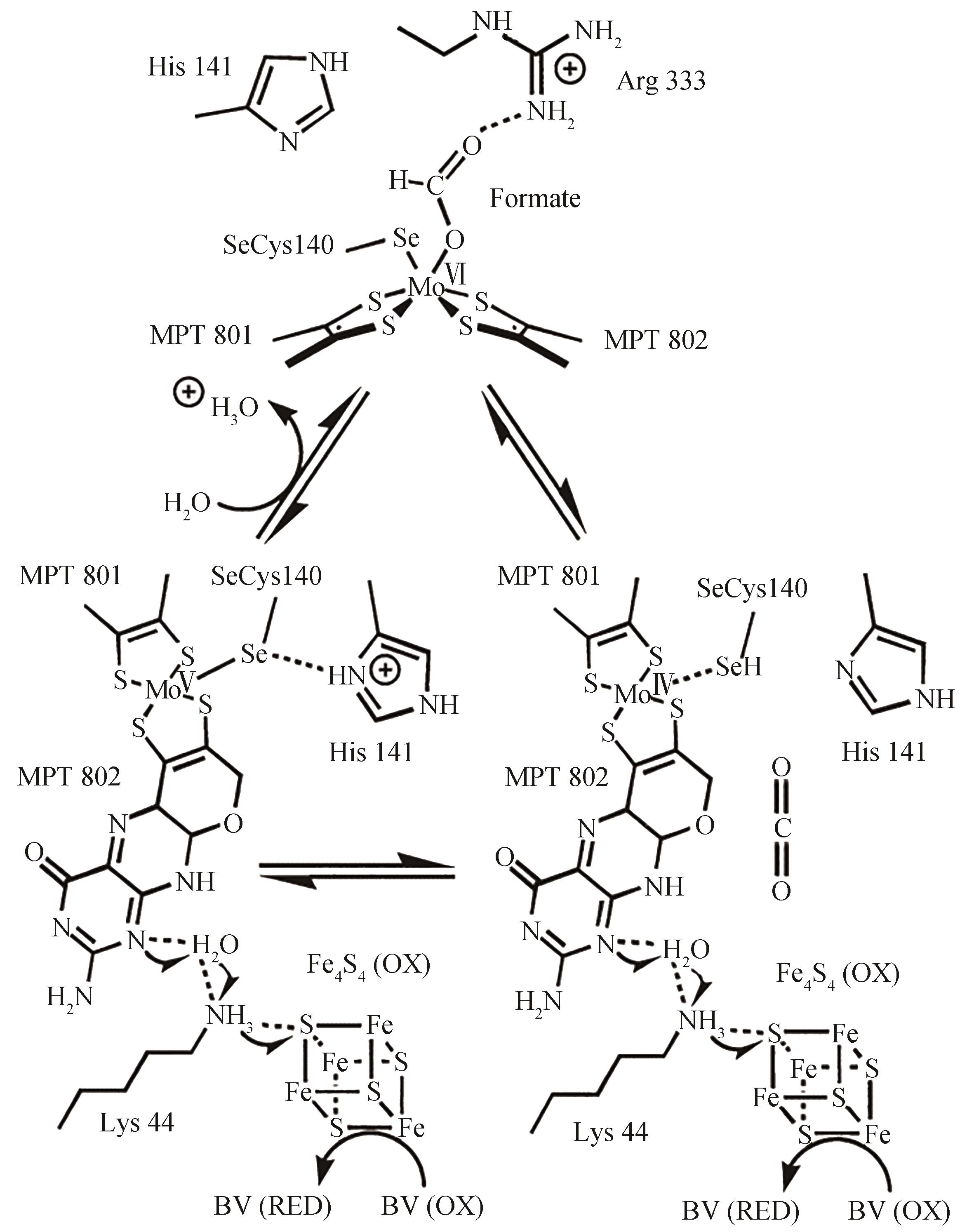
| DdFDH | Pyrolytic graphite disk | -0.5 | -0.1 | n.d.③ | Formate | [ |
| SfFDH | Pyrolytic graphite | -0.6 | -80 | n.d. | Formate | [ |
| DvFDH | Gold or graphite electrodes | -0.66 | -300 | 0.21① | Formate | [ |
| DvFDH | Gold disk electrode | -0.6 | -14.4 | n.d. | Formate | [ |
| ClFDH | Polyaniline (PANi) hydrogel | -0.6 | -3.02 | 1.42① | Formate | [ |
| Me-FoFDH | Glassy carbon | -0.54 | -20 000 | 6① | Formate | [ |
| DvFDH | P(SS-GMA-BA) | -0.59 | -533 | 0.05① | Formate | [ |
| ClFDH | Pyrolytic graphite electrode | -0.8 | -1200 | 981② | Formate | [ |
| DvFDH | IO-TiO2 electrode | n.d. | -99 | 0.185① | Formate | [ |
| DvFDH | (ITO)/TiO2 | -0.6 | -100 | 0.102① | Formate | [ |
| DvFDH | Perovskite/ITO-TiO2 | -0.47 | -4.75 | 77.3① | Formate | [ |
| DvFDH | TiO2 | n.d. | n.d. | 2.7×10-5 ② | Formate | [ |
| CcFDH, FaldDH, ADH | Cobalt phosphate/α-Fe2O3 | -0.6 | -480 | 0.021② | Methanol | [ |
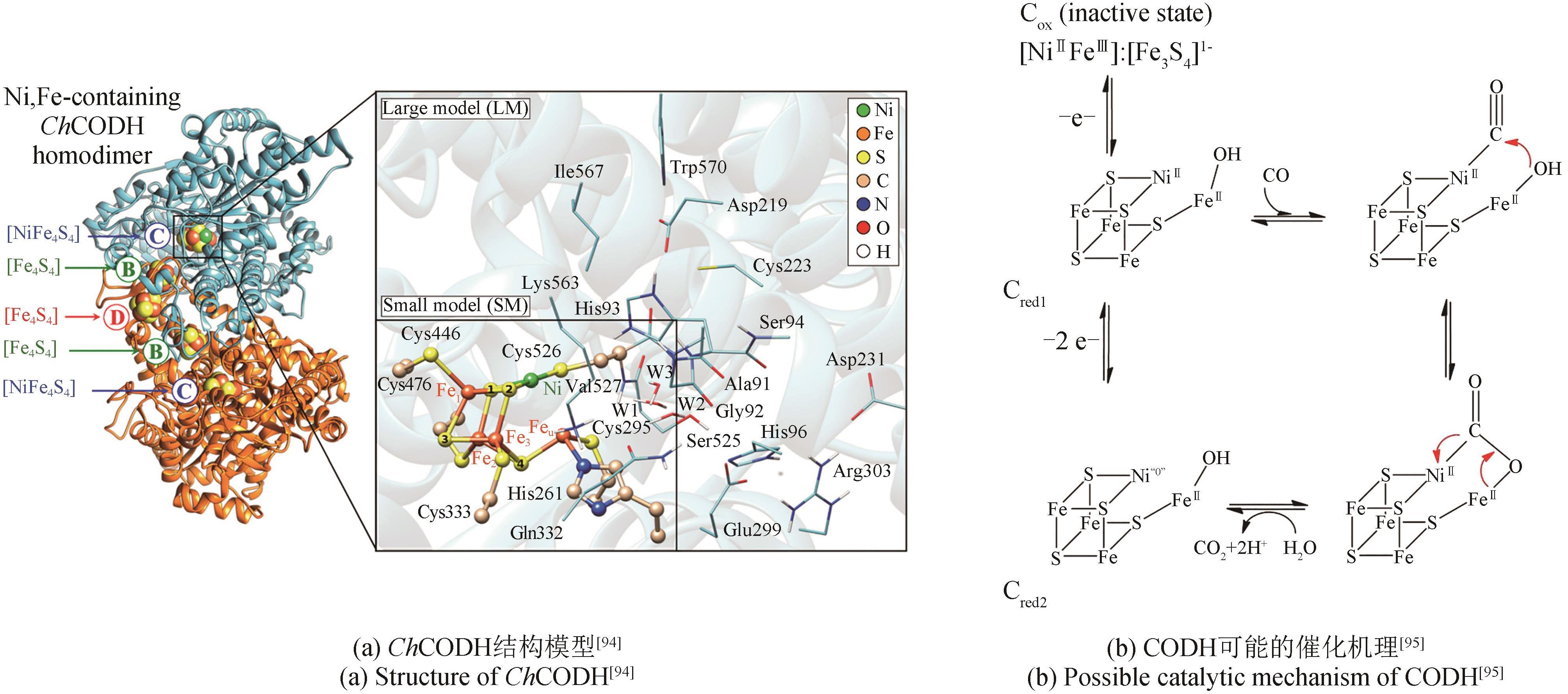
| CbFDH, FaldDH, ADH | ZIF-8 nanocrystals | -0.5 | -400 | 0.013① | Methanol | [ |
Table 2 FDH electrocatalyzes carbon dioxide reduction
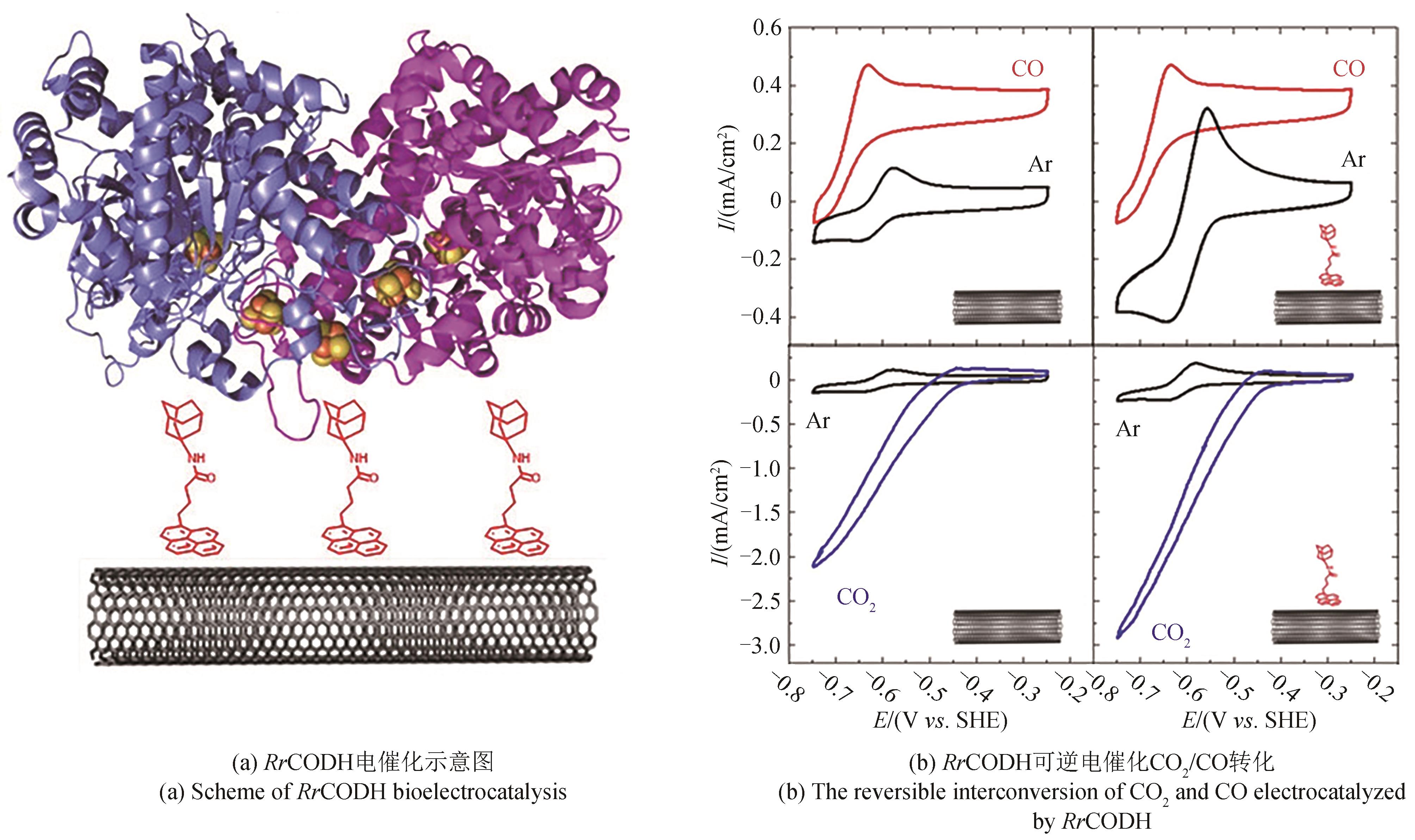
酶 Enzymes | 阴极材料 Cathode material | 电势 Potential /(V vs SHE) | 电流密度Current density /(μA/cm2) | 产率 Productivity | 产物 Product | 参考文献 References |
|---|---|---|---|---|---|---|
| PoFDH | p-type indium phosphide | -0.51 | n.d. | 1.5① | Formate | [ |
| CbFDH | Cu foil | -0.8 | n.d. | 2.1×10-3② | Formate | [ |
| CbFDH | Cu nanocrystals | -0.8 | n.d. | 6.8×10-3② | Formate | [ |
| CbFDH | Plain graphite rod | -0.8 | -3.09 | 0.15② | Formate | [ |
| CbFDH | Graphite | -0.8 | -0.20 | 28.9② | Formate | [ |
| CbFDH | Copper foam | -0.9 | n.d. | 3.6① | Formate | [ |
| CbFDH | PEI@SBA-15 | -0.51 | n.d. | 88.7② | Formate | [ |
| CbFDH | UiO-66-NH2 | -0.51 | n.d. | 101② | Formate | [ |
| CbFDH | ZIF-8 | -0.51 | n.d. | 76② | Formate | [ |
| TsFDH | Cu nanoparticles | -0.81 | n.d. | 3.6② | Formate | [ |
| CmFDH | Screen-printed gold | -0.81 | -35 | n.d.③ | Formate | [ |
| CbFDH | Carbon felt | -0.81 | n.d. | 0.02① | Formate | [ |
| DdFDH | Pyrolytic graphite disk | -0.5 | -0.1 | n.d.③ | Formate | [ |
| SfFDH | Pyrolytic graphite | -0.6 | -80 | n.d. | Formate | [ |
| DvFDH | Gold or graphite electrodes | -0.66 | -300 | 0.21① | Formate | [ |
| DvFDH | Gold disk electrode | -0.6 | -14.4 | n.d. | Formate | [ |
| ClFDH | Polyaniline (PANi) hydrogel | -0.6 | -3.02 | 1.42① | Formate | [ |
| Me-FoFDH | Glassy carbon | -0.54 | -20 000 | 6① | Formate | [ |
| DvFDH | P(SS-GMA-BA) | -0.59 | -533 | 0.05① | Formate | [ |
| ClFDH | Pyrolytic graphite electrode | -0.8 | -1200 | 981② | Formate | [ |
| DvFDH | IO-TiO2 electrode | n.d. | -99 | 0.185① | Formate | [ |
| DvFDH | (ITO)/TiO2 | -0.6 | -100 | 0.102① | Formate | [ |
| DvFDH | Perovskite/ITO-TiO2 | -0.47 | -4.75 | 77.3① | Formate | [ |
| DvFDH | TiO2 | n.d. | n.d. | 2.7×10-5 ② | Formate | [ |
| CcFDH, FaldDH, ADH | Cobalt phosphate/α-Fe2O3 | -0.6 | -480 | 0.021② | Methanol | [ |
| CbFDH, FaldDH, ADH | ZIF-8 nanocrystals | -0.5 | -400 | 0.013① | Methanol | [ |
产物 Product | 微生物 Microorganism | 阴极 Cathode | 静电位控制 Potentiostatic control /(V vs SHE) | 最高产率 Highest productionrate /[g( L·d)] | 参考文献 References |
|---|---|---|---|---|---|
| Acetate | Microbial community | Granular graphite | -0.59 | 0.24 | [ |
| Microbial community | Granular graphite | -0.59 | 1.04 | [ | |
| Microbial community | Carbon felt | -1.26 | 0.06 | [ | |
| Microbial community | Reticulated vitreous carbon foam | n.d. | 0.24 | [ | |
| Microbial community | VITO-CoRE™ | -0.40 | 0.57 | [ | |
| Microbial community | EPD-3D | -0.85 | 0.39 | [ | |
| Microbial community | Granular graphite | -0.6 | 3.10 | [ | |
| Microbial community | Reticulated vitreous carbon foam | n.d. | 18.72 | [ | |
| Microbial community | 3D-reticulated vitreous carbon | -1.10 | 77 | [ | |
| Microbial community | Carbon cloth | -0.6 | 0.03 | [ | |
| Sporomusa ovata | Graphite sticks | -0.6 | 0.045 | [ | |
| Sporomusa ovata | rGO-TEPA-modified carbon cloth | -0.69 | 0.17 | [ | |
| Sporomusa ovata | 3D-graphene/carbon felt | -0.6 | 0.12 | [ | |
| Sporomusa ovata | PEDOT: PSS modified carbon cloth | -0.69 | 0.17 | [ | |
| Sporomusa ovata | Copper foam coated graphene | -1.0 | 1.46 | [ | |
| Sporomusa ovata | Synthetic biofilm by 3D bio-printing | -0.6 | 0.68 | [ | |
| Sporomusa ovata | Ni-PHFs modified carbon nanotubes | -0.4 | 0.17 | [ | |
| Sporomusa ovata | Co-P alloy | -0.54 | 1.6 | [ | |
| Clostridium ljungdahlii | Graphite plate | -0.8 | 0.138 | [ | |
| Moorella thermoacetica | Carbon cloth | -0.4 | 0.35 | [ | |
| Butyrate | Microbial community | Carbon cloth | -0.8 | 0.16 | [ |
| Microbial community | Graphite felt | n.d. | 0.54 | [ | |
| Microbial community | Carbon felt | -0.85 | 3.2 | [ | |
| Caproate | Microbial community | Carbon felt | -0.85 | 0.95 | [ |
| Ethanol | Microbial community | VITOCore® GDE | -0.79 | 0.42 | [ |
| Microbial community | Carbon fiber brushes | -0.46 | 0.87 | [ | |
| Clostridium ljungdahlii | Microbial reverse-electrodialysis Electrosynthesis cell | -0.58 | 0.48 | [ | |
| Methane | Microbial community | Graphite fiber brush | -0.501 | 0.01① | [ |
| Microbial community | Carbon felt | -0.751 | 0.13① | [ | |
| Microbial community | Titanium mesh | -0.7 | 0.13① | [ | |
| Microbial community | Granular activated carbon and graphite granules | -0.72 | 0.14① | [ |
Table 3 The main product of microbial electrosynthesis
产物 Product | 微生物 Microorganism | 阴极 Cathode | 静电位控制 Potentiostatic control /(V vs SHE) | 最高产率 Highest productionrate /[g( L·d)] | 参考文献 References |
|---|---|---|---|---|---|
| Acetate | Microbial community | Granular graphite | -0.59 | 0.24 | [ |
