Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (4): 728-747.DOI: 10.12211/2096-8280.2021-094
• Invited Review • Previous Articles Next Articles
Biosynthesis of elastin-like polypeptides and their applications in drug delivery
YANG Zhaoying1,2, ZHANG Fan1,2, GUO Jianwen1,2, GAO Weiping1
- 1.Biomedical Engineering Department,Peking University,Beijing 100191,China
2.Institute of Medical Technology,Peking University Health Science Center,Beijing 100191,China
-
Received:2021-09-27Revised:2021-11-11Online:2022-09-08Published:2022-08-31 -
Contact:GAO Weiping
类弹性蛋白多肽的生物合成及其药物递送应用
杨兆颖1,2, 张帆1,2, 郭建文1,2, 高卫平1
- 1.北京大学跨学部生物医学工程系,北京 100191
2.北京大学医学部医学技术研究院,北京 100191
-
通讯作者:高卫平 -
作者简介:杨兆颖 (1993—),女,博士,博士后。研究方向为生物偶联物与生物材料。E-mail:yangzhaoying18@163.com张帆 (1998—),女,博士研究生。研究方向为蛋白质-高分子偶联物。E-mail:m18232657001@163.com高卫平 (1975—),男,研究员,博士生导师。研究方向为生物偶联物与生物材料。E-mail:gaoweiping@hsc.pku.edu.cn -
基金资助:国家自然科学基金重大项目(81991505)
CLC Number:
Cite this article
YANG Zhaoying, ZHANG Fan, GUO Jianwen, GAO Weiping. Biosynthesis of elastin-like polypeptides and their applications in drug delivery[J]. Synthetic Biology Journal, 2022, 3(4): 728-747.
杨兆颖, 张帆, 郭建文, 高卫平. 类弹性蛋白多肽的生物合成及其药物递送应用[J]. 合成生物学, 2022, 3(4): 728-747.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-094

Fig. 3 ELPs applications in extending half-life of drugs(a) Chemically conjugated ELP to small molecule drugs by inclusion of an intervening cleavable linker which releases the free drug intracellularly after endocytic uptake and accumulation in the acidic, enzyme-rich environment of endosomes and lysosomes; (b) Enhanced cellular uptake of ELP conjugates by functionalization of ELP with cell-penetrating peptides; (c) Schematic of ELP genetically fused to peptide and protein drugs; (d) Pharmacokinetics of NtTNF-VHHELP[43]; (e, f) Pharmacometabolic kinetics (e) and in vivo antitumor effificacy (f) after intravenous injections with IFNα-ELP and IFNα[44]

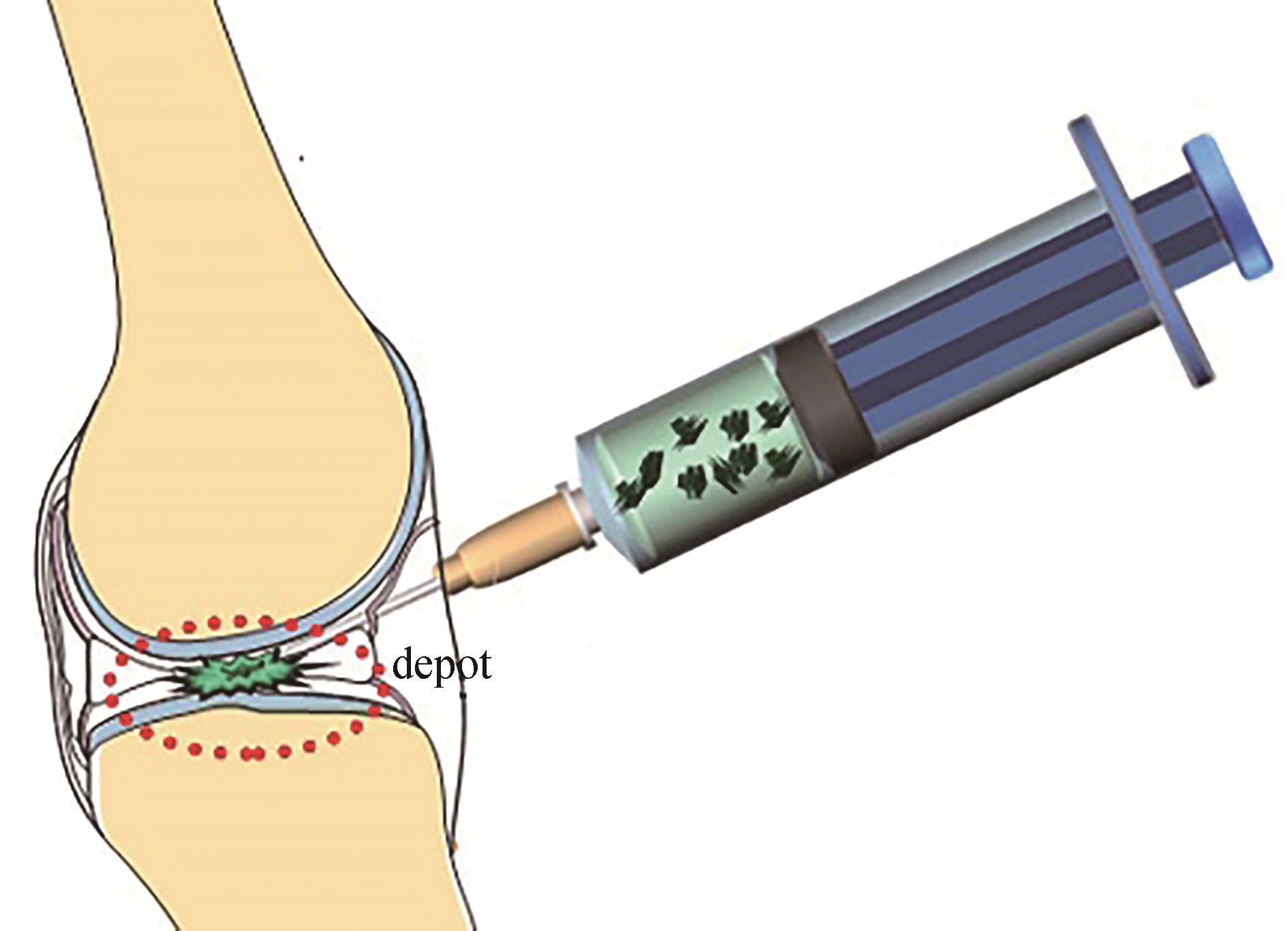
Fig. 4 Depot-forming ELP for drug delivery(a, b) Drugs form a subcutaneous insoluble coacervate upon injection and slowly dissolve from their surface to their core, steadily releasing the therapeutic into circulation; (c) The drug ELP conjugates can get into a tumor through the EPR effect and then be cleaved into free IFNα and ELP(V) by MMP-2 in the tumor, resulting in enhanced tumor penetration and antitumor efficacy[51]; (d) Fluorescence imaging of mice following MTD subcutaneous injection of Cy5-labeled IFNα-MMPS-ELP (V), IFN-MMPS-ELP (A), IFN-ELP (V), and IFNα; (e,f) pharmacokinetics (e) and antitumor efficacy (f) of mice after subcutaneous injections of IFNα-MMPS-ELP(V)、IFNα-MMPS-ELP(A)、IFNα-ELP(V) and IFNα at their MTDs.

