Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (3): 500-515.DOI: 10.12211/2096-8280.2021-070
• Invited Review • Previous Articles Next Articles
Application of enzyme catalysis in the preparation of vitamins and their derivatives
WANG Panpan1, YU Hongwei2
- 1.Zhejiang NHU Company Ltd. ,Shaoxing 312500,Zhejiang,China
2.Institute of Bioengineering,College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,Zhejiang,China
-
Received:2021-07-02Revised:2021-11-10Online:2022-07-13Published:2022-06-30 -
Contact:YU Hongwei
酶催化在维生素及其衍生物制备中的应用
王盼盼1, 于洪巍2
- 1.浙江新和成股份有限公司,浙江 绍兴 312500
2.浙江大学化学工程与生物工程学院生物工程研究所,浙江 杭州 310027
-
通讯作者:于洪巍 -
作者简介:王盼盼 (1990—),男,博士。研究方向为维生素类产品的生物合成,长期从事酶改造、酶催化和发酵工艺优化相关研发工作。E-mail:wpan2016@sina.com于洪巍 (1972—),男,博士,教授。长期从事生物催化研究,致力于利用蛋白质工程和代谢工程手段提高化学品的生物合成效率。E-mail:yuhongwei@zju.edu.cn
CLC Number:
Cite this article
WANG Panpan, YU Hongwei. Application of enzyme catalysis in the preparation of vitamins and their derivatives[J]. Synthetic Biology Journal, 2022, 3(3): 500-515.
王盼盼, 于洪巍. 酶催化在维生素及其衍生物制备中的应用[J]. 合成生物学, 2022, 3(3): 500-515.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-070
| 维生素 | 生产方法 | 主要用酶 | 催化步骤 | 特点 | 研究方向 | 参考文献 | |
|---|---|---|---|---|---|---|---|
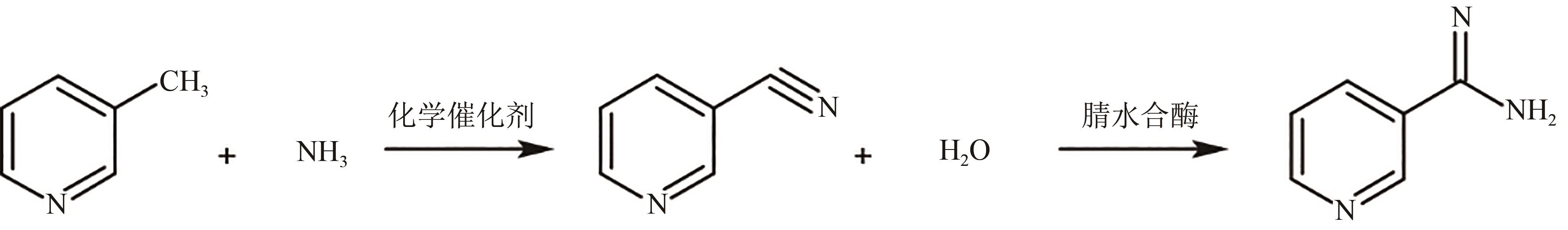
| 维生素B3 | 化学+ 生物催化 | 腈水合酶 | 催化烟腈合成烟酰胺 | 活性较高,热稳定性较差,酶的来源较为明确,已实现工业化 | 不同来源酶的异源表达;酶的工程改造 | [ | |
| 维生素B5 | 化学+ 生物催化 | 酯水解酶、天冬氨酸酶等 | DL-泛解酸内酯的拆分 | 野生菌催化活性较高,但选择性未达到100%,已实现工业化。但因基因和酶种类不明,酶活提升较困难 | 高活性和高选择性的酯水解酶的筛选和改造 | [ | |
| β-丙氨酸的酶催化合成 | 包括多种酶催化法,如通过天冬氨酸裂合酶催化富马酸合成天冬氨酸,再通过天冬氨酸脱羧酶催化天冬氨酸合成β-丙氨酸;或直接催化天冬氨酸脱羧合成β-丙氨酸;或改造天冬氨酸裂合酶催化丙烯酸合成β-丙氨酸,此方法因效率高成本低已实现工业化 | 天冬氨酸酶的改造和应用 | [ | ||||
| 维生素C | 发酵 | 醇脱氢酶 | 催化D-山梨醇合成L-山梨糖 | 因山梨醇脱氢酶来源菌株的性质,该步骤适合采用发酵法生产 | 研究氧化葡萄糖酸杆菌或醇脱氢酶本身性质 | [ | |
| 通过代谢工程手段合并两步发酵为一步发酵 | [ | ||||||
| 维生素D3 | 化学+ 生物催化 | P450酶 | 催化维生素D3合成活性25(OH)维生素D3或1α,25(OH)维生素D3 | 酶的来源广但活性低,催化转化率低 | 高活性P450酶的筛选和改造 | [ | |
| 维生素酯类衍生物 | 化学或 生物催化 | 酯酶 | 催化维生素和脂肪酸合成相应的维生素酯 | 酶的特异性较差,催化活性和转化率偏低,底物的溶解特性冲突以及酶的有机溶剂耐受问题 | 高活性、高有机溶剂耐受性的酯酶的筛选和改造 | 维生素A酯 | [ |
| 维生素C酯 | [ | ||||||
| 维生素E酯 | [ | ||||||
| 维生素C 糖苷类 | 生物催化 | 糖基转移酶、葡萄糖苷酶等 | 催化维生素C和糖基供体合成相应的维生素C葡萄糖苷 | 酶的催化活性较差,对各类糖基供体的催化活性差异大,底物转化率低 | 高活性、高转化率的糖基转移酶或同工酶的筛选和改造 | [ | |
Tab. 1 Enzymatic synthesis of various vitamins and their derivatives
| 维生素 | 生产方法 | 主要用酶 | 催化步骤 | 特点 | 研究方向 | 参考文献 | |
|---|---|---|---|---|---|---|---|
| 维生素B3 | 化学+ 生物催化 | 腈水合酶 | 催化烟腈合成烟酰胺 | 活性较高,热稳定性较差,酶的来源较为明确,已实现工业化 | 不同来源酶的异源表达;酶的工程改造 | [ | |
| 维生素B5 | 化学+ 生物催化 | 酯水解酶、天冬氨酸酶等 | DL-泛解酸内酯的拆分 | 野生菌催化活性较高,但选择性未达到100%,已实现工业化。但因基因和酶种类不明,酶活提升较困难 | 高活性和高选择性的酯水解酶的筛选和改造 | [ | |
| β-丙氨酸的酶催化合成 | 包括多种酶催化法,如通过天冬氨酸裂合酶催化富马酸合成天冬氨酸,再通过天冬氨酸脱羧酶催化天冬氨酸合成β-丙氨酸;或直接催化天冬氨酸脱羧合成β-丙氨酸;或改造天冬氨酸裂合酶催化丙烯酸合成β-丙氨酸,此方法因效率高成本低已实现工业化 | 天冬氨酸酶的改造和应用 | [ | ||||
| 维生素C | 发酵 | 醇脱氢酶 | 催化D-山梨醇合成L-山梨糖 | 因山梨醇脱氢酶来源菌株的性质,该步骤适合采用发酵法生产 | 研究氧化葡萄糖酸杆菌或醇脱氢酶本身性质 | [ | |
| 通过代谢工程手段合并两步发酵为一步发酵 | [ | ||||||
| 维生素D3 | 化学+ 生物催化 | P450酶 | 催化维生素D3合成活性25(OH)维生素D3或1α,25(OH)维生素D3 | 酶的来源广但活性低,催化转化率低 | 高活性P450酶的筛选和改造 | [ | |
| 维生素酯类衍生物 | 化学或 生物催化 | 酯酶 | 催化维生素和脂肪酸合成相应的维生素酯 | 酶的特异性较差,催化活性和转化率偏低,底物的溶解特性冲突以及酶的有机溶剂耐受问题 | 高活性、高有机溶剂耐受性的酯酶的筛选和改造 | 维生素A酯 | [ |
| 维生素C酯 | [ | ||||||
| 维生素E酯 | [ | ||||||
| 维生素C 糖苷类 | 生物催化 | 糖基转移酶、葡萄糖苷酶等 | 催化维生素C和糖基供体合成相应的维生素C葡萄糖苷 | 酶的催化活性较差,对各类糖基供体的催化活性差异大,底物转化率低 | 高活性、高转化率的糖基转移酶或同工酶的筛选和改造 | [ | |
