Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (4): 709-727.DOI: 10.12211/2096-8280.2022-001
• Invited Review • Previous Articles Next Articles
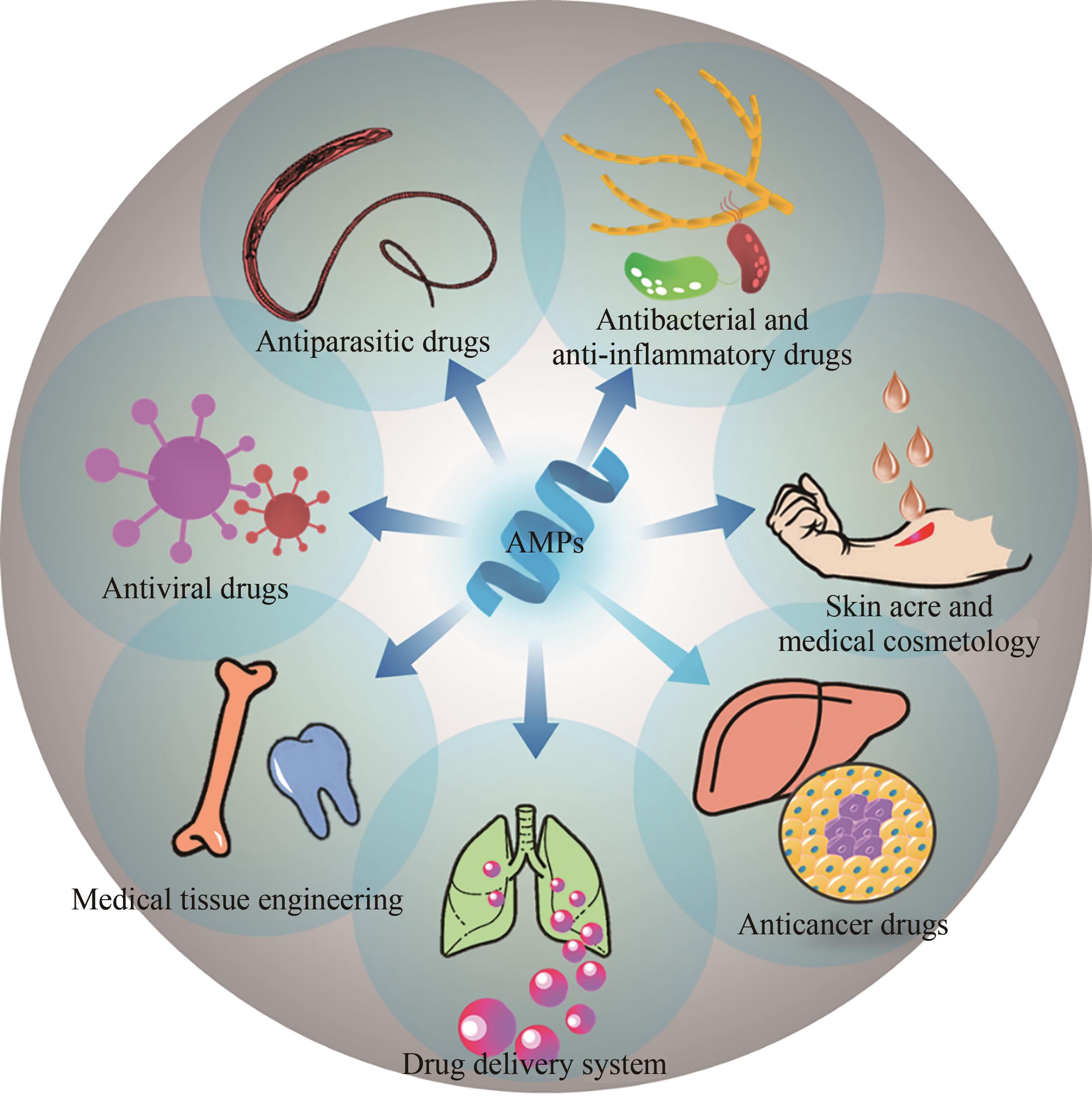
Biosynthesis of antimicrobial peptides and its medical application
WEI Daixu( ), GONG Hailun, ZHANG Xuwei
), GONG Hailun, ZHANG Xuwei
- College of Life Sciences and Medicine,Northwest University,Xi'an 710069,Shaanxi,China
-
Received:2022-01-08Revised:2022-04-26Online:2022-09-08Published:2022-08-31 -
Contact:WEI Daixu
抗菌肽的生物合成及医学应用
- 西北大学生命科学院与医学部,陕西 西安 710069
-
通讯作者:魏岱旭 -
作者简介:魏岱旭 (1986—),男,博士,教授,博士生导师。研究方向为生物材料、合成生物学、组织工程与再生医学、医用微纳米器件和智能递药系统、医学美容及化妆品、生物力学及空间生物学。E-mail:weidaixu@nwu.edu.cn; daviddxwei@163.com -
基金资助:国家自然科学基金(31900950)
CLC Number:
Cite this article
WEI Daixu, GONG Hailun, ZHANG Xuwei. Biosynthesis of antimicrobial peptides and its medical application[J]. Synthetic Biology Journal, 2022, 3(4): 709-727.
