Synthetic Biology Journal ›› 2020, Vol. 1 ›› Issue (4): 427-439.DOI: 10.12211/2096-8280.2020-045
• Invited Review • Previous Articles Next Articles
Intelligent microbial cell factory with tolerance for green biological manufacturing
XU Ke1, WANG Jingnan2, LI Chun1,2
- 1.Key Lab for Industrial Biocatalysis,Ministry of Education,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
2.Department of Biochemical Engineering,School of Chemistry and Chemical Engineering,Beijing Institute of Technology,Beijing 100081,China
-
Received:2020-04-09Revised:2020-05-02Online:2020-10-09Published:2020-08-31 -
Contact:LI Chun
智能抗逆微生物细胞工厂与绿色生物制造
许可1, 王靖楠2, 李春1,2
- 1.清华大学化学工程系,工业生物催化教育部重点实验室,北京 100084
2.北京理工大学化学与化工学院,生物化工研究所,北京 100081
-
通讯作者:李春 -
作者简介:许可(1983— ),男,博士,助理研究员,研究方向为代谢工程与合成生物学。E-mail: xuke528@tsinghua.edu.cn
李春(1970— ),男,博士生导师,教授,研究方向为代谢工程与合成生物学。E-mail: lichun@tsinghua.edu.cn -
基金资助:国家自然科学基金(22078171)
CLC Number:
Cite this article
XU Ke, WANG Jingnan, LI Chun. Intelligent microbial cell factory with tolerance for green biological manufacturing[J]. Synthetic Biology Journal, 2020, 1(4): 427-439.
许可, 王靖楠, 李春. 智能抗逆微生物细胞工厂与绿色生物制造[J]. 合成生物学, 2020, 1(4): 427-439.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2020-045
| 1 | LIU Zihe, WANG Kai, CHEN Yun, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3(3): 274-288. |
| 2 | XU Ke, Bo LYU, HUO Yixin, et al. Toward the lowest energy consumption and emission in biofuel production: combination of ideal reactors and robust hosts [J]. Current Opinion in Biotechnology, 2017, 50: 19-24. |
| 3 | 谭天伟, 苏海佳, 陈必强, 等. 绿色生物制造[J]. 北京化工大学学报(自然科学版), 2018, 45(5): 107-118. |
| TAN Tianwei, SU Haijia, CHEN Biqiang, et al. Green bio-manufacturing [J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2018, 45(5): 107-118. | |
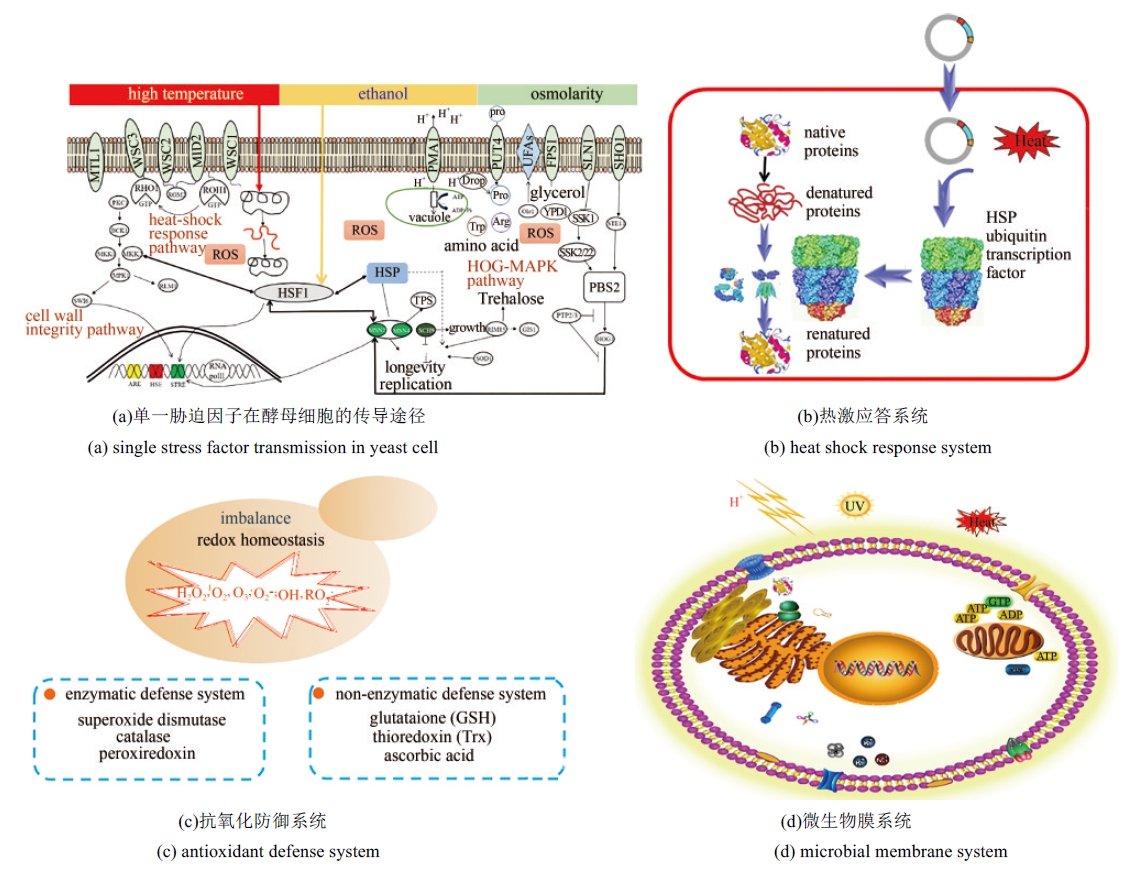
| 4 | DEPARIS Q, CLAES A, FOULQUIÉ-MORENO M R, et al. Engineering tolerance to industrially relevant stress factors in yeast cell factories [J]. FEMS Yeast Research, 2017, 17(4): fox036. |
| 5 | 赵美琳, 诸葛斌, 陆信曜, 等. 工业酵母抗逆机理研究进展[J]. 微生物学通报, 2019(5): 1155-1164. |
| ZHAO Meilin, ZHUGE bin, LU Xinyao, et al. Research progress in stress tolerance of industrial yeasts[J]. Microbiology China, 2019(5): 1155-1164. | |
| 6 | LAM F H, GHADERI A, FINK G R, et al. Biofuels. Engineering alcohol tolerance in yeast [J]. Science, 2014, 346(6205): 71-75. |
| 7 | UNREAN P. Flux control-based design of furfural-resistance strains of Saccharomyces cerevisiae for lignocellulosic biorefinery [J]. Bioprocess and Biosystems Engineering, 2017, 40(4): 611-623. |
