Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (1): 138-154.DOI: 10.12211/2096-8280.2021-029
• Invited Review • Previous Articles Next Articles
Research progress in cellulosomes and their applications in synthetic biology
FENG Yingang1,2,3, LIU Yajun1,2,3, CUI Qiu1,2,3
- 1.Shandong Provincial Key Laboratory of Synthetic Biology,CAS Key Laboratory of Biofuels,Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Sciences,Qingdao 266101,Shandong,China
2.Qingdao Engineering Laboratory of Single Cell Oil,Shandong Engineering Laboratory of Single Cell Oil,Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Sciences,Qingdao 266101,Shandong,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2021-02-26Revised:2021-04-10Online:2022-03-14Published:2022-02-28 -
Contact:FENG Yingang
纤维小体在合成生物学中的应用研究进展
冯银刚1,2,3, 刘亚君1,2,3, 崔球1,2,3
- 1.中国科学院青岛生物能源与过程研究所,中国科学院生物燃料重点实验室,山东省合成生物学重点实验室,山东 青岛 266101
2.中国科学院青岛生物能源与过程研究所,山东省单细胞油脂工程实验室,青岛市单细胞油脂工程实验室,山东 青岛 266101
3.中国科学院大学,北京 100049
-
通讯作者:冯银刚 -
作者简介:冯银刚 (1977—),男,博士,研究员,博士生导师。研究方向为能源微生物的分子生理机制与合成生物学应用、工业酶催化机制与酶工程等。E-mail:fengyg@qibebt.ac.cn -