魏岱旭, 龚海伦, 张旭维. 抗菌肽的生物合成及医学应用[J]. 合成生物学, 2022, 3(4): 709-727.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2022-001
| Expression system and engineered microorganism | Antimicrobial peptides | References |
|---|---|---|
| Prokaryotic | ||
| E. coli | Cecropin | [ |
| Perinerin | [ | |
| Adenoregulin | [ | |
| Abaecin | [ | |
| Hep-A200 | [ | |
| MLH | [ | |
| Hal18 | [ | |
| Plectasin | [ | |
| Lactoferricin | [ | |
| Buforin II | [ | |
| B.subtilis | Cecropin AD | [ |
| Cathelicidin-BF | [ | |
| T9W | [ | |
| CiMAM | [ | |
| Eukaryotic | ||
| S.cerevisiae | CecropinXJ | [ |
| Cecropin P1 | [ | |
| P.pastoris | Cecropin AD | [ |
| PaDef | [ | |
| Melittin | [ | |
| Fowlicidin-2 | [ | |
| HKABF | [ | |
| Mytichitin-A | [ | |
| ABP-CM4 | [ | |
| C.reinhardtii | 3×Mytichitin-A | [ |
| Bacteriocin LS2 | [ | |
| ToAMP4 | [ | |
| Mytichitin-CB | [ |
Tab. 1 Expression system of antimicrobial peptides in this review
| Expression system and engineered microorganism | Antimicrobial peptides | References |
|---|---|---|
| Prokaryotic | ||
| E. coli | Cecropin | [ |
| Perinerin | [ | |
| Adenoregulin | [ | |
| Abaecin | [ | |
| Hep-A200 | [ | |
| MLH | [ | |
| Hal18 | [ | |
| Plectasin | [ | |
| Lactoferricin | [ | |
| Buforin II | [ | |
| B.subtilis | Cecropin AD | [ |
| Cathelicidin-BF | [ | |
| T9W | [ | |
| CiMAM | [ | |
| Eukaryotic | ||
| S.cerevisiae | CecropinXJ | [ |
| Cecropin P1 | [ | |
| P.pastoris | Cecropin AD | [ |
| PaDef | [ | |
| Melittin | [ | |
| Fowlicidin-2 | [ | |
| HKABF | [ | |
| Mytichitin-A | [ | |
| ABP-CM4 | [ | |
| C.reinhardtii | 3×Mytichitin-A | [ |
| Bacteriocin LS2 | [ | |
| ToAMP4 | [ | |
| Mytichitin-CB | [ |
| AMPs | Indication | Deliever | Status | Clinical trial identifiers |
|---|---|---|---|---|
| Daptomycin | Bacterial skin infections | Intravenous | Approved | NCT01211470 |
| Vancomycin | Staphylococcal infections | Intravenous | Approved | NCT00175370 |
| Dalbavancin (BI397, Dalvance, Xydalba) | Acute bacterial skin infections | Intravenous | Approved | NCT03233438 |
| Colistin | Multidrug-resistant Gram-negative infections | Intravenous | Approved | NCT03397914 |
Tab. 2 Name, indication, deliever and clinical trial identification of antimicrobial peptides approved by US Food and Drug Administration (FDA)[6, 114-116]
| AMPs | Indication | Deliever | Status | Clinical trial identifiers |
|---|---|---|---|---|
| Daptomycin | Bacterial skin infections | Intravenous | Approved | NCT01211470 |
| Vancomycin | Staphylococcal infections | Intravenous | Approved | NCT00175370 |
| Dalbavancin (BI397, Dalvance, Xydalba) | Acute bacterial skin infections | Intravenous | Approved | NCT03233438 |
| Colistin | Multidrug-resistant Gram-negative infections | Intravenous | Approved | NCT03397914 |
| 1 | GONG T, FU J, SHI L X, et al. Antimicrobial peptides in gut health: a review[J]. Frontiers in Nutrition, 2021, 8: 751010. |
| 2 | PEN G H, YANG N, TENG D, et al. A review on the use of antimicrobial peptides to combat porcine viruses[J]. Antibiotics, 2020, 9(11): 801. |
| 3 | MONTALVO G, VANDENBERGHE L, SOCCOL V T, et al. The antihypertensive, antimicrobial and anticancer peptides from arthrospira with therapeutic potential: a mini review[J]. Current Molecular Medicine, 2020, 20(8): 593-606. |
| 4 | VINEETH KUMAR T V, SANIL G. A review of the mechanism of action of amphibian antimicrobial peptides focusing on peptide-membrane interaction and membrane curvature[J]. Current Protein & Peptide Science, 2017, 18(12): 1263-1272. |
| 5 | LEE J K, LUCHIAN T, PARK Y. New antimicrobial peptide kills drug-resistant pathogens without detectable resistance[J]. Oncotarget, 2018, 9(21): 15616-15634. |
| 6 | ZHANG Q Y, YAN Z B, MENG Y M, et al. Antimicrobial peptides: mechanism of action, activity and clinical potential[J]. Military Medical Research, 2021, 8(1): 48. |
| 7 | FOX J L. Antimicrobial peptides stage a comeback[J]. Nature Biotechnology, 2013, 31(5): 379-382. |
| 8 | PASUPULETI M, SCHMIDTCHEN A, MALMSTEN M. Antimicrobial peptides: key components of the innate immune system[J]. Critical Reviews in Biotechnology, 2012, 32(2): 143-171. |
| 9 | SLAVOKHOTOVA A A, NAUMANN T A, PRICE N P J, et al. Novel mode of action of plant defense peptides-hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases[J]. FEBS Journal, 2014, 281(20): 4754-4764. |