| 8 | GUBICZA K, NIEVES I U, SAGUES W J, et al. Techno-economic analysis of ethanol production from sugarcane bagasse using a Liquefaction plus Simultaneous Saccharification and co-Fermentation process [J]. Bioresource Technology, 2016, 208: 42-48. |
| 9 | QIN Lei, LI Wenchao, LIU Li, et al. Inhibition of lignin-derived phenolic compounds to cellulase [J]. Biotechnology for Biofuels, 2016, 9(1): 70. |
| 10 | MAITY S K. Opportunities, recent trends and challenges of integrated biorefinery: part I [J]. Renewable and Sustainable Energy Reviews, 2015, 43: 1427-1445. |
| 11 | 张克俞, 张明明, 赵心清, 等. 关键基因过表达提高酿酒酵母抑制剂耐受性及乙醇发酵性能[J]. 应用与环境生物学报, 2018, 24(3): 541-546. |
| ZHANG Keyu, ZHANG Mingming, ZHAO Xinqing, et al. Improvement of inhibitor stress tolerance and ethanol fermentation of Saccharomyces cerevisiae by overexpression of novel key genes [J]. Chinese Journal of Applied and Environmental Biology, 2018, 24(3): 541-546. | |
| 12 | CLOMBURG J M, CRUMBLEY A M, GONZALEZ R. Industrial biomanufacturing: the future of chemical production [J]. Science, 2017, 355(6320): aag0804. |
| 13 | ABBOTT D A, INGLEDEW W M. Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth of Saccharomyces cerevisiae and ethanol production [J]. Biotechnology Letters, 2004, 26(16): 1313-1316. |
| 14 | AUESUKAREE C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation [J]. Journal of Bioscience and Bioengineering, 2017, 124(2): 133-142. |
| 15 | XU Ke, YU Liping, BAI Wenxin, et al. Construction of thermo-tolerant yeast based on an artificial protein quality control system (APQC) to improve the production of bio-ethanol [J]. Chemical Engineering Science, 2018, 177: 410-416. |
| 16 | CASPETA L, NIELSEN J. Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral temperatures and preadaptation to other stresses [J]. mBio, 2015, 6(4): e00431-15. |
| 17 | GAO Liman, LIU Yueqin, SUN Hun, et al. Advances in mechanisms and modifications for rendering yeast thermotolerance [J]. Journal of Bioscience and Bioengineering, 2016, 121(6): 599-606. |
| 18 | LUHE A L, TAN L, WU J C, et al. Increase of ethanol tolerance of Saccharomyces cerevisiae by error-prone whole genome amplification [J]. Biotechnology Letters, 2011, 33(5): 1007-1011. |
| 19 | DIVATE N R, CHEN G H, WANG P M, et al. Engineering Saccharomyces cerevisiae for improvement in ethanol tolerance by accumulation of trehalose [J]. Bioengineered, 2016, 7(6): 445-458. |
| 20 | 朱宝生, 刘功良, 白卫东, 等. 耐高糖酵母筛选及其高糖胁迫机制的研究进展[J]. 中国酿造, 2016, 35(6): 11-14. |
| ZHU Baosheng, LIU Gongliang, BAI Weidong, et al. Screening of high-sugar-tolerant yeast and research on its high sugar stress [J]. China Brewing, 2016, 35(6): 11-14. | |