基金资助:国家自然科学基金(32070125)
CLC Number:
Cite this article
FENG Yingang, LIU Yajun, CUI Qiu. Research progress in cellulosomes and their applications in synthetic biology[J]. Synthetic Biology Journal, 2022, 3(1): 138-154.
冯银刚, 刘亚君, 崔球. 纤维小体在合成生物学中的应用研究进展[J]. 合成生物学, 2022, 3(1): 138-154.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-029
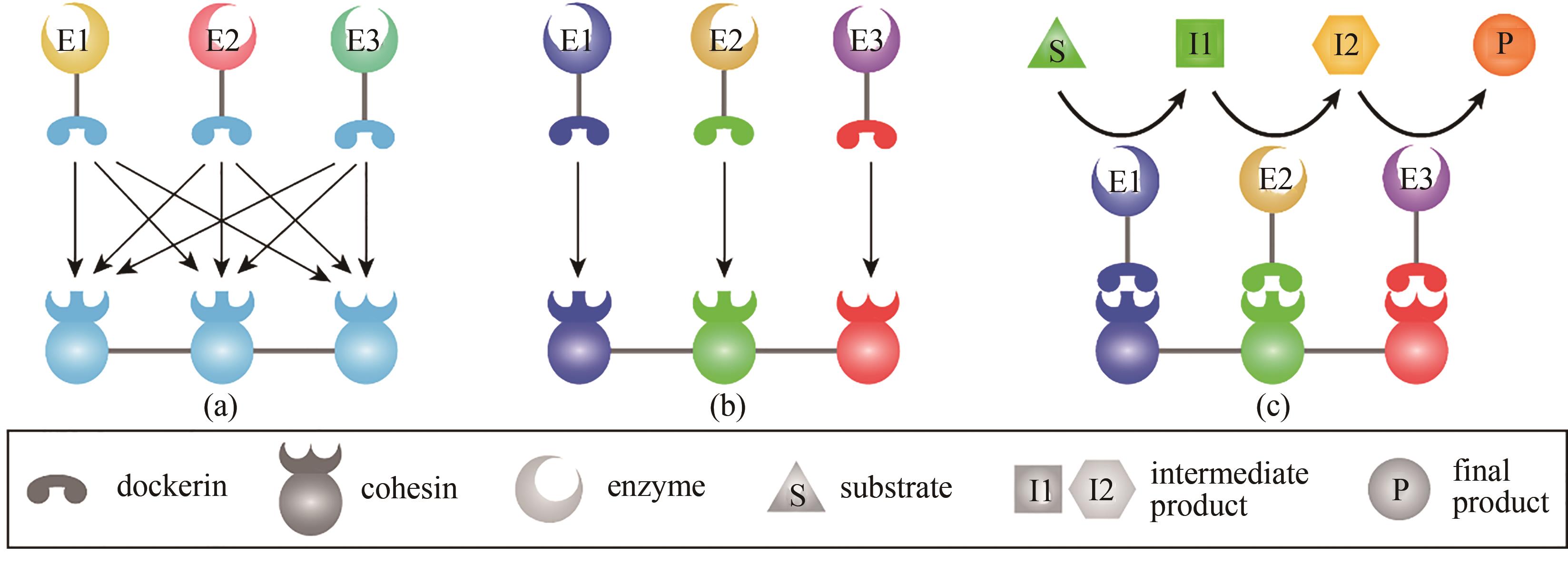
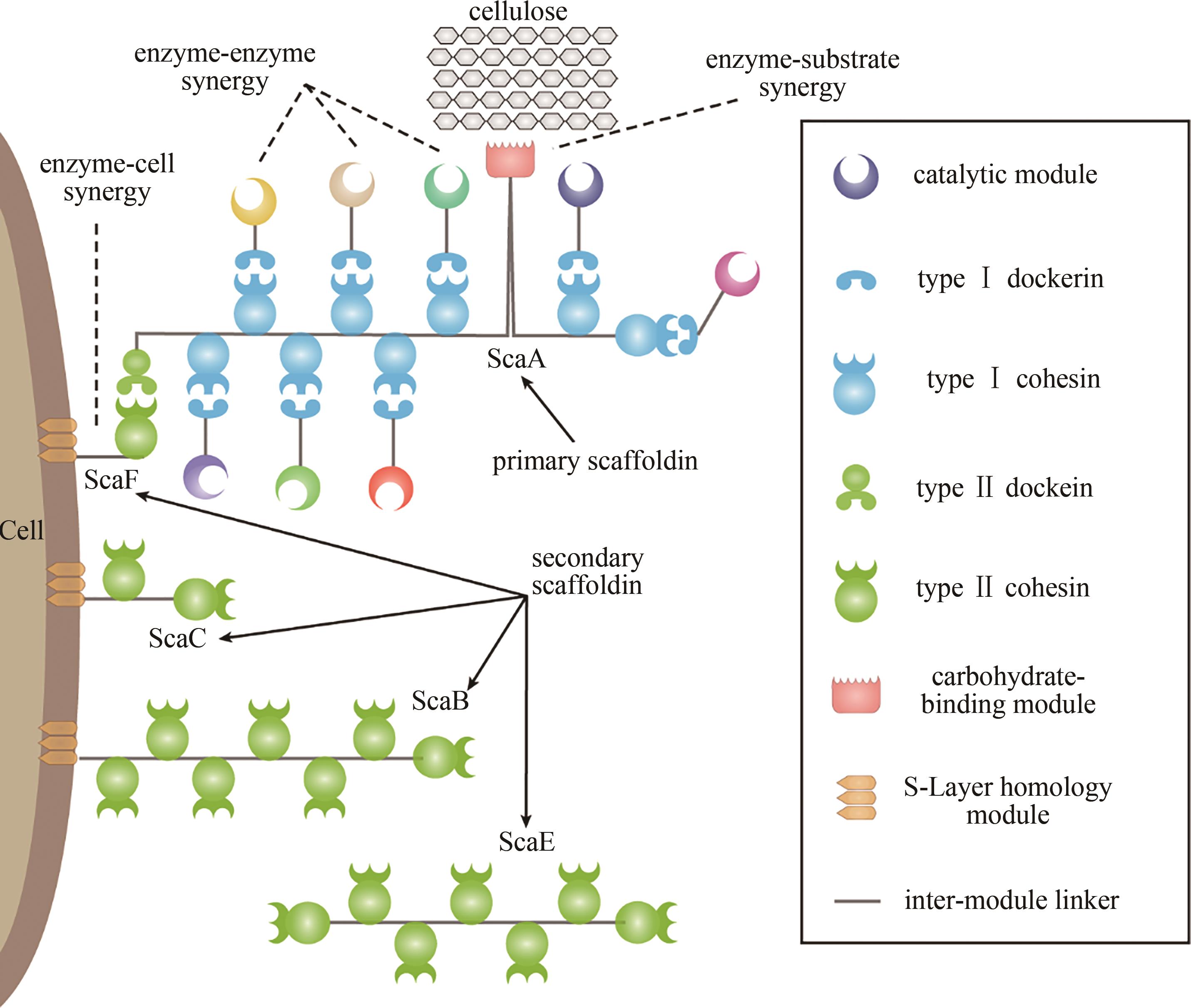
Fig. 2 Natural cellulosomes, designer cellulosomes, and substrate-channeling effects(a) naturally occurring cellulosomes contain highly homologous assembly modules, i.e. cohesins and dockerins, resulting in the random assembly of enzymes on the scaffoldin; (b) designer cellulosomes use assembly modules from different bacteria (shown in different colors), which have species-specific interactions for the enzymes to be assembled at specific positions according to the design; (c) cascading catalytic reactions are enhanced by assembling the enzyme cascade into a designer cellulosome, and the substrate is converted to the final product once it has passed through the channel, which is termed as the substrate-channeling effect