| 1 | ABANOZ B, OKAY S, KURT-KIZILDOĞAN A. Highly active and stable protease production by an extreme halophilic archaeon Haloarcula sp. TG1 isolated from Lake Tuz, Turkey[J]. Turkish Journal of Biochemistry, 2017, 42(3): 307-315. |
| 2 | 曹慧, 张腾月, 赵龙妹, 等. 土壤中高产蛋白酶菌株产酶条件及酶学性质[J]. 微生物学通报, 2020, 47(7): 2072-2081. |
| CAO H, ZHANG T Y, ZHAO L M, et al. Identification and characterization of a high protease-producing strain from soil[J]. Microbiology China, 2020, 47(7): 2072-2081. | |
| 3 | SUI M, RONG J C, ZHANG Y, et al. Screening of cellulose degrading bacteria and construction of complex microflora[J]. IOP Conference Series: Earth and Environmental Science, 2021, 632(3): 032021. |
| 4 | NIU M F, WANG S Y, PANG X P, et al. Isolation and screening of degrading cellulose strain from straw compost[C]// 2011 International Conference on Electric Technology and Civil Engineering (ICETCE). IEEE, 2011: 5142-5144. |
| 5 | 李雪英, 王建蝶, 黄毕生, 等. 高温漆酶产生菌Bacillus thuringiensis strain Lac-72的筛选、鉴定及其酶学性质研究[J]. 山东农业大学学报(自然科学版), 2020, 51(5): 797-803. |
| LI X Y, WANG J D, HUANG B S, et al. Study on the screening, identification and enzymatic properties of Bacillus thuringiensis strain Lac-72 produced by thermostable laccase[J]. Journal of Shandong Agricultural University (Natural Science Edition), 2020, 51(5): 797-803. | |
| 6 | 汪春蕾, 田菲, 张月颖, 等. 耐盐Bacillus sp.9BS的筛选及其对染料的脱色效果[J]. 湖南农业大学学报(自然科学版), 2015, 41(3): 313-317. |
| WANG C L, TIAN F, ZHANG Y Y, et al. Screening of a salt-tolerant Bacillus sp. 9BS and its decoloring effect on dye[J]. Journal of Hunan Agricultural University (Natural Sciences), 2015, 41(3): 313-317. | |
| 7 | JHA R K, STRAUSS C E M. Smart microbial cells couple catalysis and sensing to provide high-throughput selection of an organophosphate hydrolase[J]. ACS Synthetic Biology, 2020, 9(6): 1234-1239. |
| 8 | YUAN B, MAHOR D, FEI Q, et al. Water-soluble anthraquinone photocatalysts enable methanol-driven enzymatic halogenation and hydroxylation reactions[J]. ACS Catalysis, 2020, 10(15): 8277-8284. |
| 9 | SANKET A S, GHOSH S, SAHOO R, et al. Molecular identification of acidophilic manganese (Mn)-solubilizing bacteria from mining effluents and their application in mineral beneficiation[J]. Geomicrobiology Journal, 2017, 34(1): 71-80. |
| 10 | KELLY S A, MEGAW J, CASWELL J, et al. Isolation and characterisation of a halotolerant ω-transaminase from a Triassic period salt mine and its application to biocatalysis[J]. ChemistrySelect, 2017, 2(30): 9783-9791. |
| 11 | DALBØGE H, LANGE L. Using molecular techniques to identify new microbial biocatalysts[J]. Trends in Biotechnology, 1998, 16(6): 265-272. |
| 12 | DANIEL L, BURYSKA T, PROKOP Z, et al. Mechanism-based discovery of novel substrates of haloalkane dehalogenases using in silico screening[J]. Journal of Chemical Information and Modeling, 2015, 55(1): 54-62. |
| 13 | MIRETE S, MORGANTE V, GONZÁLEZ-PASTOR J E. Functional metagenomics of extreme environments[J]. Current Opinion in Biotechnology, 2016, 38: 143-149. |
| 14 | VIMAL A, KUMAR A. In vitro screening and in silico validation revealed key microbes for higher production of significant therapeutic enzyme L-asparaginase[J]. Enzyme and Microbial Technology, 2017, 98: 9-17. |
| 15 | SUN Y, WANG G, JING Z, et al. Microfluidic pneumatic printed sandwiched microdroplet array for high-throughput enzymatic reaction and screening[J]. SLAS Technology, 2020, 25(5): 446-454. |