| 21 | 毕新煜, 刘功良, 姜弘佳, 等. 酵母菌高糖耐受机制的研究进展[J]. 中国酿造, 2017(10): 1-4. |
| BI Xinyu, LIU Gongliang, JIANG Hongjia, et al. Research progress on high sugar tolerance mechanism of yeast [J]. China Brewing, 2017(10): 1-4. | |
| 22 | SHI Xinchi, ZOU Yanan, CHEN Yong, et al. Overexpression of THI4 and HAP4 improves glucose metabolism and ethanol production in Saccharomyces cerevisiae [J]. Frontiers in Microbiology, 2018, 9: 1444. |
| 23 | 岳国君, 武国庆, 郝小明. 我国燃料乙醇生产技术的现状与展望[J]. 化学进展, 2007, 19(7): 1084-1090. |
| YUE Guojun, WU Guoqing, HAO Xiaoming. The status quo and prospects of fuel ethanol process technology in China [J]. Progress in Chemistry, 2007, 19(7): 1084-1090. | |
| 24 | 赵心清, 张明明, 徐桂红, 等. 酿酒酵母乙酸耐性分子机制的功能基因组进展[J]. 生物工程学报, 2014, 30(3): 368-380. |
| ZHAO Xinqing, ZHANG Mingming, XU Guihong, et al. Advances in functional genomics studies underlying acetic acid tolerance of Saccharomyces cerevisiae [J]. Chinese Journal of Biotechnology, 2014, 30(3): 368-380. | |
| 25 | ZI Z K, WOLFRAM L, EDDA K. A quantitative study of the Hog1 MAPK response to fluctuating osmotic stress in Saccharomyces cerevisiae [J]. PLoS One, 2010, 5(3): e9522. |
| 26 | KIM Daehee, HAHN Ji-Sook. Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae's tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress [J]. Applied and Environmental Microbiology, 2013, 79(16): 5069-5077. |
| 27 | WOJDA I, ALONSO-MONGE R, BEBELMAN J P, et al. Response to high osmotic conditions and elevated temperature in Saccharomyces cerevisiae is controlled by intracellular glycerol and involves coordinate activity of MAP kinase pathways [J]. Microbiology, 2003, 149(5): 1193-1204. |
| 28 | CHAROENBHAKDI S, DOKPIKUL T, BURPHAN T, et al. Vacuolar H+-ATPase protects Saccharomyces cerevisiae cells against ethanol-induced oxidative and cell wall stresses [J]. Applied & Environmental Microbiology, 2016, 82(16): 5057. |
| 29 | HERRERO E, ROS J, BELLÍ G, et al. Redox control and oxidative stress in yeast cells [J]. BBA - General Subjects, 2008, 1780(11): 1217-1235. |
| 30 | MORANO K A, GRANT C M, MOYE-ROWLEY W S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae [J]. Genetics, 2012, 190(4): 1157-1195. |
| 31 | LIU Yueqin, ZHANG Genli, SUN Huan, et al. Enhanced pathway efficiency of Saccharomyces cerevisiae by introducing thermo-tolerant devices [J]. Bioresource Technology, 2014, 170(5): 38-44. |
| 32 | XU K, GAO L M, HASSAN J U, et al. Improving the thermo-tolerance of yeast base on the antioxidant defense system [J]. Chemical Engineering Science, 2018, 175: 335-342. |