Fig. 6 Schematic of ELPs in thermal targeting(The tumor tissue was locally heated, and the protein-ELP conjugates were phase transformed and enriched at the heating site.)
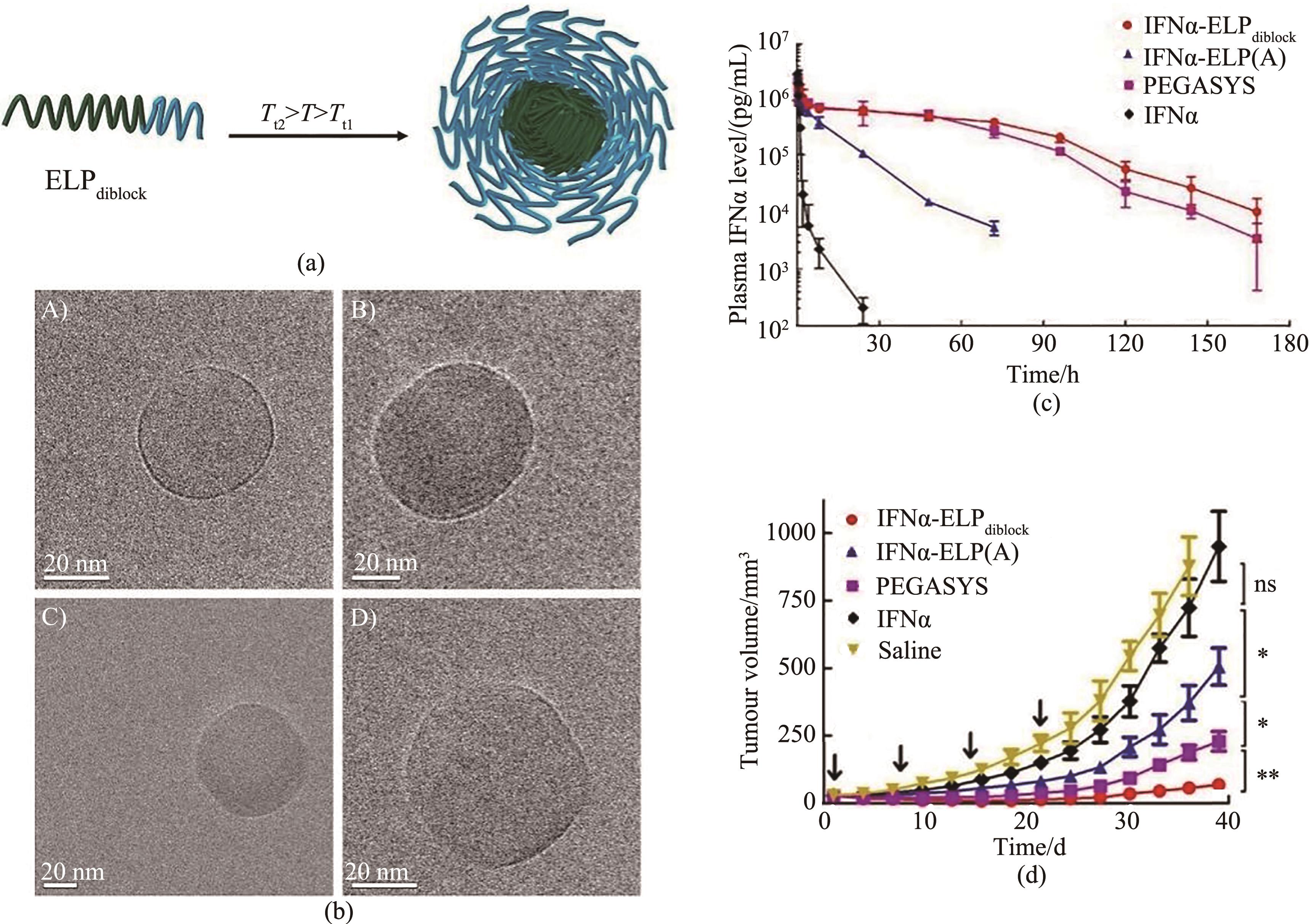
Fig. 7 (a) ELP micelles formed by diblock ELP, at a temperature between the Tt of the more hydrophobic ELP block (green, Tt 2) and the more hydrophilic ELP (blue, Tt1), the more hydrophobic block transitions and aggregates while the more hydrophilic block remains soluble, leading to self-assembly into micelles; (b) Cryo-TEM micrograph of ELP micelles[70]; (c,d) Pharmacokinetics (c) and antitumor efficacy (d) after intravenous injections with IFNα-ELPdiblock, IFNα-ELP(A), PEGASYS and IFNα [73]

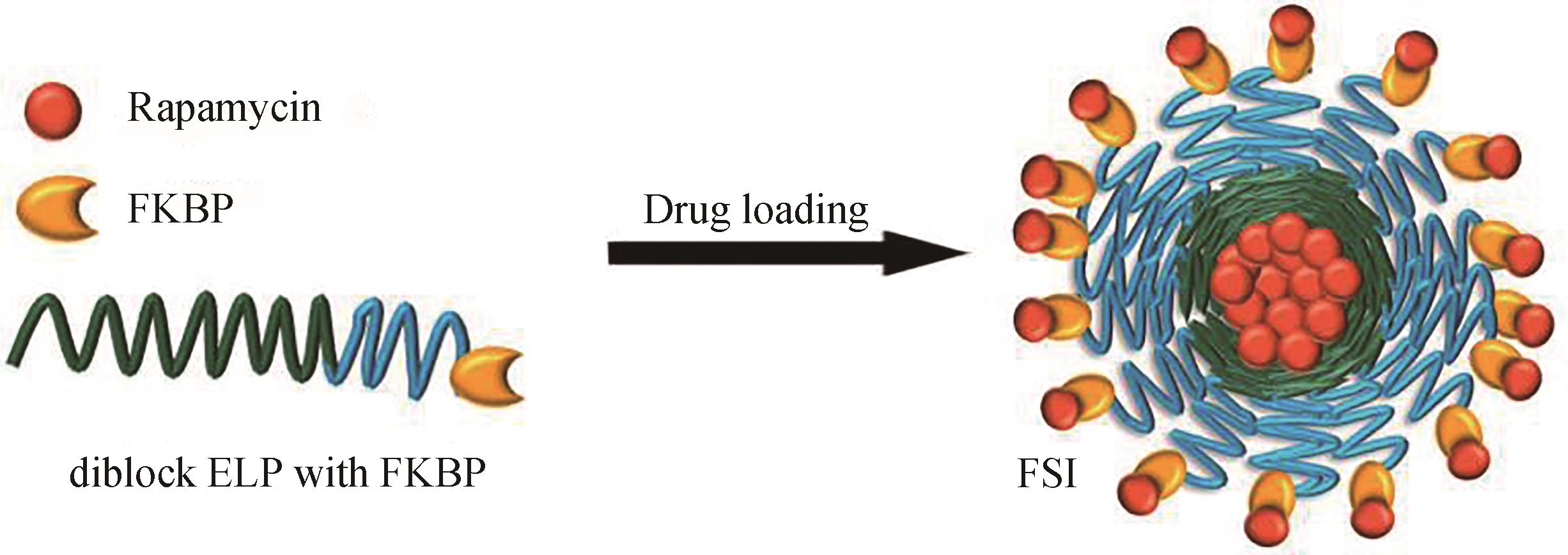
Fig. 9 (a) Schematic of ELP hybrid nanoparticles loading hydrophobic drug molecules. Hydrophobic drugs are loaded in the core; (b) Synthetic route of LHRH-ELP-DOX nanoconjugates; (c) Cryo-TEM images of LHRH-ELP2-DOX and ELP2-DOX; (d) Pharmacokinetics of LHRH-ELP2-DOX in a DOX-resistant breast tumor mouse model; (e) Tumor volume changes after LHRH-ELP-DOX plus HIFU treatment [78]
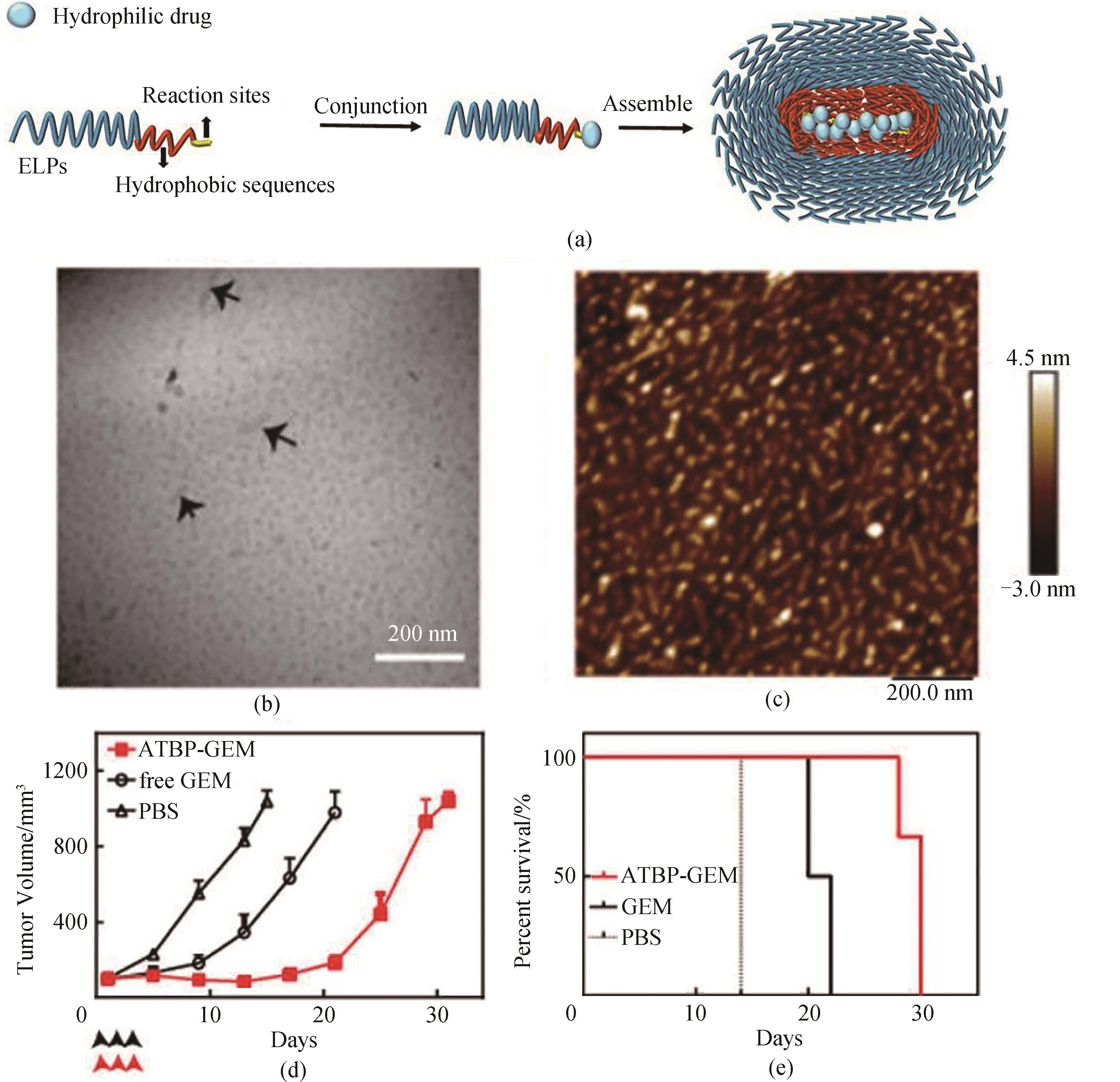
Fig. 10 (a) Schematic of ELP hybrid nanoparticles loading hydrophilic drug molecules where hydrophobic sequences act as the core of the assembly when assembled and hydrophilic drugs are loaded within the hydrophobic core; (b) Cryo-TEM micrograph of ATBP-GEM conjugate; (c) AFM image of ATBP-GEM nanoparticles; (d,e) ATBP-GEM delayed the tumor growth (d) and improved the cumulative survival (e) of mice [80]
| 药物递送策略 | 应用 | ELP药物 | ELP序列信息 | 文献 |
|---|---|---|---|---|
| 延长药物体内循环半衰期 | 髓样乳腺癌 | SynB1-ELP-DOX | (VPGXG)150 X=V5G3A2 | [ |
| 感染性休克 | NtTNF-VHHELP | (VPGXG)100 X=V5G3A2 | [ | |
| 淋巴瘤 | IFN-ELP | (VPGXG)90 X=V5G3A2 | [ | |
| 药物储库 | 2型糖尿病 | (GLP-1)-ELP | (GVGVP)120 | [ |
| 卵巢癌和黑色素瘤 | IFN-ELP | (VPGVG)90 | [ | |
| 胶质母细胞瘤 | IFN-ELP和替莫唑胺联合用药 | (VPGVG)90 | [ | |
| 黑色素瘤和卵巢癌 | IFN-MMPS-ELP | (VPGVG)90 | [ | |
| 创伤后关节炎 | xELP[IL-1Ra] | VPGKG(VPGVG)16~102 | [ | |
| 神经炎症 | ELP-curcumin | [VPGXG] L=60,80,160; X = V/I/E [1∶3∶1] | [ | |
| 骨损伤 | rhBMP-2-ELP | (VPGVG)40[(VPGVG)2(VPGCG)(VPGVG)2]2 | [ | |
| 热靶向治疗 | 卵巢癌、宫颈癌、人源咽鳞癌 | ELP1 | (VPGXG)150 X=V5G3A2 | [ |
| 两亲性自组装体 | 卵巢癌 | IFNα-ELPdiblock | ELP(A)48-ELP(V)48 | [ |
| 乳腺癌 | FKBP-ELP | G(Val-Pro-Gly-Ile-Gly)48(Val-Pro-Gly-Ser-Gly)48Y | [ | |
| 混合组装体 | 乳腺癌 | LHRH-ELP-DOX | (VPGXG)160 X=V1A8G7 | [ |
| 结肠癌 | ELP-(YG)6-(CGG)8-GEM | (VPGAG)160 | [ | |
| 乳腺癌 | DOXENC,M-ELP90A,120 | (VPGXG)120 X=A9V1 | [ | |
| 黑色素瘤 | DOX/PPy-ELP-F3 | (XGVPG)160 | [ | |
| 黑色素瘤、颈瘤、膀胱癌 | ELP-AuNP | (VPGVG)60 | [ | |
| ELP水凝胶 | 骨再生 | CDEc | (VPGVG)120 | [ |
Tab. 1 Applications of ELP in drug delivery
| 药物递送策略 | 应用 | ELP药物 | ELP序列信息 | 文献 |
|---|---|---|---|---|
| 延长药物体内循环半衰期 | 髓样乳腺癌 | SynB1-ELP-DOX | (VPGXG)150 X=V5G3A2 | [ |
| 感染性休克 | NtTNF-VHHELP | (VPGXG)100 X=V5G3A2 | [ | |
| 淋巴瘤 | IFN-ELP | (VPGXG)90 X=V5G3A2 | [ | |
| 药物储库 | 2型糖尿病 | (GLP-1)-ELP | (GVGVP)120 | [ |
| 卵巢癌和黑色素瘤 | IFN-ELP | (VPGVG)90 | [ | |
| 胶质母细胞瘤 | IFN-ELP和替莫唑胺联合用药 | (VPGVG)90 | [ | |
| 黑色素瘤和卵巢癌 | IFN-MMPS-ELP | (VPGVG)90 | [ | |
| 创伤后关节炎 | xELP[IL-1Ra] | VPGKG(VPGVG)16~102 | [ | |
| 神经炎症 | ELP-curcumin | [VPGXG] L=60,80,160; X = V/I/E [1∶3∶1] | [ | |
| 骨损伤 | rhBMP-2-ELP | (VPGVG)40[(VPGVG)2(VPGCG)(VPGVG)2]2 | [ | |