| 16 | JHA R K, KERN T L, FOX D T, et al. Engineering an Acinetobacter regulon for biosensing and high-throughput enzyme screening in E. coli via flow cytometry[J]. Nucleic Acids Research, 2014, 42(12): 8150-8160. |
| 17 | NOZERET K, PERNIN A, BUDDELMEIJER N. Click-chemistry based fluorometric assay for apolipoprotein N-acyltransferase from enzyme characterization to high-throughput screening[J]. Journal of Visualized Experiments, 2020(159): 61146. |
| 18 | ARAI K. Assay in high throughput screening[J]. Nihon Yakurigaku Zasshi Folia Pharmacologica Japonica, 2001, 118(2): 81-88. |
| 19 | WAHLER D, REYMOND J L. High-throughput screening for biocatalysts[J]. Current Opinion in Biotechnology, 2001, 12(6): 535-544. |
| 20 | ZHAO H, ARNOLD F H. Optimization of DNA shuffling for high fidelity recombination[J]. Nucleic Acids Research, 1997, 25(6): 1307-1308. |
| 21 | ZHAO H M, ARNOLD F H. Directed evolution converts subtilisin E into a functional equivalent of thermitase[J]. Protein Engineering, Design and Selection, 1999, 12(1): 47-53. |
| 22 | ZENG W Z, XU B B, DU G C, et al. Integrating enzyme evolution and high-throughput screening for efficient biosynthesis of L-DOPA[J]. Journal of Industrial Microbiology and Biotechnology, 2019, 46(12): 1631-1641. |
| 23 | CHOI J M, KIM H-S. Structure-guided rational design of the substrate specificity and catalytic activity of an enzyme [M]//TAWFIK D S. Enzyme Engineering and Evolution: General Methods. Elsevier, 2020: 181-202. |
| 24 | WIJMA H J, FLOOR R J, BJELIC S, et al. Enantioselective enzymes by computational design and in silico screening[J]. Angewandte Chemie International Edition, 2015, 54(12): 3726-3730. |
| 25 | ABDELSALAM M A, ABOULWAFA O M, BADAWEY E S A M, et al. Design, synthesis, anticancer screening, docking studies and in silico ADME prediction of some β-carboline derivatives[J]. Future Medicinal Chemistry, 2018, 10(10): 1159-1175. |
| 26 | BASSO A, SERBAN S. Industrial applications of immobilized enzymes—a review[J]. Molecular Catalysis, 2019, 479: 110607. |
| 27 | RIGOLDI F, DONINI S, REDAELLI A, et al. Review: Engineering of thermostable enzymes for industrial applications[J]. APL Bioengineering, 2018, 2(1): 011501. |
| 28 | ASANO Y, TANI Y, YAMADA H. A new enzyme "nitrile hydratase" which degrades acetonitrile in combination with amidase[J]. Agricultural and Biological Chemistry, 1980, 44(9): 2251-2252. |
| 29 | YAMADA H, KOBAYASHI M. Nitrile hydratase and its application to industrial production of acrylamide[J]. Bioscience, Biotechnology, and Biochemistry, 1996, 60(9): 1391-1400. |
| 30 | KUBÁČ D, KAPLAN O, ELIŠÁKOVÁ V, et al. Biotransformation of nitriles to amides using soluble and immobilized nitrile hydratase from Rhodococcus erythropolis A4[J]. Journal of Molecular Catalysis B: Enzymatic, 2008, 50(2/3/4): 107-113. |
| 31 | NAGASAWA T, NANBA H, RYUNO K, et al. Nitrile hydratase of Pseudomonas chlororaphis B23. Purification and characterization[J]. European Journal of Biochemistry, 1987, 162(3): 691-698. |
| 32 | KOMEDA H, KOBAYASHI M, SHIMIZU S. Characterization of the gene cluster of high-molecular-mass nitrile hydratase (H-NHase) induced by its reaction product in Rhodococcus rhodochrous J1[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(9): 4267-4272. |