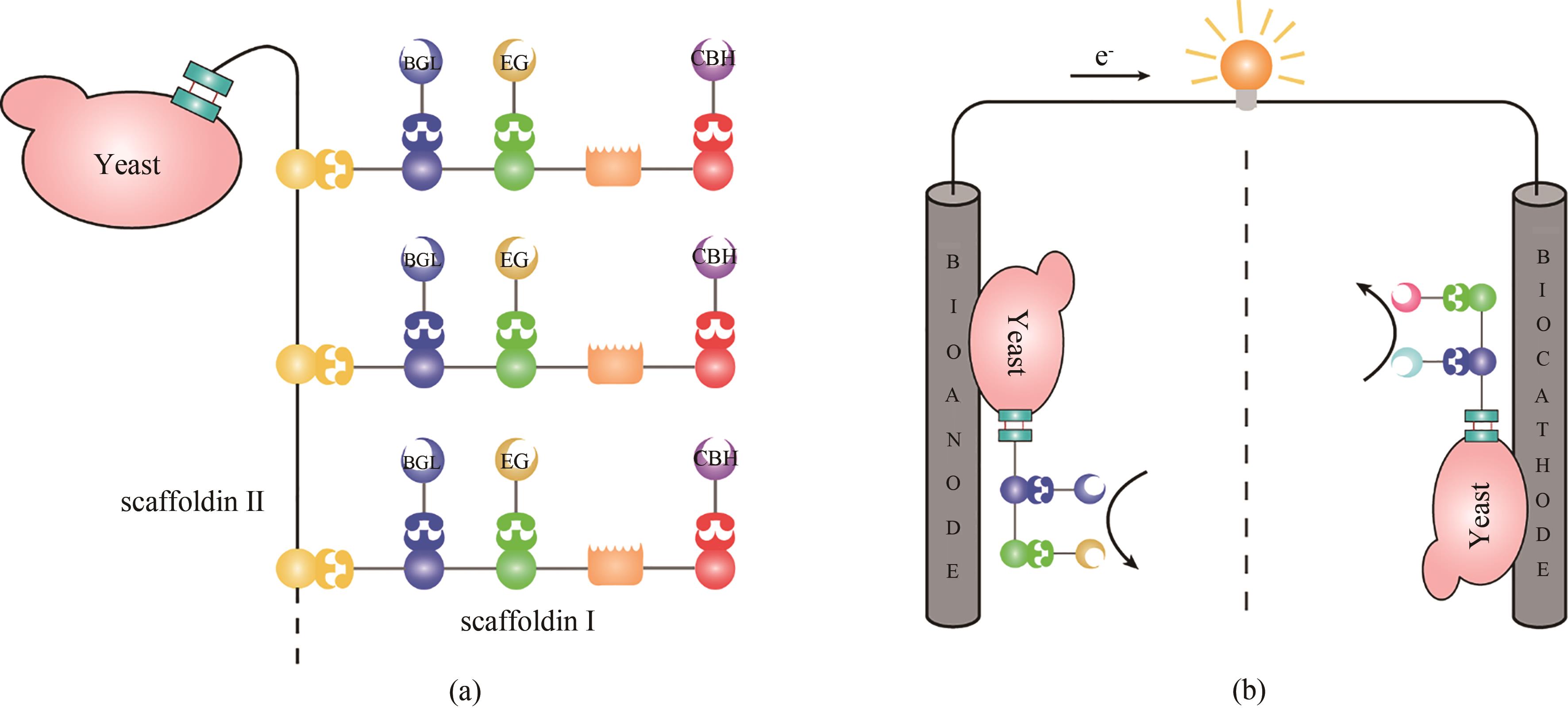
Fig. 3 Cell-display using designer cellulosomes(a) designer cellulosomes displayed on yeast cell surfaces allow the yeast to degrade cellulose; (b) using yeasts displaying designer cellulosomes containing enzyme cascades to serve as a bioanode and biocathode, the performance of the biofuel cells can be significantly enhanced
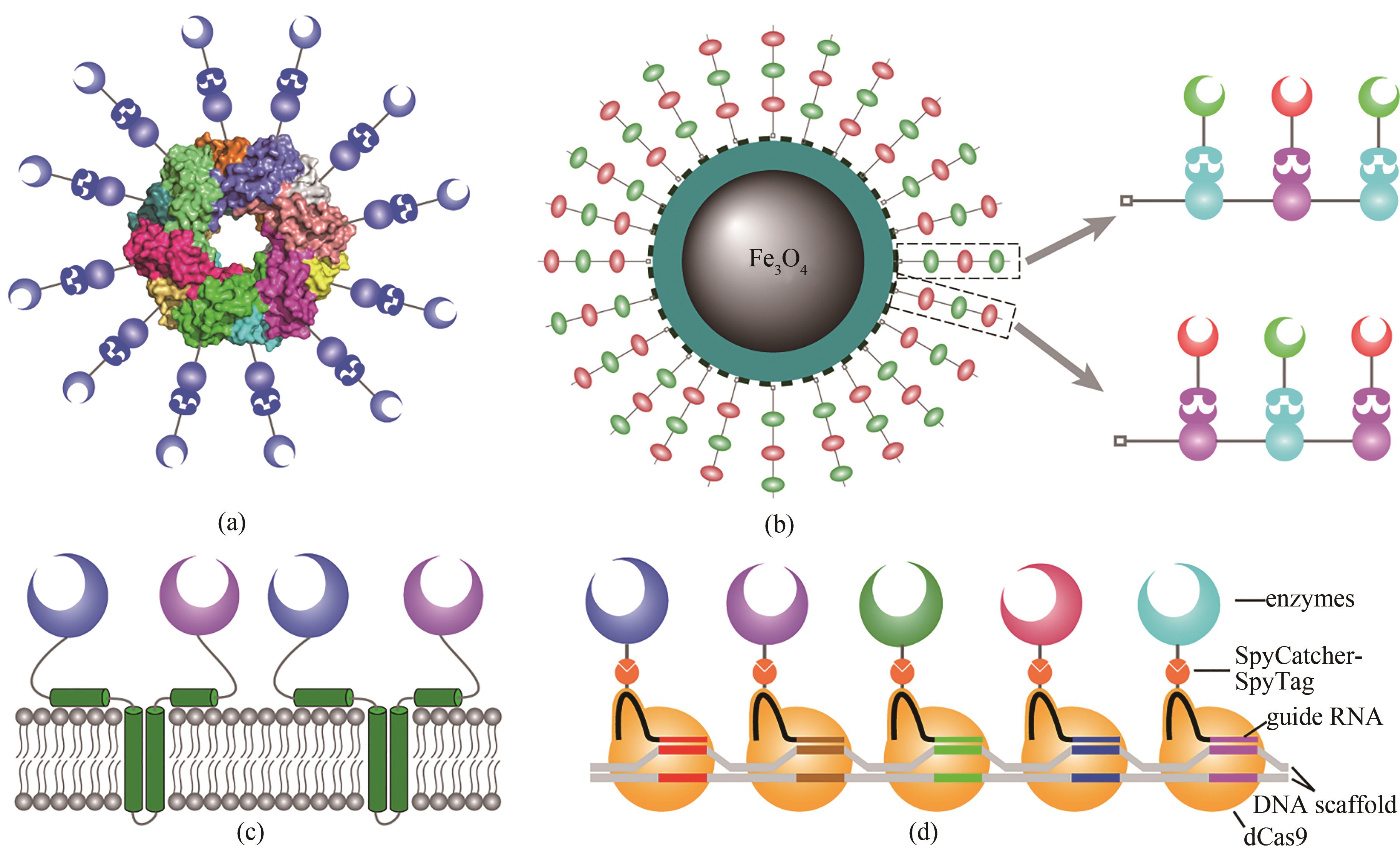
Fig. 4 Various schemes for developing artificial cellulosomes(a) Artificial cellulosomes are constructed using an oligomeric protein as scaffold; (b) using nanoparticles as scaffold for the construction of artificial cellulosomes, loading of enzymes can be significantly increased; (c) artificial cellulosomes can be constructed on surface of organelles using a membrane protein as scaffold; (d) Artificial cellulosomes can be constructed using DNA as the scaffold with the specificity of dCas9 and guide RNA.