| 热靶向治疗 | 卵巢癌、宫颈癌、人源咽鳞癌 | ELP1 | (VPGXG)150 X=V5G3A2 | [ |
| 两亲性自组装体 | 卵巢癌 | IFNα-ELPdiblock | ELP(A)48-ELP(V)48 | [ |
| 乳腺癌 | FKBP-ELP | G(Val-Pro-Gly-Ile-Gly)48(Val-Pro-Gly-Ser-Gly)48Y | [ | |
| 混合组装体 | 乳腺癌 | LHRH-ELP-DOX | (VPGXG)160 X=V1A8G7 | [ |
| 结肠癌 | ELP-(YG)6-(CGG)8-GEM | (VPGAG)160 | [ | |
| 乳腺癌 | DOXENC,M-ELP90A,120 | (VPGXG)120 X=A9V1 | [ | |
| 黑色素瘤 | DOX/PPy-ELP-F3 | (XGVPG)160 | [ | |
| 黑色素瘤、颈瘤、膀胱癌 | ELP-AuNP | (VPGVG)60 | [ | |
| ELP水凝胶 | 骨再生 | CDEc | (VPGVG)120 | [ |
| 1 | ADEPU S, RAMAKRISHNA S. Controlled drug delivery systems: Current status and future directions[J]. Molecules, 2021, 26(19): 5905. |
| 2 | MITHIEUX S M, WEISS A S. Elastin[M]//San Diego: Academic Press Inc Elsevier Science, 2005: 437-461. |
| 3 | MCPHERSON D T, MORROW C, MINEHAN D S, et al. Production and purification of a recombinant elastomeric polypeptide, G-(VPGVG)19-VPGV, from Escherichia coli [J]. Biotechnology Progress, 1992, 8(4): 347-352. |
| 4 | MACEWAN S R, CHILKOTI A. Elastin-like polypeptides: biomedical applications of tunable biopolymers[J]. Biopolymers, 2010, 94(1): 60-77. |
| 5 | ZHAO B W, LI N K, YINGLING Y G, et al. LCST behavior is manifested in a single molecule: elastin-like polypeptide (VPGVG)N[J]. Biomacromolecules, 2015, 17(1): 111-118. |
| 6 | MEYER D E, CHILKOTI A. Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides[J]. Biomacromolecules, 2004, 5(3): 846-851. |
| 7 | NAIR L S, LAURENCIN C T. Biodegradable polymers as biomaterials[J]. Progress in Polymer Science, 2007, 32(8/9): 762-798. |
| 8 | CHILKOTI A, CHRISTENSEN T, MACKAY J A. Stimulus responsive elastin biopolymers: applications in medicine and biotechnology[J]. Current Opinion in Chemical Biology, 2006, 10(6): 652-657. |
| 9 | PRASAD K U, IQBAL M A, URRY D W. Utilization of 1-hydroxybenzotriazole in mixed anhydride coupling reactions[J]. International Journal of Peptide and Protein Research, 1985, 25(4): 408-413. |
| 10 | GUDA C, ZHANG X, MCPHERSON D T, et al. Hyper expression of an environmentally friendly synthetic polymer gene[J]. Biotechnology Letters, 1995, 17(7): 745-750. |
| 11 | MCPHERSON D T, XU J, URRY D W. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP[J]. Protein Expression and Purification, 1996, 7(1): 51-57. |
| 12 | MEYER D E, CHILKOTI A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system[J]. Biomacromolecules, 2002, 3(2): 357-367. |
| 13 | MCDANIEL J R, MACKAY J A, QUIROZ F G, et al. Recursive directional ligation by plasmid reconstruction allows rapid and seamless cloning of oligomeric genes[J]. Biomacromolecules, 2010, 11(4): 944-952. |
| 14 | AMIRAM M, QUIROZ F G, CALLAHAN D J, et al. A highly parallel method for synthesizing DNA repeats enables the discovery of “smart” protein polymers[J]. Nature Materials, 2011, 10(2): 141-148. |
| 15 | TANG N C, CHILKOTI A. Combinatorial codon scrambling enables scalable gene synthesis and amplification of repetitive proteins[J]. Nature Materials, 2016, 15(4): 419-424. |
| 16 | URRY D W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions[J]. Progress in Biophysics and Molecular Biology, 1992, 57(1): 23-57. |
| 17 | URRY D W. Molecular machines: How motion and other functions of living organisms can result from reversible chemical changes[J]. Angewandte Chemie International Edition in English, 1993, 32(6): 819-841. |