| 33 | OKAMOTO S, ELTIS L D. Purification and characterization of a novel nitrile hydratase from Rhodococcus sp. RHA1[J]. Molecular Microbiology, 2007, 65(3): 828-838. |
| 34 | NAGASAWA T, MATHEW C D, MAUGER J, et al. Nitrile hydratase-catalyzed production of nicotinamide from 3-cyanopyridine in Rhodococcus rhodochrous J1[J]. Applied and Environmental Microbiology, 1988, 54(7): 1766-1769. |
| 35 | 刘丽秀, 范鲁娜. D-泛酸钙合成技术综述[J]. 湖南化工, 1999, 29(4): 13-15. |
| LIU L X, FAN L N. An introduction to synthesis of D-calcium pantothenate[J]. Hunan Chemical Industry, 1999, 29(4): 13-15. | |
| 36 | CHEN B, FAN L Q, XU J H, et al. Biocatalytic properties of a recombinant Fusarium proliferatum lactonase with significantly enhanced production by optimal expression in Escherichia coli [J]. Applied Biochemistry and Biotechnology, 2010, 162(3): 744-756. |
| 37 | 汤一新, 孙志浩, 华蕾, 等. D-泛解酸内酯水解酶产生菌的筛选及产酶条件研究[J]. 微生物学报, 2002, 42(1): 81-87. |
| TANG Y X, SUN Z H, HUA L, et al. Production of D-pantolactone hydrolase by Fusarium moniliforme SW-902[J]. Acta Microbiologica Sinica, 2002, 42(1): 81-87. | |
| 38 | SHIMIZU S, KATAOKA M, HONDA K, et al. Lactone-ring-cleaving enzymes of microorganisms: their diversity and applications[J]. Journal of Biotechnology, 2001, 92(2): 187-194. |
| 39 | 汤一新, 孙志浩, 华蕾, 等. 微生物酶拆分方法生产D-泛酸的手性中间体D-泛解酸内酯[J]. 工业微生物, 2001, 31(3): 1-5. |
| TANG Y X, SUN Z H, HUA L, et al. Optical resolution of racemic DL-pantolactone by a fungal enzyme, D-lactonohydrolase[J]. Industrial Microbiology, 2001, 31(3): 1-5. | |
| 40 | FORD J H, BUC S R, GREINER J W. An improved synthesis of β-alanine (III): The addition of ammonia to acrylonitrile at 50-150℃ [J]. Journal of the American Chemical Society, 1947, 69(4): 844-846. |
| 41 | 李博, 彭俊华, 李继涛, 等. 一种采用微通道反应器制备β-氨基丙酸的方法: CN108892621A [P]. 2018-11-27. |
| LI B, PENG J H, LI J T, et al. A method for preparing β-alanine by micro-channel reactor: CN108892621A[P]. 2018-11-27. | |
| 42 | XU J, ZHU Y, ZHOU Z M. Systematic engineering of the rate-limiting step of β-alanine biosynthesis in Escherichia coli [J]. Electronic Journal of Biotechnology, 2021, 51: 88-94. |
| 43 | ZOU X Y, GUO L X, HUANG L L, et al. Pathway construction and metabolic engineering for fermentative production of β-alanine in Escherichia coli [J]. Applied Microbiology and Biotechnology, 2020, 104(6): 2545-2559. |
| 44 | CRONAN J E Jr. Beta-alanine synthesis in Escherichia coli [J]. Journal of Bacteriology, 1980, 141(3): 1291-1297. |
| 45 | LI R F, WIJMA H J, SONG L, et al. Computational redesign of enzymes for regio- and enantioselective hydroamination[J]. Nature Chemical Biology, 2018, 14(7): 664-670. |
| 46 | 吴边, 刘洋, 李瑞峰, 等. 天冬氨酸酶变体及其制备方法与应用: CN109385415A[P]. 2019-02-26. |
| WU B, LIU Y, LI R F, et al. Aspartase variant, preparation method and applications thereof: CN109385415A[P]. 2019-02-26. | |
| 47 | 范文超, 王金刚, 梁岩, 等. 一种天冬氨酸氨裂合酶突变体及其应用: CN110791493B[P]. 2021-03-09. |
| FAN W C, WANG J G, LIANG Y, et al. Aspartate ammonia lyase mutant and application thereof: CN110791493B[P]. 2021-03-09. | |
| 48 | 尹光琳, 陶增鑫, 于龙华, 等. L-山梨糖发酵产生维生素C前体——2-酮基-L-古龙酸的研究Ⅰ.菌种的分离筛选和鉴定[J]. 微生物学报, 1980, 20(3): 246-251. |
| YIN G L, TAO Z X, YU L H, et al. Studies on the production of vitamin C precursor—2-keto-L-gulonic acid from L-sorbose by fermentation (I): Isolation, screening and identification of 2-keto-L-gulonic acid producing bacteria[J]. Acta Microbiologica Sinica, 1980, 20(3): 246-251. | |