| 33 | MORANO K A, GRANT C M, MOYE-ROWLEY W S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae [J]. Genetics, 2012, 190(4): 1157-1195. |
| 34 | FINLEY D, ULRICH H D, SOMMER T, et al. The ubiquitin-proteasome system of Saccharomyces cerevisiae [J]. Genetics, 192(2): 319-360. |
| 35 | HIPP M S, PARK S H, HARTL F U. Proteostasis impairment in protein-misfolding and-aggregation diseases [J]. Trends in Cell Biology, 2014, 24(9): 506-514. |
| 36 | BENYAIR R, RON E, LEDERKREMER G Z, et al. Protein quality control, retention, and degradation at the endoplasmic reticulum [J]. 2011, 292: 197-280. |
| 37 | SANDOVAL N R, PAPOUTSAKIS E T. Engineering membrane and cell-wall programs for tolerance to toxic chemicals: beyond solo genes [J]. Current Opinion in Microbiology, 2016, 33: 56-66. |
| 38 | RAY S, KASSAN A, BUSIJA A R, et al. The plasma membrane as a capacitor for energy and metabolism [J]. American Journal of Physiology - Cell Physiology, 2016, 310(3): C181-C192. |
| 39 | QI Yanli, LIU Hui, CHEN Xiulai, et al. Engineering microbial membranes to increase stress tolerance of industrial strains [J]. Metabolic Engineering, 2019, 53: 24-34. |
| 40 | 方华, 李灏. 海藻糖与热激蛋白在酿酒酵母耐受乙醇胁迫中的作用[J]. 中国生物工程杂志, 2014, 34(6): 84-89. |
| FANG Hua, LI Hao. The roles of trehalose and heat shock proteins for enhancing ethanol tolerance of Saccharomyces cerevisiae [J]. China Biotechnology, 2014, 34(6): 84-89. | |
| 41 | LEITE F C, LEITE D V, PEREIRA L F, et al. High intracellular trehalase activity prevents the storage of trehalose in the yeast Dekkera bruxellensis [J]. Letters in Applied Microbiology, 2016, 63(3): 210-214. |
| 42 | ELEUTHERIO E, PANEK A, MESQUITA J F D, et al. Revisiting yeast trehalose metabolism [J]. Current Genetics, 2015, 61(3): 263-274. |
| 43 | PETITJEAN M, TESTE M A, FRANCOIS J M, et al. Yeast tolerance to various stresses relies on the trehalose-6P synthase (Tps1) protein, not on trehalose [J]. Journal of Biological Chemistry, 2015, 290(26): 16177-16190. |
| 44 | BUSTI S, MAPELLI V, TRIPODI F, et al. Respiratory metabolism and calorie restriction relieve persistent endoplasmic reticulum stress induced by calcium shortage in yeast [J]. Scientific Reports, 2016, 6: 27942. |
| 45 | CASPETA L, CASTILLO T, NIELSEN J. Modifying yeast tolerance to inhibitory conditions of ethanol production processes [J]. Frontiers in Bioengineering and Biotechnology, 2015, 3: 184. |
| 46 | CASPETA L, CHEN Y, NIELSEN J. Thermotolerant yeasts selected by adaptive evolution express heat stress response at 30 ℃ [J]. Scientific Reports, 2016, 6: 27003. |