| 1 | BAYER E A, MORAG E, LAMED R. The cellulosome—a treasure-trove for biotechnology[J]. Trends in Biotechnology, 1994, 12(9): 379-386. |
| 2 | BAYER E A. Cellulosomes and designer cellulosomes: why toy with Nature?[J]. Environmental Microbiology Reports, 2017, 9(1): 14-15. |
| 3 | HU B B, ZHU M J. Reconstitution of cellulosome: research progress and its application in biorefinery[J]. Biotechnology and Applied Biochemistry, 2019, 66(5): 720-730. |
| 4 | BAYER E A, KENIG R, LAMED R. Adherence of Clostridium thermocellum to cellulose[J]. Journal of Bacteriology, 1983, 156(2): 818-827. |
| 5 | LAMED R, SETTER E, BAYER E A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum [J]. Journal of Bacteriology, 1983, 156(2): 828-836. |
| 6 | LAMED R, SETTER E, KENIG R, et al. The cellulosome: a discrete cell surface organelle of Clostridium thermocellum which exhibits separate antigenic, cellulose-binding and various cellulolytic activities[J]. Biotechnology Bioengineering Symposium, 1983, 13(13): 163-181. |
| 7 | BAYER E A, SETTER E, LAMED R. Organization and distribution of the cellulosome in Clostridium thermocellum [J]. Journal of Bacteriology, 1985, 163(2): 552-559. |
| 8 | FELIX C R, LJUNGDAHL L G. The cellulosome: the exocellular organelle of Clostridium [J]. Annual Review of Microbiology, 1993, 47: 791-819. |
| 9 | BAYER E A, SHIMON L J W, SHOHAM Y, et al. Cellulosomes—structure and ultrastructure[J]. Journal of Structural Biology, 1998, 124(2/3): 221-234. |
| 10 | BAYER E A, LAMED R, WHITE B A, et al. From cellulosomes to cellulosomics[J]. Chemical Record, 2008, 8(6): 364-377. |
| 11 | FONTES C M G A, GILBERT H J. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates[J]. Annual Review of Biochemistry, 2010, 79: 655-681. |
| 12 | SMITH S P, BAYER E A. Insights into cellulosome assembly and dynamics: from dissection to reconstruction of the supramolecular enzyme complex[J]. Current Opinion in Structural Biology, 2013, 23(5): 686-694. |
| 13 | NATAF Y, BAHARI L, KAHEL-RAIFER H, et al. Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic polysaccharides via alternative sigma factors[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(43): 18646-18651. |
| 14 | DE ORA L O, LAMED R, LIU Y J, et al. Regulation of biomass degradation by alternative σ factors in cellulolytic clostridia[J]. Scientific Reports, 2018, 8: 11036. |
| 15 | WEI Z, CHEN C, LIU Y J, et al. Alternative σI/anti-σI factors represent a unique form of bacterial σ/anti-σ complex[J]. Nucleic Acids Research, 2019, 47(11): 5988-5997. |
| 16 | MECHALY A, FIEROBE H P, BELAICH A, et al. Cohesin-dockerin interaction in cellulosome assembly: a single hydroxyl group of a dockerin domain distinguishes between nonrecognition and high affinity recognition[J]. Journal of Biological Chemistry, 2001, 276(13): 9883-9888. |
| 17 | STAHL S W, NASH M A, FRIED D B, et al. Single-molecule dissection of the high-affinity cohesin-dockerin complex[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(50): 20431-20436. |
| 18 | HEVRONI B L, MORAÏS S, BEN-DAVID Y, et al. Minimalistic cellulosome of the butanoogenic bacterium Clostridium saccharoperbutylacetonicum [J]. mBio, 2020, 11(2): e00443. |
| 19 | ZHIVIN O, DASSA B, MORAÏS S, et al. Unique organization and unprecedented diversity of the Bacteroides (Pseudobacteroides) cellulosolvens cellulosome system[J]. Biotechnology for Biofuels, 2017, 10: 211. |
| 20 | ARTZI L, BAYER E A, MORAÏS S. Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides[J]. Nature Reviews Microbiology, 2017, 15(2): 83-95. |
| 21 | BULE P, PIRES V M R, FONTES C M G A, et al. Cellulosome assembly: paradigms are meant to be broken![J]. Current Opinion in Structural Biology, 2018, 49: 154-161. |
| 22 | BULE P, PIRES V M R, ALVES V D, et al. Higher order scaffoldin assembly in Ruminococcus flavefaciens cellulosome is coordinated by a discrete cohesin-dockerin interaction[J]. Scientific Reports, 2018, 8: 6987. |
| 23 | XU Q, RESCH M G, PODKAMINER K, et al. Dramatic performance of Clostridium thermocellum explained by its wide range of cellulase modalities[J]. Science Advances, 2016, 2(2): e1501254. |
| 24 | HONG W, ZHANG J, FENG Y G, et al. The contribution of cellulosomal scaffoldins to cellulose hydrolysis by Clostridium thermocellum analyzed by using thermotargetrons[J]. Biotechnology for Biofuels, 2014, 7: 80. |
| 25 | GARCÍA-ALVAREZ B, MELERO R, DIAS F M V, et al. Molecular architecture and structural transitions of a Clostridium thermocellum mini-cellulosome[J]. Journal of Molecular Biology, 2011, 407(4): 571-580. |
| 26 | EIBINGER M, GANNER T, PLANK H, et al. A biological nanomachine at work: watching the cellulosome degrade crystalline cellulose[J]. ACS Central Science, 2020, 6(5): 739-746. |
| 27 | STEVENSON D M, WEIMER P J. Expression of 17 genes in Clostridium thermocellum ATCC 27405 during fermentation of cellulose or cellobiose in continuous culture[J]. Applied and Environmental Microbiology, 2005, 71(8): 4672-4678. |
| 28 | GOLD N D, MARTIN V J J. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis[J]. Journal of Bacteriology, 2007, 189(19): 6787-6795. |
| 29 | ZVERLOV V V, SCHWARZ W H. Bacterial cellulose hydrolysis in anaerobic environmental subsystems—Clostridium thermocellum and Clostridium stercorarium, thermophilic plant-fiber degraders[J]. Annals of the New York Academy of Sciences, 2008, 1125: 298-307. |