| 49 | URBANCE J W, BRATINA B J, STODDARD S F, et al. Taxonomic characterization of Ketogulonigenium vulgare gen. nov., sp. nov. and Ketogulonigenium robustum sp. nov., which oxidize L-sorbose to 2-keto-L-gulonic acid[J]. International Journal of Systematic and Evolutionary Microbiology, 2001, 51(Pt 3): 1059-1070. |
| 50 | WANG P P, ZENG W Z, XU S, et al. Current challenges facing one-step production of L-ascorbic acid[J]. Biotechnology Advances, 2018, 36(7): 1882-1899. |
| 51 | GLIESE N, KHODAVERDI V, GÖRISCH H. The PQQ biosynthetic operons and their transcriptional regulation in Pseudomonas aeruginosa [J]. Archives of Microbiology, 2010, 192(1): 1-14. |
| 52 | XIONG X H, ZHI J J, YANG L, et al. Complete genome sequence of the Bacterium methylovorus sp. strain MP688, a high-level producer of pyrroloquinolone quinone[J]. Journal of Bacteriology, 2011, 193(8): 2080. |
| 53 | KAY C W M, MENNENGA B, GÖRISCH H, et al. Structure of the pyrroloquinoline quinone radical in quinoprotein ethanol dehydrogenase[J]. Journal of Biological Chemistry, 2006, 281(3): 1470-1476. |
| 54 | DAVIDSON V L. Electron transfer in quinoproteins[J]. Archives of Biochemistry and Biophysics, 2004, 428(1): 32-40. |
| 55 | CHEN Y, LIU L, YU S Q, et al. Identification of gradient promoters of gluconobacter oxydans and their applications in the biosynthesis of 2-keto-L-gulonic acid[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 673844. |
| 56 | ZENG W Z, WANG P P, LI N, et al. Production of 2-keto-L-gulonic acid by metabolically engineered Escherichia coli [J]. Bioresource Technology, 2020, 318: 124069. |
| 57 | DI ROSA M, MALAGUARNERA L, NICOLOSI A, et al. Vitamin D3: an ever green molecule[J]. Frontiers in Bioscience (Scholar Edition), 2013, 5(1): 247-260. |
| 58 | HORSTING M, DELUCA H F. In vitro production of 25-hydroxycholecalciferol[J]. Biochemical and Biophysical Research Communications, 1969, 36(2): 251-256. |
| 59 | SASAKI J, MIYAZAKI A, SAITO M, et al. Transformation of vitamin D3 to 1α,25-dihydroxyvitamin D3 via 25-hydroxyvitamin D3 using Amycolata sp. strains[J]. Applied Microbiology and Biotechnology, 1992, 38(2): 152-157. |
| 60 | SASAKI J, MIKAMI A, MIZOUE K, et al. Transformation of 25- and 1α-hydroxyvitamin D3 to 1α,25-dihydroxyvitamin D3 by using Streptomyces sp. strains[J]. Applied and Environmental Microbiology, 1991, 57(10): 2841-2846. |
| 61 | FUJII Y, KABUMOTO H, NISHIMURA K, et al. Purification, characterization, and directed evolution study of a vitamin D3 hydroxylase from Pseudonocardia autotrophica [J]. Biochemical and Biophysical Research Communications, 2009, 385(2): 170-175. |
| 62 | CHEW B P. Vitamin A and β-carotene on host defense[J]. Journal of Dairy Science, 1987, 70(12): 2732-2743. |
| 63 | BLOMHOFF R, GREEN M H, NORUM K R. Vitamin A: physiological and biochemical processing[J]. Annual Review of Nutrition, 1992, 12: 37-57. |
| 64 | 金淑芳, 李传茂, 林盛杰. 维生素及其衍生物在头皮护理产品中的应用[J]. 广东化工, 2019, 46(24): 70-71. |
| JIN S F, LI C M, LIN S J. Application of vitamins and their derivatives in scalp care products[J]. Guangdong Chemical Industry, 2019, 46(24): 70-71. | |
| 65 | 沈润溥, 皮士卿, 谢斌, 等. 维生素A衍生物合成工艺的改进[J]. 应用化学, 2003, 20(12): 1211-1213. |