| 10 | SIEBERT H C, VON DER LIETH C W, KAPTEIN R, et al. Role of aromatic amino acids in carbohydrate binding of plant lectins: laser photo chemically induced dynamic nuclear polarization study of hevein domain-containing lectins[J]. Proteins, 1997, 28(2): 268-284. |
| 11 | GAO G H, LIU W, DAI J X, et al. Solution structure of PAFP-S: a new knottin-type antifungal peptide from the seeds of Phytolacca americana ,[J]. Biochemistry, 2001, 40(37): 10973-10978. |
| 12 | JONES P M, GEORGE A M. Computational analysis of the MCoTI-II plant defence knottin reveals a novel intermediate conformation that facilitates trypsin binding[J]. Scientific Reports, 2016, 6: 23174. |
| 13 | SLAVOKHOTOVA A A, ROGOZHIN E A. Defense peptides from the α-hairpinin family are components of plant innate immunity[J]. Frontiers in Plant Science, 2020, 11: 465. |
| 14 | SU T, HAN M, CAO D, et al. Molecular and biological properties of snakins: the foremost cysteine-rich plant host defense peptides[J]. Journal of Fungi, 2020, 6(4): 220. |
| 15 | HOSKIN D W, RAMAMOORTHY A. Studies on anticancer activities of antimicrobial peptides[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2008, 1778(2): 357-375. |
| 16 | GANZ T, SELSTED M E, LEHRER R I. Defensins[J]. European Journal of Haematology, 2009, 44(1): 1-8. |
| 17 | GREER A, ZENOBIA C, DARVEAU R P. Defensins and LL-37: a review of function in the gingival epithelium[J]. Periodontology 2000, 2013, 63(1): 67-79. |
| 18 | DHOPLE V, KRUKEMEYER A, RAMAMOORTHY A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2006, 1758(9): 1499-1512. |
| 19 | BATONI G, MAISETTA G, ESIN S, et al. Human beta-defensin-3: a promising antimicrobial peptide[J]. Mini Reviews in Medicinal Chemistry, 2006, 6(10): 1063-1073. |
| 20 | DELUCCA A J, BLAND J M, JACKS T J, et al. Fungicidal activity of cecropin A[J]. Antimicrobial Agents and Chemotherapy, 1997, 41(2): 481-483. |
| 21 | XIE W, QIU Q, WU H, et al. Expression of cecropin CMIV fusion protein in E. coli under T7 promoter[J]. Biochemistry and Molecular Biology International, 1996, 39(3): 487-492. |
| 22 | MENG M X, NING J F, YU J Y, et al. Antitumor activity of recombinant antimicrobial peptide penaeidin-2 against kidney cancer cells[J]. Journal of Huazhong University of Science and Technology [Medical Sciences], 2014, 34(4): 529-534. |
| 23 | YANG Y S, PONCET J, GARNIER J, et al. Solution structure of the recombinant penaeidin-3, a shrimp antimicrobial peptide[J]. Journal of Biological Chemistry, 2003, 278(38): 36859-36867. |
| 24 | PADHI A, VERGHESE B. Molecular diversity and evolution of myticin-C antimicrobial peptide variants in the Mediterranean mussel, Mytilus galloprovincialis [J]. Peptides, 2008, 29(7): 1094-1101. |
| 25 | MITTA G, HUBERT F, NOËL T, et al. Myticin, a novel cysteine-rich antimicrobial peptide isolated from haemocytes and plasma of the mussel Mytilus galloprovincialis [J]. European Journal of Biochemistry, 1999, 265(1): 71-78. |
| 26 | SABZEVARI R, ROUSHANDEH A M, MEHDIPOUR A, et al. SA/G hydrogel containing hCAP-18/LL-37-engineered WJ-MSCs-derived conditioned medium promoted wound healing in rat model of excision injury[J]. Life Sciences, 2020, 261: 118381. |
| 27 | CHAKRABORTY K, GHOSH S, KOLEY H, et al. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells[J]. Cellular Microbiology, 2008, 10(12): 2520-2537. |
| 28 | NELL M J, TJABRINGA G S, VONK M J, et al. Bacterial products increase expression of the human cathelicidin hCAP-18/LL-37 in cultured human sinus epithelial cells[J]. FEMS Immunology and Medical Microbiology, 2004, 42(2): 225-231. |
| 29 | FAHY R J, WEWERS M D. Pulmonary defense and the human cathelicidin hCAP-18/LL-37[J]. Immunologic Research, 2005, 31(2): 75-89. |
| 30 | BONANZINGA T, FERRARI M C, TANZI G, et al. The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: a literature review[J]. EFORT Open Reviews, 2019, 4(1): 10-13. |
| 31 | WYATT M C, BESWICK A D, KUNUTSOR S K, et al. The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis[J]. The Journal of Bone and Joint Surgery American Volume, 2016, 98(12): 992-1000. |
| 32 | HE W, WEI D X, ZHANG J, et al. Novel bone repairing scaffold consisting of bone morphogenetic protein-2 and human beta defensin-3[J]. Journal of Biological Engineering, 2021, 15(1): 5. |