| 18 | URRY D W. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers[J]. The Journal of Physical Chemistry B, 1997, 101(51): 11007-11028. |
| 19 | MEYER D E, CHILKOTI A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides[J]. Nature Biotechnology, 1999, 17(11): 1112-1115. |
| 20 | HASSOUNEH W, CHRISTENSEN T, CHILKOTI A. Elastin-like polypeptides as a purification tag for recombinant proteins[J]. Current Protocols in Protein Science, 2010, 61(1): 6.11.1-6.11.16. |
| 21 | LIM D W, TRABBIC-CARLSON K, MACKAY J A, et al. Improved non-chromatographic purification of a recombinant protein by cationic elastin-like polypeptides[J]. Biomacromolecules, 2007, 8(5): 1417-1424. |
| 22 | BOOTH J J, ABBOTT S, SHIMIZU S. Mechanism of hydrophobic drug solubilization by small molecule hydrotropes[J]. The Journal of Physical Chemistry B, 2012, 116(51): 14915-14921. |
| 23 | WANG Q Z. Recent advances in protein drug delivery[J]. IOP Conference Series: Materials Science and Engineering, 2020, 768(5): 052055. |
| 24 | LEADER B, BACA Q J, GOLAN D E. Protein therapeutics: a summary and pharmacological classification[J]. Nature Reviews Drug Discovery, 2008, 7(1): 21-39. |
| 25 | VERONESE F M, MERO A. The impact of PEGylation on biological therapies[J]. BioDrugs, 2008, 22(5): 315-329. |
| 26 | ABRAHAM S A, WATERHOUSE D N, MAYER L D, et al. The liposomal formulation of doxorubicin[J]. Methods in Enzymology, 2005, 391: 71-97. |
| 27 | DREHER M R, RAUCHER D, BALU N, et al. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy[J]. Journal of Controlled Release, 2003, 91(1/2): 31-43. |
| 28 | FURGESON D Y, DREHER M R, CHILKOTI A. Structural optimization of a "smart" doxorubicin-polypeptide conjugate for thermally targeted delivery to solid tumors[J]. Journal of Controlled Release, 2006, 110(2): 362-369. |
| 29 | BIDWELL G L, DAVIS A N, FOKT I, et al. A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance[J]. Investigational New Drugs, 2007, 25(4): 313-326. |
| 30 | MOKTAN S, PERKINS E, KRATZ F, et al. Thermal targeting of an acid-sensitive doxorubicin conjugate of elastin-like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo [J]. Molecular Cancer Therapeutics, 2012, 11(7): 1547-1556. |
| 31 | ROUSSELLE C, CLAIR P, LEFAUCONNIER J M, et al. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy[J]. Molecular Pharmacology, 2000, 57(4): 679-686. |
| 32 | BIDWELL G L III, RAUCHER D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery[J]. Advanced Drug Delivery Reviews, 2010, 62(15): 1486-1496. |
| 33 | BIDWELL G L III, WHITTOM A A, THOMAS E, et al. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation[J]. Peptides, 2010, 31(5): 834-841. |
| 34 | MAINI R N, ELLIOTT M J, BRENNAN F M, et al. Beneficial effects of tumour necrosis factor-alpha (TNF-α) blockade in rheumatoid arthritis (RA)[J]. Clinical and Experimental Immunology, 2013, 101(2): 207-212. |
| 35 | BEUTLER B, MILSARK I W, CERAMI A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin[J]. Science, 1985, 229(4716): 869-871. |
| 36 | VAN DULLEMEN H M, VAN DEVENTER S J H, HOMMES D W, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (CA2)[J]. Gastroenterology, 1995, 109(1): 129-135. |
| 37 | FALVO J V, TSYTSYKOVA A V, GOLDFELD A E. Transcriptional control of the TNF gene[J]. Current Directions in Autoimmunity, 2010, 11: 27-60. |