| SHEN R P, PI S Q, XIE B, et al. An improved method for synthesis of vitamin A derivatives via Wittig-Horner reaction[J]. Chinese Journal of Applied Chemistry, 2003, 20(12): 1211-1213. | |
| 66 | 刘园, 倪辉, 蔡慧农, 等. 反应条件对固定化脂肪酶转酯化合成维生素A棕榈酸酯的影响研究[J]. 中国食品学报, 2012, 12(1): 75-82. |
| LIU Y, NI H, CAI H N, et al. Study on reaction conditions for immobilized lipase synthezing retinol palmitate by transesterification[J]. Journal of Chinese Institute of Food Science and Technology, 2012, 12(1): 75-82. | |
| 67 | 李宏亮, 胡晶, 谭天伟. 固定化脂肪酶合成维生素A棕榈酸酯[J]. 生物工程学报, 2008, 24(5): 817-820. |
| LI H L, HU J, TAN T W. Immobilized lipase catalyzed synthesis of vitamin A plamitate[J]. Chinese Journal of Biotechnology, 2008, 24(5): 817-820. | |
| 68 | 于洪巍, 覃广德, 吕国锋, 等. 固定化酯酶E . coli BioH催化合成维生素A棕榈酸酯的方法: CN104673870A [P]. 2015-06-03. |
| YU H W, QIN G D, LÜ G F, et al. Synthesis of vitamin A palmitate catalyzed by immobilized esterase E . coli BioH, CN104673870A [P]. 2015-06-03. | |
| 69 | WU X F, YANG S L, YU H W, et al. Improved enantioselectivity of E. coli BioH in kinetic resolution of methyl (S)-3-cyclohexene-1-carboxylate by combinatorial modulation of steric and aromatic interactions[J]. Bioscience Biotechnology & Biochemistry, 2019, 83(7): 1263-1269. |
| 70 | 阮晖, 徐娟, 地里热巴, 等. 酵母展示脂肪酶催化合成维生素A乳酸酯的方法: CN102212602A[P]. 2011-10-12. |
| RUAN H, XU J, DILIREBA, et al. Method for synthesizing vitamin A lactate by catalysis of yeast display lipase: CN102212602A[P]. 2011-10-12. | |
| 71 | 高静, 姜艳军, 马丽, 等. 混合溶剂中酶促合成维生素A乳酸酯[J]. 分子催化, 2006, 20(4): 346-350. |
| GAO J, JIANG Y J, MA L, et al. Lipase catalyzed synthesis of vitamin A lactate in mixed solvent system[J]. Journal of Molecular Catalysis, 2006, 20(4): 346-350. | |
| 72 | ANDERSON E M, LARSSON K M, KIRK O. One biocatalyst-many applications: The use of Candida antarctica B-lipase in organic synthesis[J]. Biocatalysis and Biotransformation, 1998, 16(3): 181-204. |
| 73 | 谷雪贤. 维生素C衍生物的制备及其在化妆品中的应用[J]. 化学试剂, 2011, 33(4): 325-328. |
| GU X X. Preparation of L-ascorbic acid derivatives and their application in cosmetics[J]. Chemical Reagents, 2011, 33(4): 325-328. | |
| 74 | 张彦玲, 刘建峰, 齐永斌. 维生素C衍生物的现状与发展[J]. 河北化工, 2005, 28(3): 18-19, 21. |
| ZHANG Y L, LIU J F, QI Y B. Status and development of the VC derivates[J]. Hebei Chemical Engineering and Industry, 2005, 28(3): 18-19, 21. | |
| 75 | TAKEBAYASHI J, TAI A, GOHDA E, et al. Characterization of the radical-scavenging reaction of 2-O-substituted ascorbic acid derivatives, AA-2G, AA-2P, and AA-2S: a kinetic and stoichiometric study[J]. Biological & Pharmaceutical Bulletin, 2006, 29(4): 766-771. |
| 76 | WAKAMIYA H, SUZUKI E, YAMAMOTO I, et al. Vitamin C activity of 2-O-α-D-glucopyranosyl-L-ascorbic acid in Guinea pigs[J]. Journal of Nutritional Science and Vitaminology, 1992, 38(3): 235-245. |
| 77 | SAYO N, SEIJI K, WATARU H. Powdery cosmetic: WO2013065705A1 [P]. 2013-05-10. |
| 78 | YAMAMOTO I, MUTO N, NAGATA E, et al. Formation of a stable L-ascorbic acid α-glucoside by mammalian α-glucosidase-catalyzed transglucosylation[J]. Biochimica et Biophysica Acta, 1990, 1035(1): 44-50. |
| 79 | MUTO N, SUGA S, FUJII K, et al. Formation of a stable ascorbic acid 2-glucoside by specific transglucosylation with rice seed α-glucosidase [J]. Journal of the Agricultural Chemical Society of Japan, 2006, 54(7): 1697-703. |