| Microbial community | Granular graphite | -0.59 | 1.04 | [ | |
| Microbial community | Carbon felt | -1.26 | 0.06 | [ | |
| Microbial community | Reticulated vitreous carbon foam | n.d. | 0.24 | [ | |
| Microbial community | VITO-CoRE™ | -0.40 | 0.57 | [ | |
| Microbial community | EPD-3D | -0.85 | 0.39 | [ | |
| Microbial community | Granular graphite | -0.6 | 3.10 | [ | |
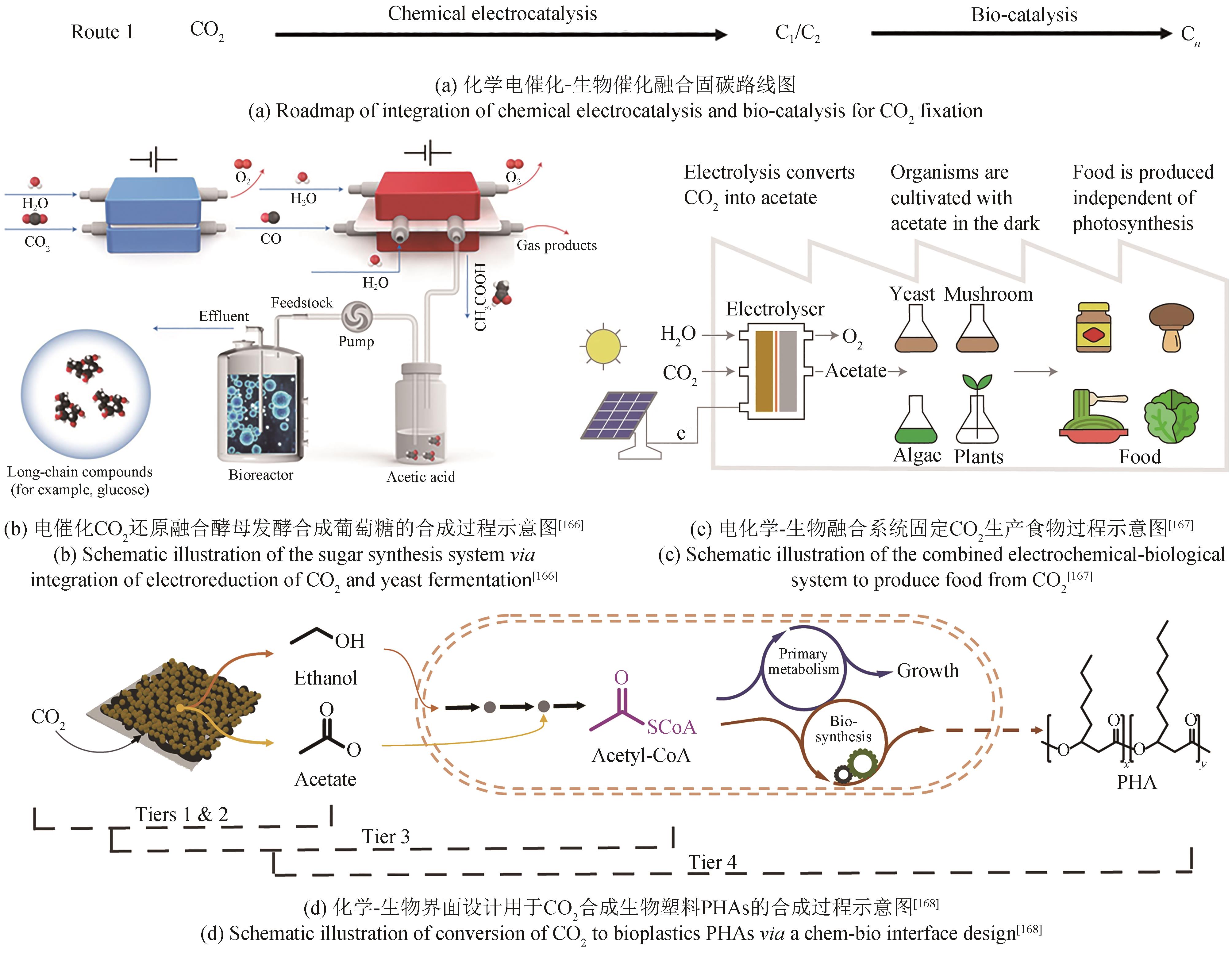
| Microbial community | Reticulated vitreous carbon foam | n.d. | 18.72 | [ | |
| Microbial community | 3D-reticulated vitreous carbon | -1.10 | 77 | [ | |
| Microbial community | Carbon cloth | -0.6 | 0.03 | [ | |
| Sporomusa ovata | Graphite sticks | -0.6 | 0.045 | [ | |
| Sporomusa ovata | rGO-TEPA-modified carbon cloth | -0.69 | 0.17 | [ | |
| Sporomusa ovata | 3D-graphene/carbon felt | -0.6 | 0.12 | [ | |
| Sporomusa ovata | PEDOT: PSS modified carbon cloth | -0.69 | 0.17 | [ | |
| Sporomusa ovata | Copper foam coated graphene | -1.0 | 1.46 | [ | |
| Sporomusa ovata | Synthetic biofilm by 3D bio-printing | -0.6 | 0.68 | [ | |
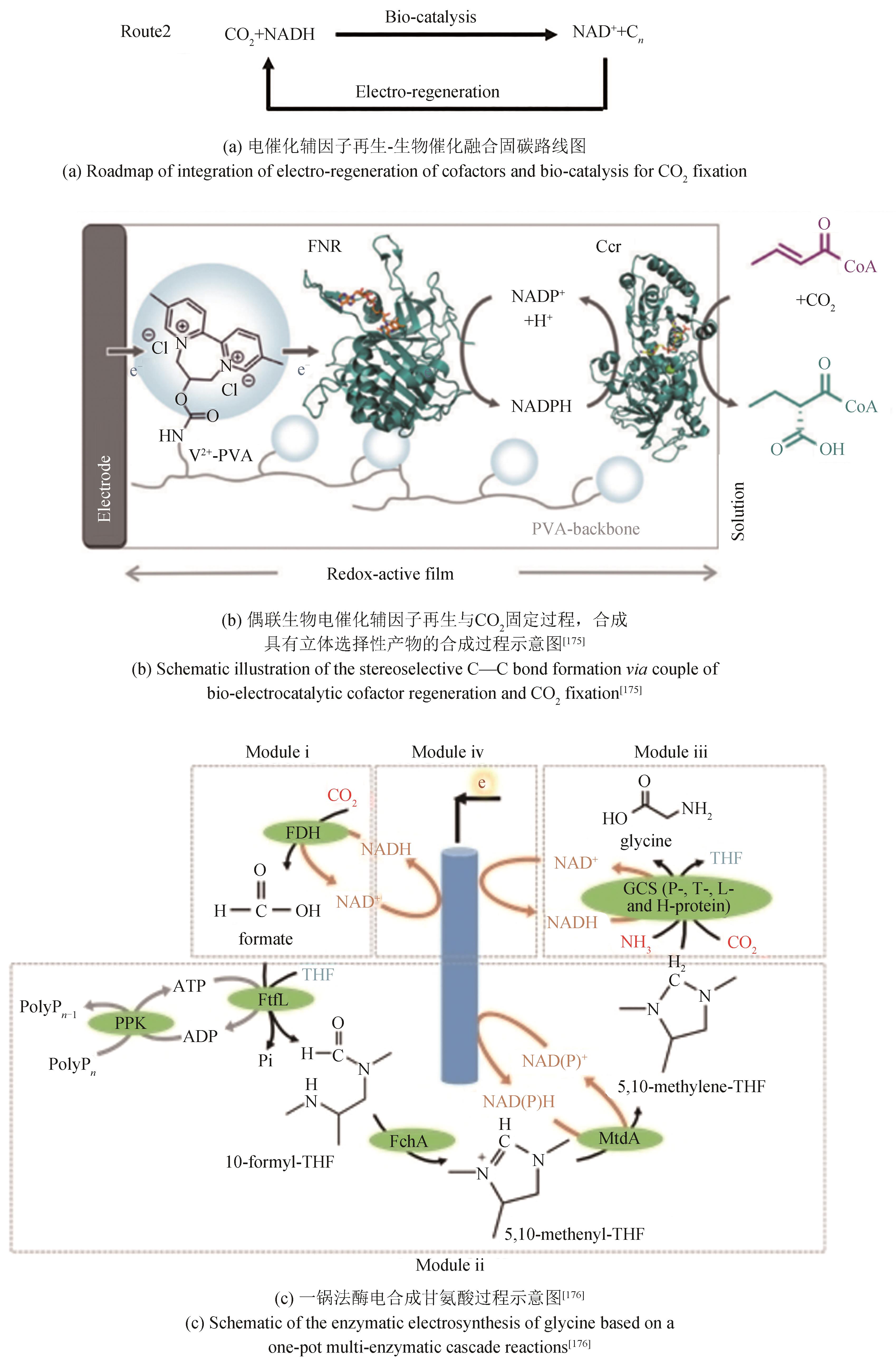
| Sporomusa ovata | Ni-PHFs modified carbon nanotubes | -0.4 | 0.17 | [ | |
| Sporomusa ovata | Co-P alloy | -0.54 | 1.6 | [ | |
| Clostridium ljungdahlii | Graphite plate | -0.8 | 0.138 | [ | |
| Moorella thermoacetica | Carbon cloth | -0.4 | 0.35 | [ | |
| Butyrate | Microbial community | Carbon cloth | -0.8 | 0.16 | [ |
| Microbial community | Graphite felt | n.d. | 0.54 | [ | |
| Microbial community | Carbon felt | -0.85 | 3.2 | [ | |
| Caproate | Microbial community | Carbon felt | -0.85 | 0.95 | [ |
| Ethanol | Microbial community | VITOCore® GDE | -0.79 | 0.42 | [ |
| Microbial community | Carbon fiber brushes | -0.46 | 0.87 | [ | |
| Clostridium ljungdahlii | Microbial reverse-electrodialysis Electrosynthesis cell | -0.58 | 0.48 | [ | |
| Methane | Microbial community | Graphite fiber brush | -0.501 | 0.01① | [ |
| Microbial community | Carbon felt | -0.751 | 0.13① | [ | |
| Microbial community | Titanium mesh | -0.7 | 0.13① | [ | |
| Microbial community | Granular activated carbon and graphite granules | -0.72 | 0.14① | [ |
| 118 | JOURDIN L, FREGUIA S, FLEXER V, et al. Bringing high-rate, CO2-based microbial electrosynthesis closer to practical implementation through improved electrode design and operating conditions[J]. Environmental Science & Technology, 2016, 50(4): 1982-1989. |
| 119 | ROVIRA-ALSINA L, PERONA-VICO E, BAÑERAS L, et al. Thermophilic bio-electro CO2 recycling into organic compounds[J]. Green Chemistry, 2020, 22(9): 2947-2955. |
| 120 | NEVIN K P, WOODARD T L, FRANKS A E, et al. Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds[J]. mBio, 2010, 1(2): e00103-10. |
| 121 | CHEN L F, TREMBLAY P L, MOHANTY S, et al. Electrosynthesis of acetate from CO2 by a highly structured biofilm assembled with reduced graphene oxide-tetraethylene pentamine[J]. Journal of Materials Chemistry A, 2016, 4(21): 8395-8401. |
| 122 | ARYAL N, HALDER A, TREMBLAY P L, et al. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis[J]. Electrochimica Acta, 2016, 217: 117-122. |
| 123 | ARYAL N, TREMBLAY P L, XU M Y, et al. Highly conductive poly(3,4-ethylenedioxythiophene) polystyrene sulfonate polymer coated cathode for the microbial electrosynthesis of acetate from carbon dioxide[J]. Frontiers in Energy Research, 2018, 6: 72. |
| 124 | ARYAL N, WAN L L, OVERGAARD M H, et al. Increased carbon dioxide reduction to acetate in a microbial electrosynthesis reactor with a reduced graphene oxide-coated copper foam composite cathode[J]. Bioelectrochemistry, 2019, 128: 83-93. |
| 125 | KRIGE A, ROVA U, CHRISTAKOPOULOS P. 3D bioprinting on cathodes in microbial electrosynthesis for increased acetate production rate using Sporomusa ovata [J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106189. |
| 126 | BIAN B, ALQAHTANI M F, KATURI K P, et al. Porous nickel hollow fiber cathodes coated with CNTs for efficient microbial electrosynthesis of acetate from CO2 using Sporomusa ovata [J]. Journal of Materials Chemistry A, 2018, 6(35): 17201-17211. |
| 127 | RODRIGUES R M, GUAN X, IÑIGUEZ J A, et al. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction[J]. Nature Catalysis, 2019, 2(5): 407-414. |
| 128 | ROY M, YADAV R, CHIRANJEEVI P, et al. Direct utilization of industrial carbon dioxide with low impurities for acetate production via microbial electrosynthesis[J]. Bioresource Technology, 2021, 320(Pt A): 124289. |
| 129 | YU L P, YUAN Y, TANG J H, et al. Thermophilic Moorella thermoautotrophica-immobilized cathode enhanced microbial electrosynthesis of acetate and formate from CO2 [J]. Bioelectrochemistry, 2017, 117: 23-28. |
| 130 | BATLLE-VILANOVA P, GANIGUÉ R, RAMIÓ-PUJOL S, et al. Microbial electrosynthesis of butyrate from carbon dioxide: production and extraction[J]. Bioelectrochemistry, 2017, 117: 57-64. |
| 131 | RAES S M T, JOURDIN L, BUISMAN C J N, et al. Continuous long-term bioelectrochemical chain elongation to butyrate[J]. ChemElectroChem, 2017, 4(2): 386-395. |
| 132 | JOURDIN L, RAES S M T, BUISMAN C J N, et al. Critical biofilm growth throughout unmodified carbon felts allows continuous bioelectrochemical chain elongation from CO2 up to caproate at high current density[J]. Frontiers in Energy Research, 2018, 6: 7. |
| 133 | GAVILANES J, REDDY C N, MIN B. Microbial electrosynthesis of bioalcohols through reduction of high concentrations of volatile fatty acids[J]. Energy & Fuels, 2019, 33(5): 4264-4271. |
| 134 | LI X H, CHEN S, LIANG D W, et al. Low-grade heat energy driven microbial electrosynthesis for ethanol and acetate production from CO2 reduction[J]. Journal of Power Sources, 2020, 477: 228990. |