| 33 | NIGRO E, COLAVITA I, SARNATARO D, et al. An ancestral host defence peptide within human β-defensin 3 recapitulates the antibacterial and antiviral activity of the full-length molecule[J]. Scientific Reports, 2016, 5: 18450. |
| 34 | LIU H W, WEI D X, DENG J Z, et al. Combined antibacterial and osteogenic in situ effects of a bifunctional titanium alloy with nanoscale hydroxyapatite coating[J]. Artificial Cells, Nanomedicine, and Biotechnology, 2018, 46(sup3): S460-S470. |
| 35 | HALE J D, HANCOCK R E. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria[J]. Expert Review of Anti-Infective Therapy, 2007, 5(6): 951-959. |
| 36 | POWERS J P S, HANCOCK R E W. The relationship between peptide structure and antibacterial activity[J]. Peptides, 2003, 24(11): 1681-1691. |
| 37 | SAINT N, MARRI L, MARCHINI D, et al. The antibacterial peptide ceratotoxin a displays alamethicin-like behavior in lipid bilayers[J]. Peptides, 2003, 24(11): 1779-1784. |
| 38 | MURZYN K, PASENKIEWICZ-GIERULA M. Construction of a toroidal model for the magainin pore[J]. Journal of Molecular Modeling, 2003, 9(4): 217-224. |
| 39 | SHAI Y, OREN Z. From "carpet" mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides[J]. Peptides, 2001, 22(10): 1629-1641. |
| 40 | DEAN R E, O'BRIEN L M, THWAITE J E, et al. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes[J]. Peptides, 2010, 31(11): 1966-1972. |
| 41 | MALINA A, SHAI Y. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide[J]. The Biochemical Journal, 2005, 390(Pt 3): 695-702. |
| 42 | REN L, HEN L, WEN H, et al. An anionic antimicrobial peptide from toad bombina maxima[J]. Biochemical and Biophysical Research Communications, 2002, 295(4): 796-799. |
| 43 | DENNISON S R, HOWE J, MORTON L H G, et al. Interactions of an anionic antimicrobial peptide with Staphylococcus aureus membranes[J]. Biochemical and Biophysical Research Communications, 2006, 347(4): 1006-1010. |
| 44 | LI S M, HAO L L, BAO W G, et al. A novel short anionic antibacterial peptide isolated from the skin of Xenopus laevis with broad antibacterial activity and inhibitory activity against breast cancer cell[J]. Archives of Microbiology, 2016, 198(5): 473-482. |
| 45 | DENNISON S R, MORTON L H, HARRIS F, et al. Low pH enhances the action of maximin H5 against Staphylococcus aureus and helps mediate lysylated phosphatidylglycerol-induced resistance[J]. Biochemistry, 2016, 55(27): 3735-3751. |
| 46 | KRAGOL G, LOVAS S, VARADI G, et al. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding[J]. Biochemistry, 2001, 40(10): 3016-3026. |
| 47 | MANDAL S M, KHAN J, MAHATA D, et al. A self-assembled clavanin A-coated amniotic membrane scaffold for the prevention of biofilm formation by ocular surface fungal pathogens[J]. Biofouling, 2017, 33(10): 881-891. |
| 48 | UYTERHOEVEN E T, BUTLER C H, KO D, et al. Investigating the nucleic acid interactions and antimicrobial mechanism of buforin II[J]. FEBS Letters, 2008, 582(12): 1715-1718. |
| 49 | LUPETTI A, DISSEL J T, BROUWER C P J M, et al. Human antimicrobial peptides' antifungal activity against Aspergillus fumigatus [J]. European Journal of Clinical Microbiology & Infectious Diseases, 2008, 27(11): 1125-1129. |
| 50 | VAN EIJK M, BOEREFIJN S, CEN L D, et al. Cathelicidin-inspired antimicrobial peptides as novel antifungal compounds[J]. Medical Mycology, 2020, 58(8): 1073-1084. |
| 51 | HOWELL M D, WOLLENBERG A, GALLO R L, et al. Cathelicidin deficiency predisposes to eczema herpeticum[J]. Journal of Allergy and Clinical Immunology, 2006, 117(4): 836-841. |
| 52 | BERGMAN P, WALTER-JALLOW L, BROLIDEN K, et al. The antimicrobial peptide LL-37 inhibits HIV-1 replication[J]. Current HIV Research, 2007, 5(4): 410-415. |
| 53 | HOWELL M D, JONES J F, KISICH K O, et al. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum[J]. Journal of Immunology, 2004, 172(3): 1763-1767. |
| 54 | LIU X W, WEI D X, ZHONG J, et al. Electrospun nanofibrous P(DLLA-CL) balloons as calcium phosphate cement filled containers for bone repair: in vitro and in vivo studies[J]. ACS Applied Materials & Interfaces, 2015, 7(33): 18540-18552. |