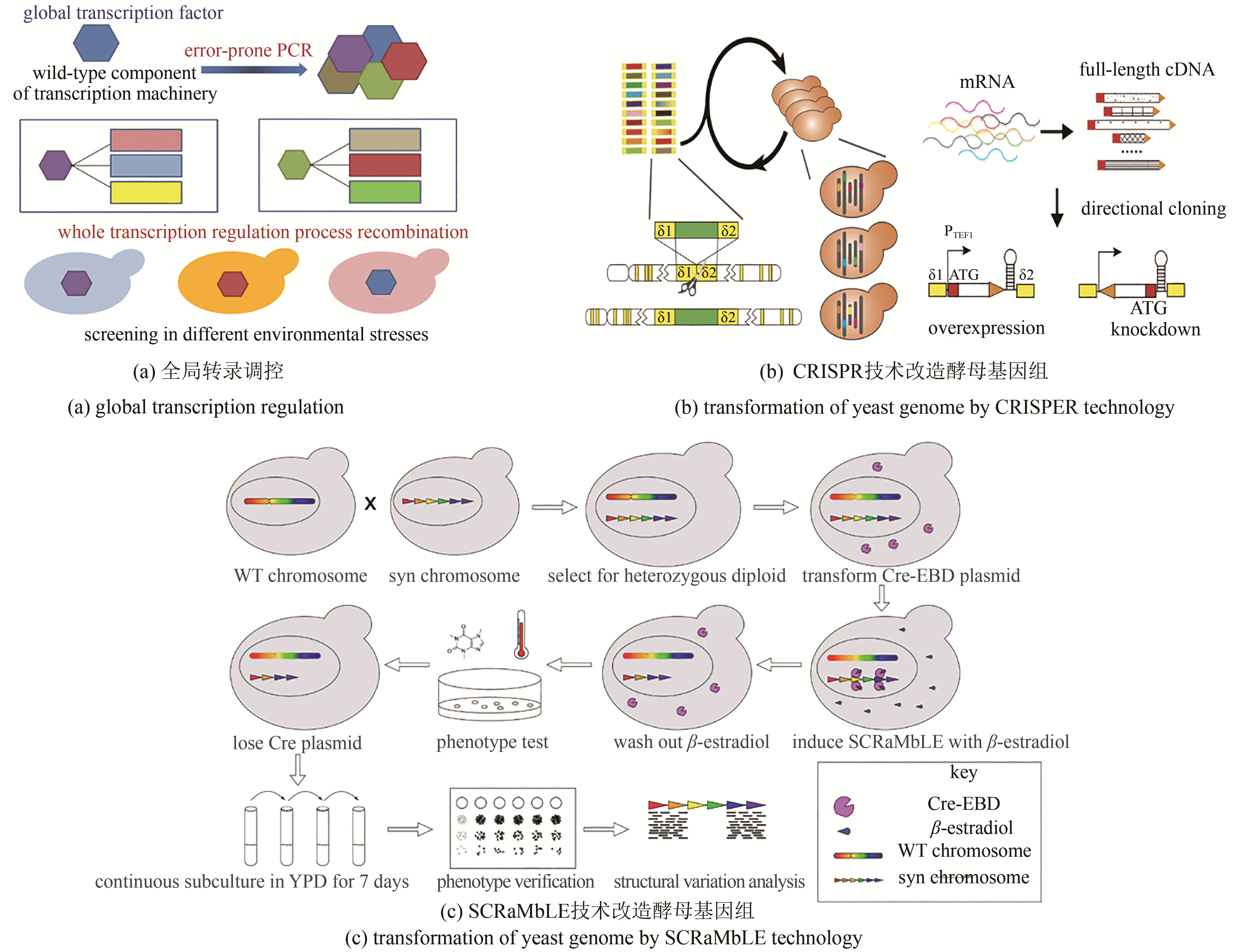
| 47 | ALPER H, MOXLEY J, NEVOIGT E, et al. Engineering yeast transcription machinery for improved ethanol tolerance and production [J]. Science, 2006, 314(5805): 1565. |
| 48 | SI Tong, CHAO Ran, MIN Yuhao, et al. Automated multiplex genome-scale engineering in yeast [J]. Nature Communications, 2017, 8: 15187. |
| 49 | SHEN M J, WU Y, YANG K, et al. Heterozygous diploid and interspecies SCRaMbLEing [J]. Nature Communications, 2018, 9(1): 1934. |
| 50 | SNOEK T, NICOLINO M P, BREMT S VAN DEN, et al. Large-scale robot-assisted genome shuffling yields industrial Saccharomyces cerevisiae yeasts with increased ethanol tolerance [J]. Biotechnology for Biofuels, 2015, 8(1): 32. |
| 51 | PARENTEAU J, MAIGNON L, BERTHOUMIEUX M, et al. Introns are mediators of cell response to starvation [J]. Nature, 2019, 565: 612-617. |
| 52 | 赵学明, 王庆昭. 合成生物学: 学科基础, 研究进展与前景展望[J]. 前沿科学, 2007(3): 56-66. |
| ZHAO Xueming, WANG Qingzhao. Synthetic biology: fundamentals, advances and prospect [J]. Frontier Science, 2007(3): 56-66. | |
| 53 | CAMERON D E, BASHOR C J, COLLINS J J. A brief history of synthetic biology [J]. Nature Reviews Microbiology, 2014, 12(5): 381-390. |
| 54 | 张先恩. 中国合成生物学发展回顾与展望[J]. 中国科学(生命科学), 2019(12): 1543-1572. |
| ZHANG X-E. Synthetic biology in China: review and prospects [J]. SCIENTIA SINICA Vitae, 2019(12): 1543-1572. | |
| 55 | 杜鹤童, 赵倚晴, 陈金春, 等. 基于嗜盐微生物合成生物学的下一代工业生物技术[J]. 生命科学, 2019, 31(4): 385-390. |
| DU Hetong, ZHAO Yiqing, CHEN Jinchun, et al. Next generation industrial biotechnology based on synthetic biology of halophiles [J]. Chinese Bulletin of Life Sciences, 2019, 31(4): 385-390. | |
| 56 | SIBANDA T, SELVARAJAN R, TEKERE M J M B. Synthetic extreme environments: overlooked sources of potential biotechnologically relevant microorganisms [J]. Microbial Biotechnology, 2017, 10(3): 570-585. |
| 57 | GONG Z W, NIELSEN J, ZHOU Y J J. Engineering robustness of microbial cell factories [J]. Biotechnology Journal, 2017, 12(10): 1700014. |
| 58 | JIA Haiyang, FAN Yanshuang, FENG Xudong, et al. Enhancing stress-resistance for efficient microbial biotransformations by synthetic biology [J]. Frontiers in Bioengineering and Biotechnology, 2014, 2: 44. |
| 59 | XU Ke, Yun Seo LEE, LI Jun, et al. Resistance mechanisms and reprogramming of microorganisms for efficient biorefinery under multiple environmental stresses [J]. Synthetic and Systems Biotechnology, 2019, 4(2): 92-98. |