| 135 | CHENG S A, XING D F, CALL D F, et al. Direct biological conversion of electrical current into methane by electromethanogenesis[J]. Environmental Science & Technology, 2009, 43(10): 3953-3958. |
| 136 | JIANG Y, SU M, ZHANG Y, et al. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate[J]. International Journal of Hydrogen Energy, 2013, 38(8): 3497-3502. |
| 137 | VAN EERTEN-JANSEN M C A A, JANSEN N C, PLUGGE C M, et al. Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(5): 963-970. |
| 138 | LIU D D, ROCA-PUIGROS M, GEPPERT F, et al. Granular carbon-based electrodes as cathodes in methane-producing bioelectrochemical systems[J]. Frontiers in Bioengineering and Biotechnology, 2018, 6: 78. |
| 139 | ANWER A H, KHAN N, UMAR M F, et al. Electrodeposited hybrid biocathode-based CO2 reduction via microbial electro-catalysis to biofuels[J]. Membranes, 2021, 11(3): 223. |
| 140 | ARENDS J B A, PATIL S A, ROUME H, et al. Continuous long-term electricity-driven bioproduction of carboxylates and isopropanol from CO2 with a mixed microbial community[J]. Journal of CO2 Utilization, 2017, 20: 141-149. |
| 141 | BLASCO-GÓMEZ R, RAMIÓ-PUJOL S, BAÑERAS L, et al. Unravelling the factors that influence the bio-electrorecycling of carbon dioxide towards biofuels[J]. Green Chemistry, 2019, 21(3): 684-691. |
| 1 | LIU E B, LU X D, WANG D C. A systematic review of carbon capture, utilization and storage: status, progress and challenges[J]. Energies, 2023, 16(6): 2865. |
| 2 | GÜR T M. Carbon dioxide emissions, capture, storage and utilization: review of materials, processes and technologies[J]. Progress in Energy and Combustion Science, 2022, 89: 100965. |
| 3 | RINGROSE P S, FURRE A K, GILFILLAN S M V, et al. Storage of carbon dioxide in saline aquifers: physicochemical processes, key constraints, and scale-up potential[J/OL]. Annual Review of Chemical and Biomolecular Engineering, 2021, 12: 471-494[2023-06-01]. . |
| 4 | BIERBAUMER S, NATTERMANN M, SCHULZ L, et al. Enzymatic conversion of CO2: from natural to artificial utilization[J]. Chemical Reviews, 2023, 123(9): 5702-5754. |
| 5 | VALLURI S, CLAREMBOUX V, KAWATRA S. Opportunities and challenges in CO2 utilization[J]. Journal of Environmental Sciences, 2022, 113: 322-344. |
| 6 | ONG M Y, NOMANBHAY S, KUSUMO F, et al. Application of microwave plasma technology to convert carbon dioxide (CO2) into high value products: a review[J]. Journal of Cleaner Production, 2022, 336: 130447. |
| 7 | LI L, LI X D, SUN Y F, et al. Rational design of electrocatalytic carbon dioxide reduction for a zero-carbon network[J]. Chemical Society Reviews, 2022, 51(4): 1234-1252. |
| 8 | LV J J, YIN R N, ZHOU L M, et al. Microenvironment engineering for the electrocatalytic CO2 reduction reaction[J]. Angewandte Chemie International Edition, 2022, 61(39): e202207252. |
| 9 | HUSSAIN I, ALASIRI H, KHAN W U, et al. Advanced electrocatalytic technologies for conversion of carbon dioxide into methanol by electrochemical reduction: recent progress and future perspectives[J]. Coordination Chemistry Reviews, 2023, 482: 215081. |
| 10 | WANG G X, CHEN J X, DING Y C, et al. Electrocatalysis for CO2 conversion: from fundamentals to value-added products[J]. Chemical Society Reviews, 2021, 50(8): 4993-5061. |
| 11 | GUAN Y Y, LIU M M, RAO X F, et al. Electrochemical reduction of carbon dioxide (CO2): bismuth-based electrocatalysts[J]. Journal of Materials Chemistry A, 2021, 9(24): 13770-13803. |
| 12 | LIN L, HE X Y, ZHANG X G, et al. A nanocomposite of bismuth clusters and Bi2O2CO3 sheets for highly efficient electrocatalytic reduction of CO2 to formate[J]. Angewandte Chemie International Edition, 2023, 62(3): e202214959. |
| 13 | SUN B, DAI M W, CAI S C, et al. Challenges and strategies towards copper-based catalysts for enhanced electrochemical CO2 reduction to multi-carbon products[J]. Fuel, 2023, 332: 126114. |
| 14 | ZHAO S Q, CHRISTENSEN O, SUN Z Z, et al. Steering carbon dioxide reduction toward C—C coupling using copper electrodes modified with porous molecular films[J]. Nature Communications, 2023, 14: 844. |
| 15 | ZHANG X Y, ZHANG Z, LI H B, et al. Insight into heterogeneous electrocatalyst design understanding for the reduction of carbon dioxide[J]. Advanced Energy Materials, 2022, 12(39): 2201461. |
| 16 | WOLDU A R, HUANG Z L, ZHAO P X, et al. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts[J]. Coordination Chemistry Reviews, 2022, 454: 214340. |
| 17 | XUE Y Y, GUO Y B, CUI H J, et al. Catalyst design for electrochemical reduction of CO2 to multicarbon products[J]. Small Methods, 2021, 5(10): 2100736. |
| 18 | YANG Q Y, GUO X X, LIU Y W, et al. Biocatalytic C—C bond formation for one carbon resource utilization[J]. International Journal of Molecular Sciences, 2021, 22(4): 1890. |
| 19 | KATAGIRI T, AMAO Y. Double-electron reduced diphenylviologen as a coenzyme for biocatalytic building carbon-carbon bonds from CO2 as a carbon feedstock[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10): 9080-9085. |
| 20 | ONYEAKA H, EKWEBELEM O C. A review of recent advances in engineering bacteria for enhanced CO2 capture and utilization[J]. International Journal of Environmental Science and Technology, 2023, 20(4): 4635-4648. |
| 21 | MARPANI F, PINELO M, MEYER A S. Enzymatic conversion of CO2 to CH3OH via reverse dehydrogenase cascade biocatalysis: quantitative comparison of efficiencies of immobilized enzyme systems[J]. Biochemical Engineering Journal, 2017, 127: 217-228. |
| 22 | MULDER D W, PETERS J W, RAUGEI S. Catalytic bias in oxidation-reduction catalysis[J]. Chemical Communications, 2021, 57(6): 713-720. |