| 80 | AGA H, YONEYAMA M, SAKAI S, et al. Synthesis of 2-O-α-D-glucopyranosyl L-ascorbic acid by cyclomaltodextrin glucanotransferase from Bacillus stearothermophilus [J]. Agricultural and Biological Chemistry, 1991, 55(7): 1751-1756. |
| 81 | VAN DER VEEN B A, VAN ALEBEEK G J, UITDEHAAG J C, et al. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms[J]. European Journal of Biochemistry, 2000, 267(3): 658-665. |
| 82 | VAN DER VEEN B A, UITDEHAAG J C M, PENNINGA D, et al. Rational design of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 to increase α-cyclodextrin production[J]. Journal of Molecular Biology, 2000, 296(4): 1027-1038. |
| 83 | JIANG Y J, ZHOU J, WU R F, et al. Heterologous expression of cyclodextrin glycosyltransferase from Paenibacillus macerans in Escherichia coli and its application in 2-O-α-D-glucopyranosyl-L-ascorbic acid production[J]. BMC Biotechnology, 2018, 18(1): 53. |
| 84 | JUN H K, BAE K M, KIM S K. Production of 2-O-α-D-glucopyranosyl L-ascorbic acid using cyclodextrin glucanotransferase from Paenibacillus sp[J]. Biotechnology Letters, 2001, 23(21): 1793-1797. |
| 85 | 李江华, 张子臣, 刘龙, 等. 响应面法优化酶法转化合成维生素C糖苷(AA-2G)条件的研究[J]. 工业微生物, 2011, 41(6): 1-5. |
| LI J H, ZHANG Z C, LIU L, et al. Process optimization of enzymatic glucopyranosyl-L-ascorbic acid (AA-2G) synthesis by response surface methodology[J]. Industrial Microbiology, 2011, 41(6): 1-5. | |
| 86 | LIU L, XU Q Y, HAN R Z, et al. Improving maltodextrin specificity for enzymatic synthesis of 2-O-D-glucopyranosyl-L-ascorbic acid by site-saturation engineering of subsite-3 in cyclodextrin glycosyltransferase from Paenibacillus macerans [J]. Journal of Biotechnology, 2013, 166(4): 198-205. |
| 87 | HAN R Z, LI J H, SHIN H D, et al. Fusion of self-assembling amphipathic oligopeptides with cyclodextrin glycosyltransferase improves 2-O-D-glucopyranosyl-L-ascorbic acid synthesis with soluble starch as the glycosyl donor[J]. Applied and Environmental Microbiology, 2014, 80(15): 4717-4724. |
| 88 | HAN R Z, LI J H, SHIN H D, et al. Carbohydrate-binding module-cyclodextrin glycosyltransferase fusion enables efficient synthesis of 2-O-D-glucopyranosyl-L-ascorbic acid with soluble starch as the glycosyl donor[J]. Applied and Environmental Microbiology, 2013, 79(10): 3234-3240. |
| 89 | TAO X M, SU L Q, WU J. Current studies on the enzymatic preparation 2-O-α-D-glucopyranosyl-L-ascorbic acid with cyclodextrin glycosyltransferase[J]. Critical Reviews in Biotechnology, 2019, 39(2): 249-257. |
| 90 | KWON T, KIM C T, LEE J H. Transglucosylation of ascorbic acid to ascorbic acid 2-glucoside by a recombinant sucrose phosphorylase from Bifidobacterium longum[J]. Biotechnology Letters, 2007, 29(4): 611-615. |
| 91 | GUDIMINCHI R K, NIDETZKY B. Walking a fine line with sucrose phosphorylase: efficient single-step biocatalytic production of L-ascorbic acid 2-glucoside from sucrose[J]. Chembiochem: A European Journal of Chemical Biology, 2017, 18(14): 1387-1390. |
| 92 | 谢若男. 生物法生产新型抗氧化剂2-O-β-D-葡萄糖基-L-抗坏血酸的研究[D]. 杭州: 浙江工业大学, 2009. |
| XIE R N. Study on enzymatic synthesis of 2-O-β-glucopyranosyl-L-ascorbid acid[D]. Hangzhou: Zhejiang University of Technology, 2009. | |
| 93 | FUJIO T, MARUYAMA A, KOIZUMI S. Process for the preparation of ascorbic acid-2-phosphate: US 5212079[P]. 1993-05-18. |
| 94 | 陈亮寰. 国外化工信息[J]. 化工进展, 2001, 20(2): 58-60. |