| 60 | JIA Haiyang, SUN Xiangying, SUN Huan, et al. Intelligent microbial heat-regulating engine (IMHeRE) for improved thermo-robustness and efficiency of bioconversion [J]. ACS Synthetic Biology, 2016, 5(4): 312-320. |
| 61 | TAN Z G, KHAKBAZ P, CHEN Y X, et al. Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables [J]. Metabolic Engineering, 2017, 44: 1-12. |
| 62 | PHAM H L, WONG A, CHUA N Y, et al. Engineering a riboswitch-based genetic platform for the self-directed evolution of acid-tolerant phenotypes [J]. Nature Communications, 2017, 8(1): 411. |
| 63 | XU Ke, QIN Lei, BAI Wenxin, et al. Multilevel defense system (MDS) relieves multiple stresses for economically boosting ethanol production of industrial Saccharomyces cerevisiae [J]. ACS Energy Letters, 2020, 5(2): 572-582. |
| 64 | QIN L, DONG S, YU J, et al. Stress-driven dynamic regulation of multiple tolerance genes improves robustness and productive capacity of Saccharomyces cerevisiae in industrial lignocellulose fermentation [J]. Metabolic Engineering, 2020, 61: 160-170. |
| [1] | WU Yujie, LIU Xinxin, LIU Jianhui, Yang Kaiguang, SUI Zhigang, ZHANG Lihua, ZHANG Yukui. Research progress of strain screening and quantitative analysis of key molecules based on high-throughput liquid chromatography and mass spectrometry [J]. Synthetic Biology Journal, 2023, 4(5): 1000-1019. |
| [2] | GAO Xianyun, NIU Lingxue, JIAN Ni, GUAN Ningzi. Applications of microbial synthetic biology in the diagnosis and treatment of diseases [J]. Synthetic Biology Journal, 2023, 4(2): 263-282. |
| [3] | REN Shichao, SUN Qiuyan, FENG Xudong, LI Chun. Biosynthesis of pentacyclic triterpenoid saponins in microbial cell factories [J]. Synthetic Biology Journal, 2022, 3(1): 168-183. |
| [4] | CHEN Jiuzhou, WANG Yu, PU Wei, ZHENG Ping, SUN Jibin. Advances and perspective on bioproduction of 5-aminolevulinic acid [J]. Synthetic Biology Journal, 2021, 2(6): 1000-1016. |
| [5] | XU Peng. In memory of Prof. Daniel I.C. Wang: Engineering Yarrowia lipolytica for the production of plant-based lipids: technical constraints and perspectives for a sustainable cellular agriculture economy [J]. Synthetic Biology Journal, 2021, 2(4): 509-527. |
| [6] | XIA Siyang, JIANG Lihong, CAI Jin, HUANG Lei, XU Zhinan, LIAN Jiazhang. Advances in genome evolution of Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2020, 1(5): 556-569. |
| [7] | Shuobo SHI, Qiongyu MENG, Weibo QIAO, Huimin ZHAO. Establishing carbon dioxide-based third-generation biorefinery for a sustainable low-carbon economy [J]. Synthetic Biology Journal, 2020, 1(1): 44-59. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||