| 30 | RAMAN B, PAN C L, HURST G B, et al. Impact of pretreated switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: a quantitative proteomic analysis[J]. PLoS One, 2009, 4(4): e5271. |
| 31 | RAMAN B, MCKEOWN C K, RODRIGUEZ M, et al. Transcriptomic analysis of Clostridium thermocellum ATCC 27405 cellulose fermentation[J]. BMC Microbiology, 2011, 11: 134. |
| 32 | RIEDERER A, TAKASUKA T E, MAKINO S I, et al. Global gene expression patterns in Clostridium thermocellum as determined by microarray analysis of chemostat cultures on cellulose or cellobiose[J]. Applied and Environmental Microbiology, 2011, 77(4): 1243-1253. |
| 33 | GRINBERG I R, YANIV O, DE ORA L O, et al. Distinctive ligand-binding specificities of tandem PA14 biomass-sensory elements from Clostridium thermocellum and Clostridium clariflavum [J]. Proteins, 2019, 87(11): 917-930. |
| 34 | DE ORA L O, MUÑOZ-GUTIÉRREZ I, BAYER E A, et al. Revisiting the regulation of the primary scaffoldin gene in Clostridium thermocellum [J]. Applied and Environmental Microbiology, 2017, 83(8): e03088. |
| 35 | XU C G, HUANG R R, TENG L, et al. Cellulosome stoichiometry in Clostridium cellulolyticum is regulated by selective RNA processing and stabilization[J]. Nature Communications, 2015, 6: 6900. |
| 36 | FIEROBE H P, MECHALY A, TARDIF C, et al. Design and production of active cellulosome chimeras: Selective incorporation of dockerin-containing enzymes into defined functional complexes[J]. Journal of Biological Chemistry, 2001, 276(24): 21257-21261. |
| 37 | PAGÈS S, BÉLAÏCH A, BÉLAÏCH J P, et al. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain[J]. Proteins-Structure Function and Genetics, 1997, 29(4): 517-527. |
| 38 | HAIMOVITZ R, BARAK Y, MORAG E, et al. Cohesin-dockerin microarray: diverse specificities between two complementary families of interacting protein modules[J]. Proteomics, 2008, 8(5): 968-979. |
| 39 | FIEROBE H P, MECHALY A, TARDIF C, et al. Designer nanosomes: selective engineering of dockerin-containing enzymes into chimeric scaffoldins to form defined nanoreactors[M]// TEERI T T, SVENSSON B, GILBERT H J, et al. Carbohydrate Bioengineering: Interdisciplinary Approaches. Cambridge, U.K.: Royal Society of Chemistry, 2002: 113-124. |
| 40 | PERRET S, BÉLAICH A, FIEROBE H P, et al. Towards designer cellulosomes in Clostridia: mannanase enrichment of the cellulosomes produced by Clostridium cellulolyticum [J]. Journal of Bacteriology, 2004, 186(19): 6544-6552. |
| 41 | FIEROBE H P, MINGARDON F, MECHALY A, et al. Action of designer cellulosomes on homogeneous versus complex substrates: controlled incorporation of three distinct enzymes into a defined trifunctional scaffoldin[J]. Journal of Biological Chemistry, 2005, 280(16): 16325-16334. |
| 42 | MINGARDON F, CHANAL A, TARDIF C, et al. Exploration of new geometries in cellulosome-like chimeras[J]. Applied and Environmental Microbiology, 2007, 73(22): 7138-7149. |
| 43 | CASPI J, IRWIN D, LAMED R, et al. Conversion of Thermobifida fusca free exoglucanases into cellulosomal components: comparative impact on cellulose-degrading activity[J]. Journal of Biotechnology, 2008, 135(4): 351-357. |
| 44 | MORAÏS S, BARAK Y, HADAR Y, et al. Assembly of xylanases into designer cellulosomes promotes efficient hydrolysis of the xylan component of a natural recalcitrant cellulosic substrate[J]. mBio, 2011, 2(6): e00233. |
| 45 | STERN J, MORAÏS S, LAMED R, et al. Adaptor scaffoldins: an original strategy for extended designer cellulosomes, inspired from nature[J]. mBio, 2016, 7(2): e00083. |
| 46 | MORAÏS S, MORAG E, BARAK Y, et al. Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes[J]. mBio, 2012, 3(6): e00508. |
| 47 | STERN J, KAHN A, VAZANA Y, et al. Significance of relative position of cellulases in designer cellulosomes for optimized cellulolysis[J]. PLoS One, 2015, 10(5): e0127326. |
| 48 | CASPI J, BARAK Y, HAIMOVITZ R, et al. Effect of linker length and dockerin position on conversion of a Thermobifida fusca endoglucanase to the cellulosomal mode[J]. Applied and Environmental Microbiology, 2009, 75(23): 7335-7342. |
| 49 | MENG D D, WANG J, YOU C. The properties of the linker in a mini-scaffoldin influence the catalytic efficiency of scaffoldin-mediated enzyme complexes[J]. Enzyme and Microbial Technology, 2020, 133: 109460. |
| 50 | VAZANA Y, BARAK Y, UNGER T, et al. A synthetic biology approach for evaluating the functional contribution of designer cellulosome components to deconstruction of cellulosic substrates[J]. Biotechnology for Biofuels, 2013, 6(1): 182. |
| 51 | LAWRIE J, SONG X, NIU W, et al. A high throughput approach for the generation of orthogonally interacting protein pairs[J]. Scientific Reports, 2018, 8: 867. |
| 52 | GEFEN G, ANBAR M, MORAG E, et al. Enhanced cellulose degradation by targeted integration of a cohesin-fused β-glucosidase into the Clostridium thermocellum cellulosome[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(26): 10298-10303. |
| 53 | ZHANG J, LIU S Y, LI R M, et al. Efficient whole-cell-catalyzing cellulose saccharification using engineered Clostridium thermocellum [J]. Biotechnology for Biofuels, 2017, 10: 124. |