| 55 | PITALE D M, KAUR G, BAGHEL M, et al. Halictine-2 antimicrobial peptide shows promising anti-parasitic activity against Leishmania spp[J]. Experimental Parasitology, 2020, 218: 107987. |
| 56 | FANG Y Q, HE X Q, ZHANG P C, et al. In vitro and in vivo antimalarial activity of LZ1, a peptide derived from snake cathelicidin[J]. Toxins, 2019, 11(7): 379. |
| 57 | RAJA Z, ANDRÉ S, ABBASSI F, et al. Insight into the mechanism of action of temporin-SHa, a new broad-spectrum antiparasitic and antibacterial agent[J]. PLoS One, 2017, 12(3): e0174024. |
| 58 | ZHENG L B, QIU J Y, LIU H H, et al. Molecular characterization and antiparasitic activity analysis of a novel piscidin 5-like type 4 from Larimichthys crocea[J]. Molecular Immunology, 2021, 129: 12-20. |
| 59 | GRABNER A N, ALFONSO J, KAYANO A M, et al. BmajPLA2-II, a basic Lys49-phospholipase A2 homologue from Bothrops marajoensis snake venom with parasiticidal potential[J]. International Journal of Biological Macromolecules, 2017, 102: 571-581. |
| 60 | VAEZI Z, BORTOLOTTI A, LUCA V, et al. Aggregation determines the selectivity of membrane-active anticancer and antimicrobial peptides: the case of killerFLIP[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2020, 1862(2): 183107. |
| 61 | FLORES-ALVAREZ L J, GUZMÁN-RODRÍGUEZ J J, LÓPEZ-GÓMEZ R, et al. PaDef defensin from avocado (Persea americana var. drymifolia) is cytotoxic to K562 chronic myeloid leukemia cells through extrinsic apoptosis[J]. The International Journal of Biochemistry & Cell Biology, 2018, 99: 10-18. |
| 62 | QIN X C, ZHU G F, HUANG L X, et al. LL-37 and its analog FF/CAP18 attenuate neutrophil migration in sepsis-induced acute lung injury[J]. Journal of Cellular Biochemistry, 2019, 120(4): 4863-4871. |
| 63 | ZHANG H D, HAN D J, LÜ T T, et al. Novel peptide myristoly-CM4 induces selective cytotoxicity in leukemia K562/MDR and Jurkat cells by necrosis and/or apoptosis pathway[J]. Drug Design, Development and Therapy, 2019, 13: 2153-2167. |
| 64 | JUNG Y, KONG B, MOON S, et al. Envelope-deforming antiviral peptide derived from influenza virus M2 protein[J]. Biochemical and Biophysical Research Communications, 2019, 517(3): 507-512. |
| 65 | WU Y M, CHEN K, WU X, et al. Superfast and water-insensitive polymerization on α-amino acid N-carboxyanhydrides to prepare polypeptides using tetraalkylammonium carboxylate as the initiator[J]. Angewandte Chemie International Edition, 2021, 60(50): 26063-26071. |
| 66 | ZHOU M, ZOU J C, LIU L Q, et al. Synthesis of poly-α/β-peptides with tunable sequence via the copolymerization on N-carboxyanhydride and N-thiocarboxyanhydride[J]. iScience, 2021, 24(10): 103124. |
| 67 | ZHOU M, XIAO X M, CONG Z H, et al. Water-insensitive synthesis of poly-β-peptides with defined architecture[J]. Angewandte Chemie International Edition, 2020, 59(18): 7240-7244. |
| 68 | MIKUT R. Computer-based analysis, visualization, and interpretation of antimicrobial peptide activities[J]. Methods in Molecular Biology, 2010, 618: 287-299. |
| 69 | BOMAN H G, AGERBERTH B, BOMAN A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine[J]. Infection and Immunity, 1993, 61(7): 2978-2984. |
| 70 | SILVESTRO L, WEISER J N, AXELSEN P H. Antibacterial and antimembrane activities of cecropin A in Escherichia coli [J]. Antimicrobial Agents and Chemotherapy, 2000, 44(3): 602-607. |
| 71 | WANG L, WU H, DOU F, et al. High-level expression of cecropin CMIV in E. coli from a fusion construct containing the human tumor necrosis factor[J]. Biochemistry and Molecular Biology International, 1997, 41(5): 1051-1056. |
| 72 | XU X X, JIN F L, YU X Q, et al. Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli [J]. Protein Expression and Purification, 2007, 53(2): 293-301. |
| 73 | ZHOU Q F, LUO X G, YE L, et al. High-level production of a novel antimicrobial peptide perinerin in Escherichia coli by fusion expression[J]. Current Microbiology, 2007, 54(5): 366-370. |
| 74 | CAO W, ZHOU Y X, MA Y S, et al. Expression and purification of antimicrobial peptide adenoregulin with C-amidated terminus in Escherichia coli [J]. Protein Expression and Purification, 2005, 40(2): 404-410. |
| 75 | KIM D S, KIM S W, SONG J M, et al. A new prokaryotic expression vector for the expression of antimicrobial peptide abaecin using SUMO fusion tag[J]. BMC Biotechnology, 2019, 19(1): 13. |