| 23 | ADAMSON H, ROBINSON M, WRIGHT J J, et al. Retuning the catalytic bias and overpotential of a [NiFe]-hydrogenase via a single amino acid exchange at the electron entry/exit site[J]. Journal of the American Chemical Society, 2017, 139(31): 10677-10686. |
| 24 | SRIKANTH S, KUMAR M, SINGH D, et al. Long-term operation of electro-biocatalytic reactor for carbon dioxide transformation into organic molecules[J]. Bioresource Technology, 2018, 265: 66-74. |
| 25 | SRIKANTH S, SINGH D, VANBROEKHOVEN K, et al. Electro-biocatalytic conversion of carbon dioxide to alcohols using gas diffusion electrode[J]. Bioresource Technology, 2018, 265: 45-51. |
| 26 | LEE Y S, LIM K, MINTEER S D. Cascaded biocatalysis and bioelectrocatalysis: overview and recent advances[J]. Annual Review of Physical Chemistry, 2021, 72: 467-488. |
| 27 | SCHLAGER S, HABERBAUER M, FUCHSBAUER A, et al. Bio-electrocatalytic application of microorganisms for carbon dioxide reduction to methane[J]. ChemSusChem, 2017, 10(1): 226-233. |
| 28 | SHI J F, JIANG Y J, JIANG Z Y, et al. Enzymatic conversion of carbon dioxide[J]. Chemical Society Reviews, 2015, 44(17): 5981-6000. |
| 142 | MA L Q, FANG Z, WANG Y Z, et al. Photo-driven highly efficient one-step CO2 biomethanation with engineered photo-synthetic bacteria Rhodopseudomonas palustris [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(26): 9616-9621. |
| 143 | NEVIN K P, HENSLEY S A, FRANKS A E, et al. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms[J]. Applied and Environmental Microbiology, 2011, 77(9): 2882-2886. |
| 144 | LEHTINEN T, EFIMOVA E, TREMBLAY P L, et al. Production of long chain alkyl esters from carbon dioxide and electricity by a two-stage bacterial process[J]. Bioresource Technology, 2017, 243: 30-36. |
| 145 | BLANCHET E, DUQUENNE F, RAFRAFI Y, et al. Importance of the hydrogen route in up-scaling electrosynthesis for microbial CO2 reduction[J]. Energy & Environmental Science, 2015, 8(12): 3731-3744. |
| 146 | SHI X C, TREMBLAY P L, WAN L L, et al. Improved robustness of microbial electrosynthesis by adaptation of a strict anaerobic microbial catalyst to molecular oxygen[J]. Science of the Total Environment, 2021, 754: 142440. |
| 147 | KRACKE F, VIRDIS B, BERNHARDT P V, et al. Redox dependent metabolic shift in Clostridium autoethanogenum by extracellular electron supply[J]. Biotechnology for Biofuels, 2016, 9: 249. |
| 148 | LIU H X, SONG T S, FEI K Q, et al. Microbial electrosynthesis of organic chemicals from CO2 by Clostridium scatologenes ATCC 25775T [J]. Bioresources and Bioprocessing, 2018, 5: 7. |
| 149 | BAJRACHARYA S, HEIJNE A TER, DOMINGUEZ BENETTON X, et al. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode[J]. Bioresource Technology, 2015, 195: 14-24. |
| 150 | WANG G R, HUANG Q, SONG T S, et al. Enhancing microbial electrosynthesis of acetate and butyrate from CO2 reduction involving engineered Clostridium ljungdahlii with a nickel-phosphide-modified electrode[J]. Energy & Fuels, 2020, 34(7): 8666-8675. |
| 151 | HA B N, PHAM D M, MASUDA D, et al. Humin-promoted microbial electrosynthesis of acetate from CO2 by Moorella thermoacetica [J]. Biotechnology and Bioengineering, 2022, 119(12): 3487-3496. |
| 152 | CHEN S S, FANG Y L, JING X Y, et al. Enhanced electrosynthesis performance of Moorella thermoautotrophica by improving cell permeability[J]. Bioelectrochemistry, 2018, 121: 151-159. |
| 153 | SAKIMOTO K K, WONG A B, YANG P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 29 | WINDLE C D, PERUTZ R N. Advances in molecular photocatalytic and electrocatalytic CO2 reduction[J]. Coordination Chemistry Reviews, 2012, 256(21/22): 2562-2570. |
| 30 | CADOUX C, MILTON R D. Recent enzymatic electrochemistry for reductive reactions[J]. ChemElectroChem, 2020, 7(9): 1974-1986. |
| 31 | CHEN H, HUANG Y, SHA C, et al. Enzymatic carbon dioxide to formate: mechanisms, challenges and opportunities[J]. Renewable and Sustainable Energy Reviews, 2023, 178: 113271. |
| 32 | SAHM H, WAGNER F. Mikrobielle Verwertung von Methanol. Eigenschaften der formaldehyddehydrogenase und der formiatdehydrogenase aus Candida boidinii[J/OL]. Archiv Für Mikrobiologie, 1973, 90(3): 263-268[2023-06-01]. . |
| SAHM H, WAGNER F. Microbial assimilation of methanol. Properties of formaldehyde dehydrogenase and formate dehydrogenase from Candida boidinii [J]. Archives of Microbiology, 1973, 90(3): 263-268[2023-06-01]. . | |
| 33 | CHOE H, JOO J C, CHO D H, et al. Efficient CO2-reducing activity of NAD-dependent formate dehydrogenase from Thiobacillus sp. KNK65MA for formate production from CO2 gas[J]. PLoS One, 2014, 9(7): e103111. |
| 34 | ALTAŞ N, ASLAN A S, KARATAŞ E, et al. Heterologous production of extreme alkaline thermostable NAD+-dependent formate dehydrogenase with wide-range pH activity from Myceliophthora thermophila [J]. Process Biochemistry, 2017, 61: 110-118. |
| 35 | AVILOVA T V, EGOROVA O A, IOANESYAN L S, et al. Biosynthesis, isolation and properties of NAD-dependent formate dehydrogenase from the yeast Candida methylica [J]. European Journal of Biochemistry, 1985, 152(3): 657-662. |
| 36 | ŸZGÜN G, KARAGÜLER N G, TURUNEN O, et al. Characterization of a new acidic NAD+-dependent formate dehydrogenase from thermophilic fungus Chaetomium thermophilum [J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 122: 212-217. |
| 37 | MULLER U, WILLNOW P, RUSCHIG U, et al. Formate dehydrogenase from Pseudomonas oxalaticus [J]. European Journal of Biochemistry, 1978, 83(2): 485-498. |
| 38 | DE BOK F A M, HAGEDOORN P L, SILVA P J, et al. Two W-containing formate dehydrogenases (CO2-reductases) involved in syntrophic propionate oxidation by Syntrophobacter fumaroxidans [J]. European Journal of Biochemistry, 2003, 270(11): 2476-2485. |