| 54 | LIU S Y, LIU Y J, FENG Y G, et al. Construction of consolidated bio-saccharification biocatalyst and process optimization for highly efficient lignocellulose solubilization[J]. Biotechnology for Biofuels, 2019, 12: 35. |
| 55 | LIU Y J, LI B, FENG Y G, et al. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world[J]. Biotechnology Advances, 2020, 40: 107535. |
| 56 | KAHN A, MORAÏS S, GALANOPOULOU A P, et al. Creation of a functional hyperthermostable designer cellulosome[J]. Biotechnology for Biofuels, 2019, 12: 44. |
| 57 | KAHN A, MORAÏS S, CHUNG D, et al. Glycosylation of hyperthermostable designer cellulosome components yields enhanced stability and cellulose hydrolysis[J]. The FEBS Journal, 2020, 287(20): 4370-4388. |
| 58 | ARFI Y, SHAMSHOUM M, ROGACHEV I, et al. Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(25): 9109-9114. |
| 59 | DAVIDI L, MORAÏS S, ARTZI L, et al. Toward combined delignification and saccharification of wheat straw by a laccase-containing designer cellulosome[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(39): 10854-10859. |
| 60 | HAN Z L, SU W W. Intein-mediated assembly of tunable scaffoldins for facile synthesis of designer cellulosomes[J]. Applied Microbiology and Biotechnology, 2018, 102(3): 1331-1342. |
| 61 | HYEON J E, JEON W J, WHANG S Y, et al. Production of minicellulosomes for the enhanced hydrolysis of cellulosic substrates by recombinant Corynebacterium glutamicum [J]. Enzyme and Microbial Technology, 2011, 48(4-5): 371-377. |
| HYEON J E, JEON W J, WHANG S Y, et al. Production of minicellulosomes for the enhanced hydrolysis of cellulosic substrates by recombinant corynebacterium glutamicum[J]. Enzyme and Microbial Technology, 2011, 48(4/5): 371-377. | |
| 62 | CHO H Y, YUKAWA H, INUI M, et al. Production of minicellulosomes from Clostridium cellulovorans in Bacillus subtilis WB800[J]. Applied and Environmental Microbiology, 2004, 70(9): 5704-5707. |
| 63 | CHANG J J, ANANDHARAJ M, HO C Y, et al. Biomimetic strategy for constructing Clostridium thermocellum cellulosomal operons in Bacillus subtilis [J]. Biotechnology for Biofuels, 2018, 11: 157. |
| 64 | ARAI T, MATSUOKA S, CHO H Y, et al. Synthesis of Clostridium cellulovorans minicellulosomes by intercellular complementation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(5): 1456-1460. |
| 65 | BEN-DAVID Y, MORAÏS S, BAYER E A, et al. Rapid adaptation for fibre degradation by changes in plasmid stoichiometry within Lactobacillus plantarumat the synthetic community level[J]. Microbial Biotechnology, 2020, 13(6): 1748-1764. |
| 66 | HIRANO N, HASEGAWA H, NIHEI S, et al. Cell-free protein synthesis and substrate specificity of full-length endoglucanase CelJ (Cel9D-Cel44A), the largest multi-enzyme subunit of the Clostridium thermocellum cellulosome[J]. FEMS Microbiology Letters, 2013, 344(1): 25-30. |
| 67 | HIRANO K, KUROSAKI M, NIHEI S, et al. Enzymatic diversity of the Clostridium thermocellum cellulosome is crucial for the degradation of crystalline cellulose and plant biomass[J]. Scientific Reports, 2016, 6: 35709. |
| 68 | HIRANO K, NIHEI S, HASEGAWA H, et al. Stoichiometric assembly of the cellulosome generates maximum synergy for the degradation of crystalline cellulose, as revealed by in vitro reconstitution of the Clostridium thermocellum cellulosome[J]. Applied and Environmental Microbiology, 2015, 81(14): 4756-4766. |
| 69 | HIRANO K, SAITO T, SHINODA S, et al. In vitro assembly and cellulolytic activity of a β-glucosidase-integrated cellulosome complex[J]. FEMS Microbiology Letters, 2019, 366(17): fnz209. |
| 70 | YOU C, ZHANG Y H P. Self-assembly of synthetic metabolons through synthetic protein scaffolds: one-step purification, co-immobilization, and substrate channeling[J]. ACS Synthetic Biology, 2013, 2(2): 102-110. |
| 71 | LIU F, BANTA S, CHEN W. Functional assembly of a multi-enzyme methanol oxidation cascade on a surface-displayed trifunctional scaffold for enhanced NADH production[J]. Chemical Communications, 2013, 49(36): 3766-3768. |
| 72 | JINDOU S, ITO Y, MITO N, et al. Engineered platform for bioethylene production by a Cyanobacterium expressing a chimeric complex of plant enzymes[J]. ACS Synthetic Biology, 2014, 3(7): 487-496. |
| 73 | KIM S, HAHN J S. Synthetic scaffold based on a cohesin-dockerin interaction for improved production of 2,3-butanediol in Saccharomyces cerevisiae [J]. Journal of Biotechnology, 2014, 192: 192-196. |
| 74 | LIN J L, ZHU J, WHEELDON I. Synthetic protein scaffolds for biosynthetic pathway colocalization on lipid droplet membranes[J]. ACS Synthetic Biology, 2017, 6(8): 1534-1544. |
| 75 | YANG K X, LI F, QIAO Y G, et al. Design of a new multienzyme complex synthesis system based on Yarrowia lipolytica simultaneously secreted and surface displayed fusion proteins for sustainable production of fatty acid-derived hydrocarbons[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 17035-17043. |
| 76 | LI F, YANG K X, XU Y, et al. A genetically-encoded synthetic self-assembled multienzyme complex of lipase and P450 fatty acid decarboxylase for efficient bioproduction of fatty alkenes[J]. Bioresource Technology, 2019, 272: 451-457. |
| 77 | YOU C, ZHANG X Z, SATHITSUKSANOH N, et al. Enhanced microbial utilization of recalcitrant cellulose by an ex vivo cellulosome-microbe complex[J]. Applied and Environmental Microbiology, 2012, 78(5): 1437-1444. |