| 76 | COSTA A DA, PEREIRA A M, GOMES A C, et al. Production of bioactive hepcidin by recombinant DNA tagging with an elastin-like recombinamer[J]. New Biotechnology, 2018, 46: 45-53. |
| 77 | GONG G L, WEI Y, WANG Z Z. Functional expression, purification, and antimicrobial activity of a novel antimicrobial peptide MLH in Escherichia coli [J]. Preparative Biochemistry & Biotechnology, 2018, 48(1): 57-63. |
| 78 | WEI Q D, KIM Y S, SEO J H, et al. Facilitation of expression and purification of an antimicrobial peptide by fusion with baculoviral polyhedrin in Escherichia coli [J]. Applied and Environmental Microbiology, 2005, 71(9): 5038-5043. |
| 79 | JING X L, LUO X G, TIAN W J, et al. High-level expression of the antimicrobial peptide plectasin in Escherichia coli [J]. Current Microbiology, 2010, 61(3): 197-202. |
| 80 | KIM H K, CHUN D S, KIM J S, et al. Expression of the cationic antimicrobial peptide lactoferricin fused with the anionic peptide in Escherichia coli [J]. Applied Microbiology and Biotechnology, 2006, 72(2): 330-338. |
| 81 | LEE J H, MINN I, PARK C B, et al. Acidic peptide-mediated expression of the antimicrobial peptide buforin II as tandem repeats in Escherichia coli [J]. Protein Expression and Purification, 1998, 12(1): 53-60. |
| 82 | WANG Q, ZHU F F, XIN Y Q, et al. Expression and purification of antimicrobial peptide buforin IIb in Escherichia coli [J]. Biotechnology Letters, 2011, 33(11): 2121-2126. |
| 83 | CHEN X, ZHU F M, CAO Y H, et al. Novel expression vector for secretion of cecropin AD in Bacillus subtilis with enhanced antimicrobial activity[J]. Antimicrobial Agents and Chemotherapy, 2009, 53(9): 3683-3689. |
| 84 | LUAN C, ZHANG H W, SONG D G, et al. Expressing antimicrobial peptide cathelicidin-BF in Bacillus subtilis using SUMO technology[J]. Applied Microbiology and Biotechnology, 2014, 98(8): 3651-3658. |
| 85 | ZHANG L C, LI G Q, ZHAN N, et al. Expression of a Pseudomonas aeruginosa-targeted antimicrobial peptide T9W in Bacillus subtilis using a maltose-inducible vector[J]. Process Biochemistry, 2019, 81: 22-27. |
| 86 | LEE B C, TSAI J C, LIN C Y, et al. Using Bacillus subtilis as a host cell to express an antimicrobial peptide from the marine chordate ciona intestinalis[J]. Marine Drugs, 2021, 19(2): 111. |
| 87 | XIA L J, LIU Z Y, MA J, et al. Expression, purification and characterization of cecropin antibacterial peptide from Bombyx mori in Saccharomyces cerevisiae [J]. Protein Expression and Purification, 2013, 90(1): 47-54. |
| 88 | JIANG R J, ZHANG P F, WU X L, et al. Expression of antimicrobial peptide cecropin P1 in Saccharomyces cerevisiae and its antibacterial and antiviral activity in vitro [J]. Electronic Journal of Biotechnology, 2021, 50: 16-22. |
| 89 | AHMAD M, HIRZ M, PICHLER H, et al. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production[J]. Applied Microbiology and Biotechnology, 2014, 98(12): 5301-5317. |
| 90 | MENG D M, ZHAO J F, LING X, et al. Recombinant expression, purification and antimicrobial activity of a novel antimicrobial peptide PaDef in Pichia pastoris [J]. Protein Expression and Purification, 2017, 130: 90-99. |
| 91 | MORIDI K, HEMMATY M, AKBARI EIDGAHI M R, et al. Construction, cloning, and expression of Melittin antimicrobial peptide using Pichia pastoris expression system[J]. Gene Reports, 2020, 21: 100900. |
| 92 | XING L W, TIAN S X, GAO W, et al. Recombinant expression and biological characterization of the antimicrobial peptide fowlicidin-2 in Pichia pastoris [J]. Experimental and Therapeutic Medicine, 2016, 12(4): 2324-2330. |
| 93 | WANG L, LAI C E, WU Q F, et al. Production and characterization of a novel antimicrobial peptide HKABF by Pichia pastoris [J]. Process Biochemistry, 2008, 43(10): 1124-1131. |
| 94 | MENG D M, DAI H X, GAO X F, et al. Expression, purification and initial characterization of a novel recombinant antimicrobial peptide Mytichitin-A in Pichia pastoris [J]. Protein Expression and Purification, 2016, 127: 35-43. |
| 95 | ZHANG J, ZHANG S Q, WU X, et al. Expression and characterization of antimicrobial peptide ABP-CM4 in methylotrophic yeast Pichia pastoris [J]. Process Biochemistry, 2006, 41(2): 251-256. |