| 38 | TSAI E Y, YIE J, THANOS D, et al. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN[J]. Molecular and Cellular Biology, 1996, 16(10): 5232-5244. |
| 39 | 高世勇, 李丹. 肿瘤坏死因子与癌症相关研究进展[J]. 中国药理学通报, 2020, 36(9): 1209-1213. |
| GAO S Y, LI D. Research advances in tumor necrosis factor and cancer[J]. Chinese Pharmacological Bulletin, 2020, 36(9): 1209-1213. | |
| 40 | SMITH C A, FARRAH T, GOODWIN R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death[J]. Cell, 1994, 76(6): 959-962. |
| 41 | COPPIETERS K, DREIER T, SILENCE K, et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis[J]. Arthritis and Rheumatism, 2006, 54(6): 1856-1866. |
| 42 | PLAGMANN I, CHALARIS A, KRUGLOV A A, et al. Transglutaminase-catalyzed covalent multimerization of Camelidae anti-human TNF single domain antibodies improves neutralizing activity[J]. Journal of Biotechnology, 2009, 142(2): 170-178. |
| 43 | CONRAD U, PLAGMANN I, MALCHOW S, et al. ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock[J]. Plant Biotechnology Journal, 2011, 9(1): 22-31. |
| 44 | HU J, WANG G L, LIU X Y, et al. Enhancing pharmacokinetics, tumor accumulation, and antitumor efficacy by elastin-like polypeptide fusion of interferon alpha[J]. Advanced Materials, 2015, 27(45): 7320-7324. |
| 45 | MIRANDA L P, WINTERS K A, GEGG C V, et al. Design and synthesis of conformationally constrained glucagon-like peptide-1 derivatives with increased plasma stability and prolonged in vivo activity[J]. Journal of Medicinal Chemistry, 2008, 51(9): 2758-2765. |
| 46 | 桑延霞. 胰高血糖素样肽1类似物长效化的策略[J]. 中国新药与临床杂志, 2021, 40(7): 481-488. |
| SANG Y X. Strategies for long-acting effect of glucagon-like peptide 1 analogs[J]. Chinese Journal of New Drugs and Clinical Remedies, 2021, 40(7): 481-488. | |
| 47 | AMIRAM M, LUGINBUHL K M, LI X, et al. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection[J]. Journal of Controlled Release, 2013, 172(1): 144-151. |
| 48 | MALM-ERJEFÄLT M, BJØRNSDOTTIR I, VANGGAARD J, et al. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase[J]. Drug Metabolism and Disposition: the Biological Fate of Chemicals, 2010, 38(11): 1944-1953. |
| 49 | WANG Z R, GUO J W, NING J, et al. One-month zero-order sustained release and tumor eradication after a single subcutaneous injection of interferon alpha fused with a body-temperature-responsive polypeptide[J]. Biomaterials Science, 2018, 7(1): 104-112. |
| 50 | LIANG P, WANG G H, LIU X Y, et al. Spatiotemporal combination of thermosensitive polypeptide fused interferon and temozolomide for post-surgical glioblastoma immunochemotherapy[J]. Biomaterials, 2021, 264: 120447. |
| 51 | WANG Z R, GUO J W, SUN J W, et al. Thermoresponsive and protease-cleavable interferon-polypeptide conjugates with spatiotemporally programmed two-step release kinetics for tumor therapy[J]. Advanced Science, 2019, 6(16): 1900586. |
| 52 | CHEVALIER X, GOUPILLE P, BEAULIEU A D, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study[J]. Arthritis and Rheumatism, 2009, 61(3): 344-352. |
| 53 | KIMMERLING K A, FURMAN B D, MANGIAPANI D S, et al. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis[J]. European Cells & Materials, 2015, 29: 124-139. |