| 78 | CHEN L, MULCHANDANI A, GE X. Spore-displayed enzyme cascade with tunable stoichiometry[J]. Biotechnology Progress, 2017, 33(2): 383-389. |
| 79 | PARK M, SUN Q, LIU F, et al. Positional assembly of enzymes on bacterial outer membrane vesicles for cascade reactions[J]. PLoS One, 2014, 9(5): e97103. |
| 80 | VITA N, BORNE R, FIEROBE H P. Cell-surface exposure of a hybrid 3-cohesin scaffoldin allowing the functionalization of Escherichia coli envelope[J]. Biotechnology and Bioengineering, 2020, 117(3): 626-636. |
| 81 | WIECZOREK A S, MARTIN V J J. Effects of synthetic cohesin-containing scaffold protein architecture on binding dockerin-enzyme fusions on the surface of Lactococcus lactis [J]. Microbial Cell Factories, 2012, 11: 160. |
| 82 | STERN J, MORAÏS S, BEN-DAVID Y, et al. Assembly of synthetic functional cellulosomal structures onto the cell surface of Lactobacillus plantarum, a potent member of the gut microbiome[J]. Applied and Environmental Microbiology, 2018, 84(8): e00282. |
| 83 | DVOŘÁK P, BAYER E A, DE LORENZO V. Surface display of designer protein scaffolds on genome-reduced strains of Pseudomonas putida [J]. ACS Synthetic Biology, 2020, 9(10): 2749-2764. |
| 84 | OLSON D G, MCBRIDE J E, SHAW A J, et al. Recent progress in consolidated bioprocessing[J]. Current Opinion in Biotechnology, 2012, 23(3): 396-405. |
| 85 | LILLY M, FIEROBE H P, VAN ZYL W H, et al. Heterologous expression of a Clostridium minicellulosome in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2009, 9(8): 1236-1249. |
| 86 | TSAI S L, OH J, SINGH S, et al. Functional assembly of minicellulosomes on the Saccharomyces cerevisiae cell surface for cellulose hydrolysis and ethanol production[J]. Applied and Environmental Microbiology, 2009, 75(19): 6087-6093. |
| 87 | FAN L H, ZHANG Z J, YU X Y, et al. Self-surface assembly of cellulosomes with two miniscaffoldins on Saccharomyces cerevisiae for cellulosic ethanol production[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(33): 13260-13265. |
| 88 | TSAI S L, DASILVA N A, CHEN W. Functional display of complex cellulosomes on the yeast surface via adaptive assembly[J]. ACS Synthetic Biology, 2013, 2(1): 14-21. |
| 89 | TANG H T, WANG J J, WANG S H, et al. Efficient yeast surface-display of novel complex synthetic cellulosomes[J]. Microbial Cell Factories, 2018, 17(1): 122. |
| 90 | SRIKRISHNAN S, CHEN W, SILVA N A DA. Functional assembly and characterization of a modular xylanosome for hemicellulose hydrolysis in yeast[J]. Biotechnology and Bioengineering, 2013, 110(1): 275-285. |
| 91 | OU J S, CAO Y C. Incorporation of Nasutitermes takasagoensis endoglucanase into cell surface-displayed minicellulosomes in Pichia pastoris X33[J]. Journal of Microbiology and Biotechnology, 2014, 24(9): 1178-1188. |
| 92 | DONG C, QIAO J, WANG X P, et al. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 108. |
| 93 | SMITH M R, GAO H, PRABHU P, et al. Elucidating structure-performance relationships in whole-cell cooperative enzyme catalysis[J]. Nature Catalysis, 2019, 2(9): 809-819. |
| 94 | ANANDHARAJ M, LIN Y J, RANI R P, et al. Constructing a yeast to express the largest cellulosome complex on the cell surface[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(5): 2385-2394. |
| 95 | SZCZUPAK A, AIZIK D, MORAÏS S, et al. The electrosome: A surface-displayed enzymatic cascade in a biofuel cell's anode and a high-density surface-displayed biocathodic enzyme[J]. Nanomaterials, 2017, 7(7): 153. |
| 96 | FAN S Q, LIANG B, XIAO X X, et al. Controllable display of sequential enzymes on yeast surface with enhanced biocatalytic activity toward efficient enzymatic biofuel cells[J]. Journal of the American Chemical Society, 2020, 142(6): 3222-3230. |
| 97 | YOU C, CHEN H G, MYUNG S, et al. Enzymatic transformation of nonfood biomass to starch[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(18): 7182-7187. |
| 98 | LI M, YUE Y, ZHANG Z J, et al. Site-specific and high-loading immobilization of proteins by using cohesin-dockerin and CBM-cellulose interactions[J]. Bioconjugate Chemistry, 2016, 27(7): 1579-1583. |
| 99 | HEYMAN A, BARAK Y, CASPI J, et al. Multiple display of catalytic modules on a protein scaffold: nano-fabrication of enzyme particles[J]. Journal of Biotechnology, 2007, 131(4): 433-439. |
| 100 | MORAÏS S, HEYMAN A, BARAK Y, et al. Enhanced cellulose degradation by nano-complexed enzymes: synergism between a scaffold-linked exoglucanase and a free endoglucanase[J]. Journal of Biotechnology, 2010, 147(3/4): 205-211. |
| 101 | MITSUZAWA S, KAGAWA H, LI Y F, et al. The rosettazyme: a synthetic cellulosome[J]. Journal of Biotechnology, 2009, 143(2): 139-144. |
| 102 | CHUNDAWAT S P S, PAAVOLA C D, RAMAN B, et al. Saccharification of thermochemically pretreated cellulosic biomass using native and engineered cellulosomal enzyme systems[J]. Reaction Chemistry & Engineering, 2016, 1(6): 616-628. |
| 103 | LEE C C, KIBBLEWHITE R E, PAAVOLA C D, et al. Production of D-xylonic acid from hemicellulose using artificial enzyme complexes[J]. Journal of Microbiology and Biotechnology, 2017, 27(1): 77-83. |
| 104 | MCCONNELL S A, CANNON K A, MORGAN C, et al. Designed protein cages as scaffolds for building multienzyme materials[J]. ACS Synthetic Biology, 2020, 9(2): 381-391. |