| 96 | DONG B, CHENG R Q, LIU Q Y, et al. Multimer of the antimicrobial peptide Mytichitin - A expressed in Chlamydomonas reinhardtii exerts a broader antibacterial spectrum and increased potency[J]. Journal of Bioscience and Bioengineering, 2018, 125(2): 175-179. |
| 97 | LI A G, HUANG R H, WANG C G, et al. Expression of anti-lipopolysaccharide factor isoform 3 in Chlamydomonas reinhardtii showing high antimicrobial activity[J]. Marine Drugs, 2021, 19(5): 239. |
| 98 | XUE B, DONG C M, HU H H, et al. Chlamydomonas reinhardtii-expressed multimer of ToAMP4 inhibits the growth of bacteria of both Gram-positive and Gram-negative[J]. Process Biochemistry, 2020, 91: 311-318. |
| 99 | HADIATULLAH H, WANG H, LIU Y X, et al. Chlamydomonas reinhardtii-derived multimer Mytichitin-CB possesses potent antibacterial properties[J]. Process Biochemistry, 2020, 96: 21-29. |
| 100 | CHAHARDOLI M, FAZELI A, NIAZI A, et al. Recombinant expression of LFchimera antimicrobial peptide in a plant-based expression system and its antimicrobial activity against clinical and phytopathogenic bacteria[J]. Biotechnology & Biotechnological Equipment, 2018, 32(3): 714-723. |
| 101 | LI C, BLENCKE H M, PAULSEN V, et al. Powerful workhorses for antimicrobial peptide expression and characterization[J]. Bioengineered Bugs, 2010, 1(3): 217-220. |
| 102 | SINHA R, SHUKLA P. Antimicrobial peptides: recent insights on biotechnological interventions and future perspectives[J]. Protein and Peptide Letters, 2019, 26(2): 79-87. |
| 103 | ISHIDA H, NGUYEN L T, GOPAL R, et al. Overexpression of antimicrobial, anticancer, and transmembrane peptides in Escherichia coli through a calmodulin-peptide fusion system[J]. Journal of the American Chemical Society, 2016, 138(35): 11318-11326. |
| 104 | SCHÜTZ M, SCHÖPPE J, SEDLÁK E, et al. Directed evolution of G protein-coupled receptors in yeast for higher functional production in eukaryotic expression hosts[J]. Scientific Reports, 2016, 6: 21508. |
| 105 | KARBALAEI M, REZAEE S A, FARSIANI H. Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins[J]. Journal of Cellular Physiology, 2020, 235(9): 5867-5881. |
| 106 | BI X N, WANG C, DONG W B, et al. Antimicrobial properties and interaction of two Trp-substituted cationic antimicrobial peptides with a lipid bilayer[J]. The Journal of Antibiotics, 2014, 67(5): 361-368. |
| 107 | TRIPATHI A K, KUMARI T, TANDON A, et al. Selective phenylalanine to proline substitution for improved antimicrobial and anticancer activities of peptides designed on phenylalanine heptad repeat[J]. Acta Biomaterialia, 2017, 57: 170-186. |
| 108 | SUN Y, DONG W B, SUN L, et al. Insights into the membrane interaction mechanism and antibacterial properties of chensinin-1b[J]. Biomaterials, 2015, 37: 299-311. |
| 109 | DONG W B, MAO X M, GUAN Y, et al. Antimicrobial and anti-inflammatory activities of three chensinin-1 peptides containing mutation of glycine and histidine residues[J]. Scientific Reports, 2017, 7: 40228. |
| 110 | CARDOSO M H, OROZCO R Q, REZENDE S B, et al. Computer-aided design of antimicrobial peptides: are we generating effective drug candidates? [J]. Frontiers in Microbiology, 2020, 10: 3097. |
| 111 | LEE E Y, FULAN B M, WONG G C L, et al. Mapping membrane activity in undiscovered peptide sequence space using machine learning[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(48): 13588-13593. |
| 112 | DAS P, SERCU T, WADHAWAN K, et al. Accelerated antimicrobial discovery via deep generative models and molecular dynamics simulations[J]. Nature Biomedical Engineering, 2021, 5(6): 613-623. |
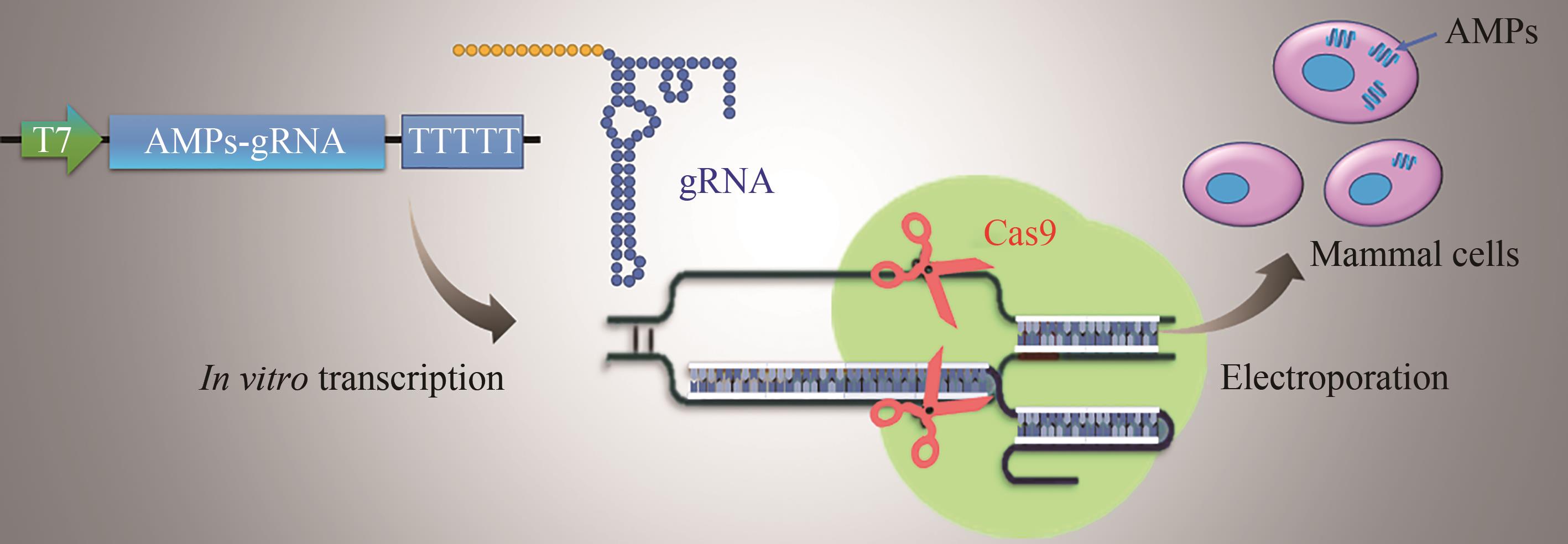
| 113 | PARK J W, YU J H, KIM S-W, et al. Enhancement of antimicrobial peptide genes expression in cactus mutated Bombyx mori cells by CRISPR/Cas9[J]. International Journal of Industrial Entomology, 2018, 37(1): 21-28. |
| 114 | MOOKHERJEE N, ANDERSON M A, HAAGSMAN H P, et al. Antimicrobial host defence peptides: functions and clinical potential[J]. Nature Reviews Drug Discovery, 2020, 19(5): 311-332. |
| 115 | MCCARTHY M W, KEYLOUN K R, GILLARD P, et al. Dalbavancin reduces hospital stay and improves productivity for patients with acute bacterial skin and skin structure infections: the ENHANCE trial[J]. Infectious Diseases and Therapy, 2020, 9(1): 53-67. |