| 54 | ADAMS S B, SHAMJI M F, NETTLES D L, et al. Sustained release of antibiotics from injectable and thermally responsive polypeptide depots[J]. Journal of Biomedical Materials Research Part B, Applied Biomaterials, 2009, 90(1): 67-74. |
| 55 | BETRE H, LIU W G, ZALUTSKY M R, et al. A thermally responsive biopolymer for intra-articular drug delivery[J]. Journal of Controlled Release, 2006, 115(2): 175-182. |
| 56 | ANAND P, THOMAS S G, KUNNUMAKKARA A B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature[J]. Biochemical Pharmacology, 2008, 76(11): 1590-1611. |
| 57 | 刘伟, 顾秀竹, 吴筱霓, 等. 姜黄素药理作用的研究进展[J]. 华西药学杂志, 2021, 36(3): 336-340. |
| LIU W, GU X Z, WU X N, et al. Research on the pharmacological actions of curcumin[J]. West China Journal of Pharmaceutical Sciences, 2021, 36(3): 336-340. | |
| 58 | 张洪英. 中药姜黄的研究进展[J]. 菏泽医专学报, 2001, 13(4): 84-87. |
| ZHANG H Y. Research progress of traditional Chinese medicine turmeric [J]. Journal of Heze Medical College, 2001, 13(4): 84-87. | |
| 59 | SINCLAIR S M, BHATTACHARYYA J, MCDANIEL J R, et al. A genetically engineered thermally responsive sustained release curcumin depot to treat neuroinflammation[J]. Journal of Controlled Release, 2013, 171(1): 38-47. |
| 60 | CARREIRA A C, ALVES G G, ZAMBUZZI W F, et al. Bone morphogenetic proteins: structure, biological function and therapeutic applications[J]. Archives of Biochemistry and Biophysics, 2014, 561: 64-73. |
| 61 | MIMATSU K, KISHI S, HASHIZUME Y. Experimental chronic compression on the spinal cord of the rabbit by ectopic bone formation in the ligamentum flavum with bone morphogenetic protein[J]. Spinal Cord, 1997, 35(11): 740-746. |
| 62 | MCCARTHY B, YUAN Y, KORIA P. Elastin-like-polypeptide based fusion proteins for osteogenic factor delivery in bone healing[J]. Biotechnology Progress, 2016, 32(4): 1029-1037. |
| 63 | DEWHIRST M W, PROSNITZ L, THRALL D, et al. Hyperthermic treatment of malignant diseases: current status and a view toward the future[J]. Seminars in Oncology, 1997, 24(6): 616-625. |
| 64 | FEYERABEND T, STEEVES R, WIEDEMANN G J, et al. Rationale and clinical status of local hyperthermia, radiation, and chemotherapy in locally advanced malignancies[J]. Anticancer Research, 1997, 17(4B): 2895-2897. |
| 65 | ISSELS R. Hyperthermia combined with chemotherapy-biological rationale, clinical application, and treatment results[J]. Oncology Research and Treatment, 1999, 22(5): 374-381. |
| 66 | RAUCHER D, CHILKOTI A. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition[J]. Cancer Research, 2001, 61(19): 7163-7170. |
| 67 | MEYER D E, KONG G A, DEWHIRST M W, et al. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia[J]. Cancer Research, 2001, 61(4): 1548-1554. |
| 68 | WHITESIDES G M, MATHIAS J P, SETO C T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures[J]. Science, 1991, 254(5036): 1312-1319. |
| 69 | RODRÍGUEZ-HERNÁNDEZ J, CHÉCOT F, GNANOU Y, et al. Toward "smart" nano-objects by self-assembly of block copolymers in solution[J]. Progress in Polymer Science, 2005, 30(7): 691-724. |
| 70 | DREHER M R, SIMNICK A J, FISCHER K, et al. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles[J]. Journal of the American Chemical Society, 2008, 130(2): 687-694. |
| 71 | 杨国新, 侯佳男, 胡学军, 等. 自组装ELP多肽的从头设计及其在生物医药领域中的应用进展[J]. 生命的化学, 2021, 41(2): 296-305. |