| 39 | OLIVEIRA A R, MOTA C, MOURATO C, et al. Toward the mechanistic understanding of enzymatic CO2 reduction[J]. ACS Catalysis, 2020, 10(6): 3844-3856. |
| 154 | ZHANG H, LIU H, TIAN Z Q, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nature Nanotechnology, 2018, 13(10): 900-905. |
| 155 | CHEN H, LI J W, FAN Q C, et al. A feasible strategy for microbial electrocatalytic CO2 reduction via whole-cell-packed and exogenous-mediator-free rGO/Shewanella biohydrogel[J]. Chemical Engineering Journal, 2023, 460: 141863. |
| 156 | WANG K, DA Y Y, BI H R, et al. A one-carbon chemicals conversion strategy to produce precursor of biofuels with Saccharomyces cerevisiae [J]. Renewable Energy, 2023, 208: 331-340. |
| 157 | LI C F, YIN L J, WANG J W, et al. Light-driven biosynthesis of volatile, unstable and photosensitive chemicals from CO2 [J/OL]. Nature Synthesis, 2023[2023-06-01]. . |
| 158 | SUO D, FANG Z, YU Y Y, et al. Synthetic curli enables efficient microbial electrocatalysis with stainless-steel electrode[J]. AIChE Journal, 2020, 66(4): e16897. |
| 159 | MOSALI V S S, ZHANG X L, ZHANG Y, et al. Electrocatalytic CO2 reduction to formate on Cu based surface alloys with enhanced selectivity[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(24): 19453-19462. |
| 160 | KUHL K P, HATSUKADE T, CAVE E R, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces[J]. Journal of the American Chemical Society, 2014, 136(40): 14107-14113. |
| 161 | CHEN X Y, CHEN J F, ALGHORAIBI N M, et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes[J]. Nature Catalysis, 2021, 4(1): 20-27. |
| 162 | KIM S, SHIN D, PARK J, et al. Grain boundary-rich copper nanocatalysts generated from metal-organic framework nanoparticles for CO2-to-C2+ electroconversion[J]. Advanced Science, 2023, 10(9): 2207187. |
| 163 | NAVARRO-JAÉN S, VIRGINIE M, BONIN J, et al. Highlights and challenges in the selective reduction of carbon dioxide to methanol[J]. Nature Reviews Chemistry, 2021, 5(8): 564-579. |
| 164 | WANG C, LIU Y P, REN H A, et al. Diminishing the uncoordinated N species in Co-N-C catalysts toward highly efficient electrochemical CO2 reduction[J]. ACS Catalysis, 2022, 12(4): 2513-2521. |
| 165 | LIEW F E, NOGLE R, ABDALLA T, et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale[J]. Nature Biotechnology, 2022, 40(3): 335-344. |
| 166 | ZHENG T T, ZHANG M L, WU L H, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| 40 | SCHWARZ F M, SCHUCHMANN K, MÜLLER V. Hydrogenation of CO2 at ambient pressure catalyzed by a highly active thermostable biocatalyst[J]. Biotechnology for Biofuels, 2018, 11: 237. |
| 41 | ALISSANDRATOS A, KIM H K, MATTHEWS H, et al. Clostridium carboxidivorans strain P7T recombinant formate dehydrogenase catalyzes reduction of CO2 to formate[J]. Applied and Environmental Microbiology, 2013, 79(2): 741-744. |
| 42 | BASSEGODA A, MADDEN C, WAKERLEY D W, et al. Reversible interconversion of CO2 and formate by a molybdenum-containing formate dehydrogenase[J]. Journal of the American Chemical Society, 2014, 136(44): 15473-15476. |
| 43 | ÇAKAR M M, MANGAS-SANCHEZ J, BIRMINGHAM W R, et al. Discovery of a new metal and NAD+-dependent formate dehydrogenase from Clostridium ljungdahlii [J]. Preparative Biochemistry & Biotechnology, 2018, 48(4): 327-334. |
| 44 | HARTMANN T, LEIMKÜHLER S. The oxygen-tolerant and NAD+-dependent formate dehydrogenase from Rhodobacter capsulatus is able to catalyze the reduction of CO2 to formate[J]. The FEBS Journal, 2013, 280(23): 6083-6096. |
| 45 | MAIA L B, FONSECA L, MOURA I, et al. Reduction of carbon dioxide by a molybdenum-containing formate dehydrogenase: a kinetic and mechanistic study[J]. Journal of the American Chemical Society, 2016, 138(28): 8834-8846. |
| 46 | YU X J, NIKS D, MULCHANDANI A, et al. Efficient reduction of CO2 by the molybdenum-containing formate dehydrogenase from Cupriavidus necator (Ralstonia eutropha)[J]. Journal of Biological Chemistry, 2017, 292(41): 16872-16879. |
| 47 | MIN K, PARK Y S, PARK G W, et al. Elevated conversion of CO2 to versatile formate by a newly discovered formate dehydrogenase from Rhodobacter aestuarii [J]. Bioresource Technology, 2020, 305: 123155. |
| 48 | SCHUCHMANN K, MÜLLER V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase[J]. Science, 2013, 342(6164): 1382-1385. |
| 49 | NILOV D K, SHABALIN I G, POPOV V O, et al. Molecular modeling of formate dehydrogenase: the formation of the Michaelis complex[J]. Journal of Biomolecular Structure and Dynamics, 2012, 30(2): 170-179. |
| 50 | YILMAZER B, ISUPOV M N, DE ROSE S A, et al. Structural insights into the NAD+-dependent formate dehydrogenase mechanism revealed from the NADH complex and the formate NAD+ ternary complex of the Chaetomium thermophilum enzyme[J]. Journal of Structural Biology, 2020, 212(3): 107657. |
| 51 | PARKINSON B A, WEAVER P F. Photoelectrochemical pumping of enzymatic CO2 reduction[J]. Nature, 1984, 309(5964): 148-149. |
| 167 | HANN E C, OVERA S, HARLAND-DUNAWAY M, et al. A hybrid inorganic-biological artificial photosynthesis system for energy-efficient food production[J]. Nature Food, 2022, 3(6): 461-471. |
| 168 | ZHANG P, CHEN K N, XU B, et al. Chem-bio interface design for rapid conversion of CO2 to bioplastics in an integrated system[J]. Chem, 2022, 8(12): 3363-3381. |
| 169 | HAAS T, KRAUSE R, WEBER R, et al. Technical photosynthesis involving CO2 electrolysis and fermentation[J]. Nature Catalysis, 2018, 1(1): 32-39. |
| 170 | CHEN X L, CAO Y X, LI F, et al. Enzyme-assisted microbial electrosynthesis of poly(3-hydroxybutyrate) via CO2 bioreduction by engineered Ralstonia eutropha [J]. ACS Catalysis, 2018, 8(5): 4429-4437. |