| CHEN L H. Chemical engineering information [J]. Chemical Industry and Engineering Progress, 2001, 20(2): 58-60. | |
| 95 | 汤鲁宏, 张浩. 非水相脂肪酶催化合成L-抗坏血酸棕榈酸酯的研究Ⅰ[J]. 生物工程学报, 2000, 16(3): 363-367. |
| TANG L H, ZHANG H. Studies on lipase-catalyzed synthesis of L-ascorbyl palmitate in non-aqueous phase[J]. Chinese Journal of Biotechnology, 2000, 16(3): 363-367. | |
| 96 | ZAKS A, KLIBANOV A M. The effect of water on enzyme action in organic media[J]. Journal of Biological Chemistry, 1988, 263(17): 8017-8021. |
| 97 | HUMEAU C, GIRARDIN M, ROVEL B, et al. Effect of the thermodynamic water activity and the reaction medium hydrophobicity on the enzymatic synthesis of ascorbyl palmitate[J]. Journal of Biotechnology, 1998, 63(1): 1-8. |
| 98 | LERIN L A, RICHETTI A, DALLAGO R, et al. Enzymatic synthesis of ascorbyl palmitate in organic solvents: process optimization and kinetic evaluation[J]. Food and Bioprocess Technology, 2012, 5(3): 1068-1076. |
| 99 | KARMEE S K. A two step chemo-enzymatic method for the synthesis of fatty acid ascorbyl esters[J]. Journal of Oil Palm Research, 2012, 24: 1518-1523. |
| 100 | BOUTS T, GASTHUYS F. The importance of vitamin E in zoo mammals[J]. Vlaams Diergeneeskundig Tijdschrift, 2003, 72(2): 125-129. |
| 101 | JIANG X J, HU Y, JIANG L, et al. Synthesis of vitamin E succinate from Candida rugosa lipase in organic medium[J]. Chemical Research in Chinese Universities, 2013, 29(2): 223-226. |
| 102 | YIN C H, ZHANG C, GAO M. Enzyme-catalyzed synthesis of vitamin E succinate using a chemically modified novozym-435[J]. Chinese Journal of Chemical Engineering, 2011, 19(1): 135-139. |
| 103 | DE CARVALHO C C C R. Enzymatic and whole cell catalysis: finding new strategies for old processes[J]. Biotechnology Advances, 2011, 29(1): 75-83. |
| 104 | KIRK O, BORCHERT T V, FUGLSANG C C. Industrial enzyme applications[J]. Current Opinion in Biotechnology, 2002, 13(4): 345-351. |
| [1] | LIU Xiaonan, LI Jing, ZHU Xiaoxi, XU Zishuo, QI Jian, JIANG Huifeng. Research advances on paclitaxel biosynthesis [J]. Synthetic Biology Journal, 2024, 5(3): 527-547. |
| [2] | Chaofan YANG, Yuchao JIANG, Moli SANG, Shengying LI, Wei ZHANG. Studies on the functional modulating effect of redox partners on the cytochrome P450 enzyme MycG [J]. Synthetic Biology Journal, 2022, 3(3): 587-601. |
| [3] | TU Tao, LUO Huiying, YAO Bin. Progress in the application of protein engineering in the developing of feed enzymes [J]. Synthetic Biology Journal, 2022, 3(3): 487-499. |
| [4] | Huibin WANG, Changli CHE, Song YOU. Recent advances of enzymatic synthesis of organohalogens catalyzed by Fe/αKG-dependent halogenases [J]. Synthetic Biology Journal, 2022, 3(3): 545-566. |
| [5] | Zhi LIN, Zhiwei HU, Xudong QU, Shuangjun LIN. Advances and challenges in microbial production of benzylisoquinoline alkaloids [J]. Synthetic Biology Journal, 2021, 2(5): 716-733. |
| [6] | Feiyan YUAN, Yang YU, Chun LI. Artificial enzyme designs and its application based on non-natural structural elements [J]. Synthetic Biology Journal, 2020, 1(6): 685-696. |
| [7] | Meixia LIU, Qiangzi LI, Dongdong MENG, Xinlei WEI, Chun YOU. Protein engineering of nicotinamide coenzyme-dependent oxidoreductases for coenzyme preference and its application in synthetic biology [J]. Synthetic Biology Journal, 2020, 1(5): 570-582. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||