| YANG G X, HOU J N, HU X J, et al. De novo design of self-assembled ELP peptides and their applications in biomedicine[J]. Chemistry of Life, 2021, 41(2): 296-305. | |
| 72 | ABDELGHANI M, SHAO J X, LE D H T, et al. Self-assembly or coassembly of multiresponsive histidine-containing elastin-like polypeptide block copolymers[J]. Macromolecular Bioscience, 2021, 21(6): e2100081. |
| 73 | WANG Z R, GUO J W, LIU X Y, et al. Temperature-triggered micellization of interferon alpha-diblock copolypeptide conjugate with enhanced stability and pharmacology[J]. Journal of Controlled Release, 2020, 328: 444-453. |
| 74 | SIMAMORA P, ALVAREZ J M, YALKOWSKY S H. Solubilization of rapamycin[J]. International Journal of Pharmaceutics, 2001, 213(1/2): 25-29. |
| 75 | SHI P, ALURI S, LIN Y A, et al. Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo [J]. Journal of Controlled Release, 2013, 171(3): 330-338. |
| 76 | 郑鹏生, 冀静. mTOR信号通路与肿瘤的研究进展[J]. 西安交通大学学报(医学版), 2010, 31(1): 1-9. |
| ZHENG P S, JI J. Advance in research on the relationship between mTOR signaling pathway and tumors[J]. Journal of Xi'an Jiaotong University (Medical Sciences), 2010, 31(1): 1-9. | |
| 77 | MCDANIEL J R, BHATTACHARYYA J, VARGO K B, et al. Self-assembly of thermally responsive nanoparticles of a genetically encoded peptide polymer by drug conjugation[J]. Angewandte Chemie International Edition, 2013, 52(6): 1683-1687. |
| 78 | WANG Z R, HE Q, ZHAO W G, et al. Tumor-homing, pH- and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy[J]. Journal of Controlled Release, 2017, 264: 66-75. |
| 79 | DHARAP S S, WANG Y, CHANDNA P, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(36): 12962-12967. |
| 80 | BHATTACHARYYA J, WEITZHANDLER I, HO S B, et al. Encapsulating a hydrophilic chemotherapeutic into rod-like nanoparticles of a genetically encoded asymmetric triblock polypeptide improves its efficacy[J]. Advanced Functional Materials, 2017, 27(12): 1605421. |
| 81 | LUGINBUHL K M, MOZHDEHI D, DZURICKY M, et al. Recombinant synthesis of hybrid lipid-peptide polymer fusions that self-assemble and encapsulate hydrophobic drugs[J]. Angewandte Chemie International Edition, 2017, 56(45): 13979-13984. |
| 82 | SUN M M, GUO J W, HAO H J, et al. Tumour-homing chimeric polypeptide-conjugated polypyrrole nanoparticles for imaging-guided synergistic photothermal and chemical therapy of cancer[J]. Theranostics, 2018, 8(10): 2634-2645. |
| 83 | 黄晚秋, 高苗苗, 窦红静. 聚吡咯及其纳米复合材料在光热治疗领域的应用[J]. 化学进展, 2020, 32(4): 371-380. |
| HUANG W Q, GAO M M, DOU H J. Polypyrrole and its nanocomposites applied in photothermal therapy[J]. Progress in Chemistry, 2020, 32(4): 371-380. | |
| 84 | PALLAVICINI P, DONÀ A, CASU A, et al. Triton X-100 for three-plasmon gold nanostars with two photothermally active NIR (near IR) and SWIR (short-wavelength IR) channels[J]. Chemical Communications, 2013, 49(56): 6265-6267. |
| 85 | LIU X S, HUANG N, LI H, et al. Multidentate polyethylene glycol modified gold nanorods for in vivo near-infrared photothermal cancer therapy[J]. ACS Applied Materials & Interfaces, 2014, 6(8): 5657-5668. |
| 86 | KHLEBTSOV N, BOGATYREV V, DYKMAN L, et al. Analytical and theranostic applications of gold nanoparticles and multifunctional nanocomposites[J]. Theranostics, 2013, 3(3): 167-180. |
| 87 | GRABINSKI C, SCHAEUBLIN N, WIJAYA A, et al. Effect of gold nanorod surface chemistry on cellular response[J]. ACS Nano, 2011, 5(4): 2870-2879. |