| 171 | PAUL C E, HOLLMANN F. A survey of synthetic nicotinamide cofactors in enzymatic processes[J]. Applied Microbiology and Biotechnology, 2016, 100(11): 4773-4778. |
| 172 | DI SPIRIDIONE C, ARESTA M, DIBENEDETTO A. Improving the enzymatic cascade of reactions for the reduction of CO2 to CH3OH in water: from enzymes immobilization strategies to cofactor regeneration and cofactor suppression[J]. Molecules, 2022, 27(15): 4913. |
| 173 | IMMANUEL S, SIVASUBRAMANIAN R, GUL R, et al. Recent progress and perspectives on electrochemical regeneration of reduced nicotinamide adenine dinucleotide (NADH)[J]. Chemistry-an Asian Journal, 2020, 15(24): 4256-4270. |
| 174 | MORRISON C S, ARMIGER W B, DODDS D R, et al. Improved strategies for electrochemical 1,4-NAD(P)H2 regeneration: a new era of bioreactors for industrial biocatalysis[J]. Biotechnology Advances, 2018, 36(1): 120-131. |
| 175 | CASTAÑEDA-LOSADA L, ADAM D, PACZIA N, et al. Bioelectrocatalytic cofactor regeneration coupled to CO2 fixation in a redox-active hydrogel for stereoselective C—C bond formation[J]. Angewandte Chemie International Edition, 2021, 60(38): 21056-21061. |
| 176 | WU R R, LI F, CUI X Y, et al. Enzymatic electrosynthesis of glycine from CO2 and NH3 [J]. Angewandte Chemie International Edition, 2023, 62(14): e202218387. |
| 177 | TORELLA J P, GAGLIARDI C J, CHEN J S, et al. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system [J]. Proceedings of the National Academy of Sciences, 2015, 112(8): 2337-2342. |
| 178 | CAI T, SUN H B, QIAO J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide[J]. Science, 2021, 373(6562): 1523-1527. |
| 52 | KIM S, KIM M K, LEE S H, et al. Conversion of CO2 to formate in an electroenzymatic cell using Candida boidinii formate dehydrogenase[J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 102: 9-15. |
| 53 | KIM S H, CHUNG G Y, KIM S H, et al. Electrochemical NADH regeneration and electroenzymatic CO2 reduction on Cu nanorods/glassy carbon electrode prepared by cyclic deposition[J]. Electrochimica Acta, 2016, 210: 837-845. |
| 54 | SRIKANTH S, MAESEN M, DOMINGUEZ-BENETTON X, et al. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES)[J]. Bioresource Technology, 2014, 165: 350-354. |
| 55 | ZHANG L J, LIU J Y, ONG J, et al. Specific and sustainable bioelectro-reduction of carbon dioxide to formate on a novel enzymatic cathode[J]. Chemosphere, 2016, 162: 228-234. |
| 56 | BARIN R, BIRIA D, RASHID-NADIMI S, et al. Enzymatic CO2 reduction to formate by formate dehydrogenase from Candida boidinii coupling with direct electrochemical regeneration of NADH[J]. Journal of CO2 Utilization, 2018, 28: 117-125. |
| 57 | LIU G H, CHEN H X, ZHAO H, et al. Accelerating electroenzymatic CO2 reduction by immobilizing formate dehydrogenase on polyethylenimine-modified mesoporous silica[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(1): 633-644. |
| 58 | YAN L H, LIU G H, LIU J Q, et al. Hierarchically porous metal organic framework immobilized formate dehydrogenase for enzyme electrocatalytic CO2 reduction[J]. Chemical Engineering Journal, 2022, 450: 138164. |
| 59 | LI Y X, YAN L H, LIU G H, et al. Enhanced electroenzymatic CO2 reduction by a multifunctional ZIF-8 layer on silica nanoflower with immobilized enzyme[J]. Chemical Engineering Journal, 2023, 466: 143198. |
| 60 | SONG H Y, MA C L, LIU P, et al. A hybrid CO2 electroreduction system mediated by enzyme-cofactor conjugates coupled with Cu nanoparticle-catalyzed cofactor regeneration[J]. Journal of CO2 Utilization, 2019, 34: 568-575. |
| 61 | REGINALD S S, KIM M J, LEE H, et al. Direct electrical contact of NAD+/NADH-dependent dehydrogenase on electrode surface enabled by non-native solid-binding peptide as a molecular binder[J]. Electrochimica Acta, 2022, 421: 140480. |
| 62 | BARIN R, BIRIA D, RASHID-NADIMI S, et al. Investigating the enzymatic CO2 reduction to formate with electrochemical NADH regeneration in batch and semi-continuous operations[J]. Chemical Engineering and Processing-Process Intensification, 2019, 140: 78-84. |
| 63 | ALI I, GILL A, OMANOVIC S. Direct electrochemical regeneration of the enzymatic cofactor 1,4-NADH employing nano-patterned glassy carbon/Pt and glassy carbon/Ni electrodes[J]. Chemical Engineering Journal, 2012, 188: 173-180. |
| 179 | YANG X Y, JIANG Y F, ZOU R, et al. Green electricity-driven simultaneous ammonia recovery and in situ upcycling for microbial protein production[J]. Chemical Engineering Journal, 2022, 430(Pt_2): 132890. |
| 180 | LIN L, HUANG H N, ZHANG X, et al. Hydrogen-oxidizing bacteria and their applications in resource recovery and pollutant removal[J]. Science of the Total Environment, 2022, 835: 155559. |
| 181 | WILDE E. Untersuchungen über wachstum und speicherstoffsynthese von hydrogenomonas[J/OL]. Archiv Für Mikrobiologie, 1962, 43(2): 109-137[2023-06-01]. . |
| 182 | MA Z, LIU D, LIU M X, et al. From CO2 to high value-added products: advances on carbon sequestration by Ralstonia eutropha H16[J]. Chinese Science Bulletin, 2021, 66(33): 4218-4230. |
| 183 | BAJRACHARYA S, KRIGE A, MATSAKAS L, et al. Dual cathode configuration and headspace gas recirculation for enhancing microbial electrosynthesis using Sporomusa ovata [J]. Chemosphere, 2022, 287(Pt 3): 132188. |
| 184 | LIU C, COLÓN B C, ZIESACK M, et al. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis[J]. Science, 2016, 352(6290): 1210-1213. |
| 185 | TEETZ N, HOLTMANN D, HARNISCH F, et al. Upgrading kolbe electrolysis — Highly efficient production of green fuels and solvents by coupling biosynthesis and electrosynthesis[J]. Angewandte Chemie International Edition, 2022, 61(50): e202210596. |
| 64 | WANG X D, YIU H H P. Heterogeneous catalysis mediated cofactor NADH regeneration for enzymatic reduction[J]. ACS Catalysis, 2016, 6(3): 1880-1886. |