| 105 | BUDINOVA G A L G, MORI Y, TANAKA T, et al. Casein-based scaffold for artificial cellulosome design[J]. Process Biochemistry, 2018, 66: 140-145. |
| 106 | HAN Z L, ZHANG B, WANG Y E, et al. Self-assembled amyloid-like oligomeric-cohesin scaffoldin for augmented protein display on the Saccharomyces cerevisiae cell surface[J]. Applied and Environmental Microbiology, 2012, 78(9): 3249-3255. |
| 107 | KIM H J, LEE E J, PARK J S, et al. Reversible and multi-cyclic protein-protein interaction in bacterial cellulosome-mimic system using rod-shaped viral nanostructure[J]. Journal of Biotechnology, 2016, 221: 101-106. |
| 108 | KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux[J]. Nature Communications, 2019, 10: 4248. |
| 109 | LIU Z J, CAO S, LIU M, et al. Self-assembled multienzyme nanostructures on synthetic protein scaffolds[J]. ACS Nano, 2019, 13(10): 11343-11352. |
| 110 | BEHRENDORFF J B Y H, SANDOVAL-IBAÑEZ O A, SHARMA A, et al. Membrane-bound protein scaffolding in diverse hosts using thylakoid protein CURT1A[J]. ACS Synthetic Biology, 2019, 8(4): 611-620. |
| 111 | KIM D M, NAKAZAWA H, UMETSU M, et al. A nanocluster design for the construction of artificial cellulosomes[J]. Catalysis Science & Technology, 2012, 2(3): 499-503. |
| 112 | CHO E J, JUNG S, KIM H J, et al. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose[J]. Chemical Communications, 2012, 48(6): 886-888. |
| 113 | YUE Y, LU Y Y, LI M, et al. Co-localization of proteins with defined sequential order and controlled stoichiometric ratio on magnetic nanoparticles[J]. Nanoscale, 2017, 9(13): 4397-4400. |
| 114 | LU L, ZHANG L B, YUAN L, et al. Artificial cellulosome complex from the self-assembly of Ni-NTA-functionalized polymeric micelles and cellulases[J]. ChemBioChem, 2019, 20(11): 1394-1399. |
| 115 | MORI Y, OZASA S, KITAOKA M, et al. Aligning an endoglucanase Cel5A from Thermobifida fusca on a DNA scaffold: potent design of an artificial cellulosome[J]. Chemical Communications, 2013, 49(62): 6971-6973. |
| 116 | SUN Q, MADAN B, TSAI S L, et al. Creation of artificial cellulosomes on DNA scaffolds by zinc finger protein-guided assembly for efficient cellulose hydrolysis[J]. Chemical Communications, 2014, 50(12): 1423-1425. |
| 117 | SUN Q, CHEN W. HaloTag mediated artificial cellulosome assembly on a rolling circle amplification DNA template for efficient cellulose hydrolysis[J]. Chemical Communications, 2016, 52(40): 6701-6704. |
| 118 | CHEN Q, YU S, MYUNG N, et al. DNA-guided assembly of a five-component enzyme cascade for enhanced conversion of cellulose to gluconic acid and H2O2 [J]. Journal of Biotechnology, 2017, 263: 30-35. |
| 119 | BERCKMAN E A, CHEN W. Exploiting dCas9 fusion proteins for dynamic assembly of synthetic metabolons[J]. Chemical Communications, 2019, 55(57): 8219-8222. |
| 120 | BERCKMAN E A, CHEN W. A modular approach for dCas9-mediated enzyme cascading via orthogonal bioconjugation[J]. Chemical Communications, 2020, 56(77): 11426-11428. |
| 121 | LIM S, KIM J, KIM Y, et al. CRISPR/Cas-directed programmable assembly of multi-enzyme complexes[J]. Chemical Communications, 2020, 56(36): 4950-4953. |
| 122 | YAO X Z, CHEN C, WANG Y F, et al. Discovery and mechanism of a pH-dependent dual-binding-site switch in the interaction of a pair of protein modules[J]. Science Advances, 2020, 6(43): eabd7182. |
| 123 | CHEN C, YANG H W, XUAN J S, et al. Resonance assignments of a cellulosomal double-dockerin from Clostridium thermocellum [J]. Biomolecular NMR Assignments, 2019, 13(1): 97-101. |
| 124 | BRÁS J L A, CARTMELL A, CARVALHO A L M, et al. Structural insights into a unique cellulase fold and mechanism of cellulose hydrolysis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(13): 5237-5242. |
| 125 | ARTZI L, MORAG E, SHAMSHOUM M, et al. Cellulosomal expansin: functionality and incorporation into the complex[J]. Biotechnology for Biofuels, 2016, 9: 61. |
| 126 | CHEN C, CUI Z L, SONG X F, et al. Integration of bacterial expansin-like proteins into cellulosome promotes the cellulose degradation[J]. Applied Microbiology and Biotechnology, 2016, 100(5): 2203-2212. |
| 127 | ZONG Y Q, ZHANG H Q M, LYU C, et al. Insulated transcriptional elements enable precise design of genetic circuits[J]. Nature Communications, 2017, 8: 52. |
| 128 | PINTO D, VECCHIONE S, WU H, et al. Engineering orthogonal synthetic timer circuits based on extracytoplasmic function σ factors[J]. Nucleic Acids Research, 2018, 46(14): 7450-7464. |
| 129 | HOLWERDA E K, OLSON D G, RUPPERTSBERGER N M, et al. Metabolic and evolutionary responses of Clostridium thermocellum to genetic interventions aimed at improving ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 40. |
| 130 | YAN F, WEI R, CUI Q, et al. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum [J]. Microbial Biotechnology, 2021, 14(2): 374-385. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | ZHAI Tingting, GU Hongzhou, FAN Chunhai. Enzyme immobilization assisted by protein assemblies for highly efficient biocatalysis in organic systems [J]. Synthetic Biology Journal, 2022, 3(2): 256-259. |
| [3] | Yi-Heng ZHANG. Remembering Professor Daniel I.C. Wang’s contribution to biorefining and my perspective on the progress [J]. Synthetic Biology Journal, 2021, 2(4): 497-508. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||