| 88 | WANG S T, CHEN K J, WU T H, et al. Photothermal effects of supramolecularly assembled gold nanoparticles for the targeted treatment of cancer cells[J]. Angewandte Chemie International Edition, 2010, 49(22): 3777-3781. |
| 89 | SUN M M, PENG D, HAO H J, et al. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy[J]. ACS Applied Materials & Interfaces, 2017, 9(12): 10453-10460. |
| 90 | BETRE H, SETTON L A, MEYER D E, et al. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair[J]. Biomacromolecules, 2002, 3(5): 910-916. |
| 91 | URRY D W, HAYNES B, ZHANG H, et al. Mechanochemical coupling in synthetic polypeptides by modulation of an inverse temperature transition[J]. Proceedings of the National Academy of Sciences of the United States of America, 1988, 85(10): 3407-3411. |
| 92 | TRABBIC-CARLSON K, SETTON L A, CHILKOTI A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides[J]. Biomacromolecules, 2003, 4(3): 572-580. |
| 93 | HRABCHAK C, ROULEAU J, MOSS I, et al. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits[J]. Acta Biomaterialia, 2010, 6(6): 2108-2115. |
| 94 | SUN F, ZHANG W B, MAHDAVI A, et al. Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(31): 11269-11274. |
| 95 | PAL P, NGUYEN Q C, BENTON A H, et al. Drug-loaded elastin-like polypeptide-collagen hydrogels with high modulus for bone tissue engineering[J]. Macromolecular Bioscience, 2019, 19(9): e1900142. |
| 96 | ZHANG W B, SUN F, TIRRELL D A, et al. Controlling macromolecular topology with genetically encoded SpyTag-SpyCatcher chemistry[J]. Journal of the American Chemical Society, 2013, 135(37): 13988-13997. |
| 97 | WANG X W, ZHANG W B. Cellular synthesis of protein catenanes[J]. Angewandte Chemie International Edition, 2016, 55(10): 3442-3446. |
| 98 | LIU D, WU W H, LIU Y J, et al. Topology engineering of proteins in vivo using genetically encoded, mechanically interlocking SpyX modules for enhanced stability[J]. ACS Central Science, 2017, 3(5): 473-481. |
| 99 | DA X D, ZHANG W B. Active template synthesis of protein heterocatenanes[J]. Angewandte Chemie International Edition, 2019, 58(32): 11097-11104. |
| 100 | ROSANO G L, CECCARELLI E A. Recombinant protein expression in Escherichia coli: advances and challenges[J]. Frontiers in Microbiology, 2014, 5: 172. |
| [1] | LI Shikai, ZENG Dong′ao, DU Fangzhou, ZHANG Jingzhong, YU Shuang. The construction approaches and biomaterials for vascularized organoids [J]. Synthetic Biology Journal, 2024, 5(4): 851-866. |
| [2] | JI Botao, QIAN Zhigang, XIA Xiaoxia. Application of cell-free synthesis strategy in biomaterial research [J]. Synthetic Biology Journal, 2022, 3(4): 658-675. |
| [3] | LI Jingjing, MA Chao, WANG Fan, ZHANG Hongjie, LIU Kai. Biosynthesis of high-performance protein materials and their applications [J]. Synthetic Biology Journal, 2022, 3(4): 638-657. |
| [4] | ZHANG Can, SHI Liyang, DAI Jianwu. Cultured meat from biomaterials: challenges and prospects [J]. Synthetic Biology Journal, 2022, 3(4): 676-689. |
| [5] | ZHENG Hanqi, WU Qing, LI Hongjun, GU Zhen. Integration of synthetic biology and nanobiotechnology for biomedical applications [J]. Synthetic Biology Journal, 2022, 3(2): 279-301. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||