| 65 | ZHANG Z B, VASILIU T, LI F F, et al. Electrochemically driven efficient enzymatic conversion of CO2 to formic acid with artificial cofactors[J]. Journal of CO2 Utilization, 2021, 52: 101679. |
| 66 | CORDAS C M, CAMPANIÇO M, BAPTISTA R, et al. Direct electrochemical reduction of carbon dioxide by a molybdenum-containing formate dehydrogenase[J]. Journal of Inorganic Biochemistry, 2019, 196: 110694. |
| 67 | REDA T, PLUGGE C M, ABRAM N J, et al. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(31): 10654-10658. |
| 68 | ALVAREZ-MALMAGRO J, OLIVEIRA A R, GUTIÉRREZ-SÁNCHEZ C, et al. Bioelectrocatalytic activity of W-formate dehydrogenase covalently immobilized on functionalized gold and graphite electrodes[J]. ACS Applied Materials & Interfaces, 2021, 13(10): 11891-11900. |
| 69 | BADIANI V M, COBB S J, WAGNER A, et al. Elucidating film loss and the role of hydrogen bonding of adsorbed redox enzymes by electrochemical quartz crystal microbalance analysis[J]. ACS Catalysis, 2022, 12(3): 1886-1897. |
| 70 | KUK S K, GOPINATH K, SINGH R K, et al. NADH-free electroenzymatic reduction of CO2 by conductive hydrogel-conjugated formate dehydrogenase[J]. ACS Catalysis, 2019, 9(6): 5584-5589. |
| 71 | SAKAI K, KITAZUMI Y, SHIRAI O, et al. Efficient bioelectrocatalytic CO2 reduction on gas-diffusion-type biocathode with tungsten-containing formate dehydrogenase[J]. Electrochemistry Communications, 2016, 73: 85-88. |
| 72 | LI W J, GAO Y X, SUN X A, et al. Direct detection of a single [4Fe-4S]cluster in a tungsten-containing enzyme: electrochemical conversion of CO2 into formate by formate dehydrogenase[J]. Carbon Energy, 2023, 5(5): e304. |
| 73 | SOKOL K P, ROBINSON W E, OLIVEIRA A R, et al. Photoreduction of CO2 with a formate dehydrogenase driven by photosystemⅡusing a semi-artificial Z-scheme architecture[J]. Journal of the American Chemical Society, 2018, 140(48): 16418-16422. |
| 74 | MILLER M, ROBINSON W E, OLIVEIRA A R, et al. Interfacing formate dehydrogenase with metal oxides for the reversible electrocatalysis and solar-driven reduction of carbon dioxide[J]. Angewandte Chemie International Edition, 2019, 58(14): 4601-4605. |
| 75 | EDWARDES MOORE E, ANDREI V, OLIVEIRA A R, et al. A semi-artificial photoelectrochemical tandem leaf with a CO2-to-formate efficiency approaching 1%[J]. Angewandte Chemie International Edition, 2021, 60(50): 26303-26307. |
| 76 | LAM E, MILLER M, LINLEY S, et al. Comproportionation of CO2 and cellulose to formate using a floating semiconductor-enzyme photoreforming catalyst[J]. Angewandte Chemie International Edition, 2023, 62(20): e202215894. |
| 77 | KUK S K, SINGH R K, NAM D H, et al. Photoelectrochemical reduction of carbon dioxide to methanol through a highly efficient enzyme cascade[J]. Angewandte Chemie International Edition, 2017, 56(14): 3827-3832. |
| 78 | ZHANG Z B, LI J J, JI M B, et al. Encapsulation of multiple enzymes in a metal-organic framework with enhanced electro-enzymatic reduction of CO2 to methanol[J]. Green Chemistry, 2021, 23(6): 2362-2371. |
| 79 | STRIPP S T, DUFFUS B R, FOURMOND V, et al. Second and outer coordination sphere effects in nitrogenase, hydrogenase, formate dehydrogenase, and CO dehydrogenase[J]. Chemical Reviews, 2022, 122(14): 11900-11973. |
| 80 | BOYINGTON J C, GLADYSHEV V N, KHANGULOV S V, et al. Crystal structure of formate dehydrogenase H: catalysis involving Mo, molybdopterin, selenocysteine, and an Fe4S4 cluster[J]. Science, 1997, 275(5304): 1305-1308. |
| 81 | RADON C, MITTELSTÄDT G, DUFFUS B R, et al. Cryo-EM structures reveal intricate Fe-S cluster arrangement and charging in Rhodobacter capsulatus formate dehydrogenase[J]. Nature Communications, 2020, 11: 1912. |
| 82 | STEINHILPER R, HÖFF G, HEIDER J, et al. Structure of the membrane-bound formate hydrogenlyase complex from Escherichia coli [J]. Nature Communications, 2022, 13: 5395. |
| 83 | DIETRICH H M, RIGHETTO R D, KUMAR A, et al. Membrane-anchored HDCR nanowires drive hydrogen-powered CO2 fixation[J]. Nature, 2022, 607(7920): 823-830. |
| 84 | JOHNSON M K, REES D C, ADAMS M W W. Tungstoenzymes[J]. Chemical Reviews, 1996, 96(7): 2817-2840. |
| 85 | SZCZESNY J, RUFF A, OLIVEIRA A R, et al. Electroenzymatic CO2 fixation using redox polymer/enzyme-modified gas diffusion electrodes[J]. ACS Energy Letters, 2020, 5(1): 321-327. |
| 86 | COBB S J, DHARANI A M, OLIVEIRA A R, et al. Carboxysome-inspired electrocatalysis using enzymes for the reduction of CO2 at low concentrations[J]. Angewandte Chemie International Edition, 2023, 62(26): e202218782. |
| 87 | ADACHI T, KITAZUMI Y, SHIRAI O, et al. Construction of a bioelectrochemical formate generating system from carbon dioxide and dihydrogen[J]. Electrochemistry Communications, 2018, 97: 73-76. |
| 88 | GAO Y X, LI W J, SUN X A, et al. Boosting the performance of formate dehydrogenase by silver nanoclusters for photoreduction of CO2 to formate[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(45): 14888-14896. |
| 89 | BADIANI V M, CASADEVALL C, MILLER M, et al. Engineering electro- and photocatalytic carbon materials for CO2 reduction by formate dehydrogenase[J]. Journal of the American Chemical Society, 2022, 144(31): 14207-14216. |
| 90 | SCHLAGER S, DUMITRU L M, HABERBAUER M, et al. Electrochemical reduction of carbon dioxide to methanol by direct injection of electrons into immobilized enzymes on a modified electrode[J]. ChemSusChem, 2016, 9(6): 631-635. |
| 91 | SEELAJAROEN H, BAKANDRITSOS A, OTYEPKA M, et al. Immobilized enzymes on graphene as nanobiocatalyst[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 250-259. |
| 92 | VALLE GOMES M Z DO, MASDEU G, EIRING P, et al. Improved biocatalytic cascade conversion of CO2 to methanol by enzymes Co-immobilized in tailored siliceous mesostructured cellular foams[J]. Catalysis Science & Technology, 2021, 11(21): 6952-6959. |
| 93 | JEOUNG J H, MARTINS B M, DOBBEK H. Carbon monoxide dehydrogenases[J]. Methods in Molecular Biology, 2019, 1876: 37-54. |
| 94 | BREGLIA R, ARRIGONI F, SENSI M, et al. First-principles calculations on Ni, Fe-containing carbon monoxide dehydrogenases reveal key stereoelectronic features for binding and release of CO2 to/from the C-cluster[J]. Inorganic Chemistry, 2021, 60(1): 387-402. |
| 95 | CONTALDO U, GUIGLIARELLI B, PERARD J, et al. Efficient electrochemical CO2/CO interconversion by an engineered carbon monoxide dehydrogenase on a gas-diffusion carbon nanotube-based bioelectrode[J]. ACS Catalysis, 2021, 11(9): 5808-5817. |
| 96 | SHIN W, LEE S H, SHIN J W, et al. Highly selective electrocatalytic conversion of CO2 to CO at -0.57 V (NHE) by carbon monoxide dehydrogenase from Moorella thermoacetica [J]. Journal of the American Chemical Society, 2003, 125(48): 14688-14689. |
| 97 | PARKIN A, SERAVALLI J, VINCENT K A, et al. Rapid and efficient electrocatalytic CO2/CO interconversions by carboxydothermus hydrogenoformans CO dehydrogenaseⅠon an electrode[J]. Journal of the American Chemical Society, 2007, 129(34): 10328-10329. |
| 98 | WANG V C C, CAN M, PIERCE E, et al. A unified electrocatalytic description of the action of inhibitors of nickel carbon monoxide dehydrogenase[J]. Journal of the American Chemical Society, 2013, 135(6): 2198-2206. |
| 99 | DILWORTH M J, EADY R R, ROBSON R L, et al. Ethane formation from acetylene as a potential test for vanadium nitrogenase in vivo [J]. Nature, 1987, 327(6118): 167-168. |
| 100 | SCHNEIDER K, GOLLAN U, DROTTBOOM M, et al. Comparative biochemical characterization of the iron-only nitrogenase and the molybdenum nitrogenase from Rhodobacter capsulatus [J]. European Journal of Biochemistry, 1997, 244(3): 789-800. |
| 101 | KHADKA N, DEAN D R, SMITH D, et al. CO2 reduction catalyzed by nitrogenase: pathways to formate, carbon monoxide, and methane[J]. Inorganic Chemistry, 2016, 55(17): 8321-8330. |
| 102 | SEEFELDT L C, HOFFMAN B M, DEAN D R. Mechanism of Mo-dependent nitrogenase[J]. Annual Review of Biochemistry, 2009, 78: 701-722. |
| 103 | REBELEIN J G, HU Y L, RIBBE M W. Widening the product profile of carbon dioxide reduction by vanadium nitrogenase[J]. ChemBioChem, 2015, 16(14): 1993-1996. |
| 104 | HU B, HARRIS D F, DEAN D R, et al. Electrocatalytic CO2 reduction catalyzed by nitrogenase MoFe and FeFe proteins[J]. Bioelectrochemistry, 2018, 120: 104-109. |
| 105 | CAI R, MILTON R D, ABDELLAOUI S, et al. Electroenzymatic C—C bond formation from CO2 [J]. Journal of the American Chemical Society, 2018, 140(15): 5041-5044. |
| 106 | ERB T J. Carboxylases in natural and synthetic microbial pathways[J]. Applied and Environmental Microbiology, 2011, 77(24): 8466-8477. |
| 107 | ÜNLÜ A, DUMAN-ÖZDAMAR Z E, ÇALOĞLU B, et al. Enzymes for efficient CO2 conversion[J]. The Protein Journal, 2021, 40(4): 489-503. |
| 108 | DESSÌ P, ROVIRA-ALSINA L, SÁNCHEZ C, et al. Microbial electrosynthesis: towards sustainable biorefineries for production of green chemicals from CO2 emissions[J]. Biotechnology Advances, 2021, 46: 107675. |
| 109 | VASSILEV I, HERNANDEZ P A, BATLLE-VILANOVA P, et al. Microbial electrosynthesis of isobutyric, butyric, caproic acids, and corresponding alcohols from carbon dioxide[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8485-8493. |
| 110 | MARSHALL C W, ROSS D E, FICHOT E B, et al. Electrosynthesis of commodity chemicals by an autotrophic microbial community[J]. Applied and Environmental Microbiology, 2012, 78(23): 8412-8420. |
| 111 | MARSHALL C W, ROSS D E, FICHOT E B, et al. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes[J]. Environmental Science & Technology, 2013, 47(11): 6023-6029. |
| 112 | PATIL S A, ARENDS J B A, VANWONTERGHEM I, et al. Selective enrichment establishes a stable performing community for microbial electrosynthesis of acetate from CO2 [J]. Environmental Science & Technology, 2015, 49(14): 8833-8843. |
| 113 | JOURDIN L, FREGUIA S, DONOSE B C, et al. A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis[J]. Journal of Materials Chemistry A, 2014, 2(32): 13093-13102. |
| 114 | MOHANAKRISHNA G, SEELAM J S, VANBROEKHOVEN K, et al. An enriched electroactive homoacetogenic biocathode for the microbial electrosynthesis of acetate through carbon dioxide reduction[J]. Faraday Discussions, 2015, 183: 445-462. |
| 115 | JOURDIN L, GRIEGER T, MONETTI J, et al. High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide[J]. Environmental Science & Technology, 2015, 49(22): 13566-13574. |
| 116 | LABELLE E V, MARSHALL C W, GILBERT J A, et al. Influence of acidic pH on hydrogen and acetate production by an electrosynthetic microbiome[J]. PLoS One, 2014, 9(10): e109935. |
| 117 | LABELLE E V, MAY H D. Energy efficiency and productivity enhancement of microbial electrosynthesis of acetate[J]. Frontiers in Microbiology, 2017, 8: 756. |
| [1] | LEI Hangbin, HE Ning, LI Feixuan, DONG Lingling, WANG Shizhen. Advance in the immobilization of hydrogenases [J]. Synthetic Biology Journal, 2024, 5(6): 1485-1497. |
| [2] | Xinyu CUI, Ranran WU, Yuanming WANG, Zhiguang ZHU. Construction and enhancement of enzymatic bioelectrocatalytic systems [J]. Synthetic Biology Journal, 2022, 3(5): 1006-1030. |
| [3] | Zixuan YOU, Feng LI, Hao SONG. Design and construction of electroactive cells by synthetic biology strategies [J]. Synthetic Biology Journal, 2022, 3(5): 1031-1059. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||