| 116 | BÜYÜKKIRAZ M E, KESMEN Z. Antimicrobial peptides (AMPs): a promising class of antimicrobial compounds[J]. Journal of Applied Microbiology, 2022, 132(3): 1573-1596. |
| 117 | BAREFOOT S F, KLAENHAMMER T R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus [J]. Applied and Environmental Microbiology, 1983, 45(6): 1808-1815. |
| 118 | CHEIGH C I, PYUN Y R. Nisin biosynthesis and its properties[J]. Biotechnology Letters, 2005, 27(21): 1641-1648. |
| 119 | DE ARAUZ L J, JOZALA A F, MAZZOLA P G, et al. Nisin biotechnological production and application: a review[J]. Trends in Food Science & Technology, 2009, 20(3/4): 146-154. |
| 120 | GALVÁN MÁRQUEZ I J, MCKAY B, WONG A, et al. Mode of action of nisin on Escherichia coli [J]. Canadian Journal of Microbiology, 2020, 66(2): 161-168. |
| 121 | FIELD D, BAGHOU I, REA M C, et al. Nisin in combination with cinnamaldehyde and EDTA to control growth of Escherichia coli strains of swine origin[J]. Antibiotics, 2017, 6(4): 35 |
| 122 | TONG Z C, ZHANG Y J, LING J Q, et al. An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis [J]. PLoS One, 2014, 9(2): e89209. |
| 123 | CASCIARO B, LOFFREDO M R, LUCA V, et al. Esculentin-1a derived antipseudomonal peptides: limited induction of resistance and synergy with aztreonam[J]. Protein and Peptide Letters, 2018, 25(12): 1155-1162. |
| 124 | ARCIOLA C R, CAMPOCCIA D, MONTANARO L. Implant infections: adhesion, biofilm formation and immune evasion[J]. Nature Reviews Microbiology, 2018, 16(7): 397-409. |
| 125 | CRÉMET L, BROQUET A, JACQUELINE C, et al. Innate immune evasion of Escherichia coli clinical strains from orthopedic implant infections[J]. European Journal of Clinical Microbiology & Infectious Diseases, 2016, 35(6): 993-999. |
| 126 | LEVY O, SISSON R B, FRYER H E, et al. Neutrophil defense in patients undergoing bone marrow transplantation: bactericidal/permeability-increasing protein (BPI) and defensins in graft-derived neutrophils[J]. Transplantation, 2002, 73(9): 1522-1526. |
| 127 | HOOVER D M, BOULEGUE C, YANG D, et al. The structure of human macrophage inflammatory protein-3alpha/CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins[J]. The Journal of Biological Chemistry, 2002, 277(40): 37647-37654. |
| 128 | AGERBERTH B, CHARO J, WERR J, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations[J]. Blood, 2000, 96(9): 3086-3093. |
| 129 | BLOMQVIST M, BERGQUIST J, WESTMAN A, et al. Identification of defensins in human lymphocyte nuclei[J]. European Journal of Biochemistry, 1999, 263(2): 312-318. |
| 130 | SRIVASTAVA M D, SRIVASTAVA B I S. Expression of mRNA and proteins for toll-like receptors, associated molecules, defensins and LL-37 by SRIK-NKL, a CD8+ NK/T cell line[J]. Leukemia Research, 2005, 29(7): 813-820. |
| 131 | HOLLY M K, DIAZ K, SMITH J G. Defensins in viral infection and pathogenesis[J]. Annual Review of Virology, 2017, 4(1): 369-391. |
| 132 | NIYONSABA F, USHIO H, NAKANO N, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines[J]. The Journal of Investigative Dermatology, 2007, 127(3): 594-604. |
| 133 | MI B B, LIU J, LIU Y, et al. The designer antimicrobial peptide A-hBD-2 facilitates skin wound healing by stimulating keratinocyte migration and proliferation[J]. Cellular Physiology and Biochemistry, 2018, 51(2): 647-663. |
| 134 | BARLOW P G, SVOBODA P, MACKELLAR A, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37[J]. PLoS One, 2011, 6(10): e25333. |
| 135 | TRIPATHI S, TECLE T, VERMA A, et al. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins[J]. The Journal of General Virology, 2013, 94(Pt 1): 40-49. |
| 136 | TODOROV S D, WACHSMAN M B, KNOETZE H, et al. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans[J]. International Journal of Antimicrobial Agents, 2005, 25(6): 508-513. |
| 137 | LIANG X L, ZHANG X J, LIAN K Q, et al. Antiviral effects of bovine antimicrobial peptide against TGEV in vivo and in vitro [J]. Journal of Veterinary Science, 2020, 21(5): e80. |
| 138 | MARCOCCI M E, AMATORE D, VILLA S, et al. The amphibian antimicrobial peptide temporin B inhibits in vitro herpes simplex virus 1 infection[J]. Antimicrobial Agents and Chemotherapy, 2018, 62(5): e02367-e02317. |
| 139 | BHATTACHARYA R, GUPTA A M, MITRA S, et al. A natural food preservative peptide nisin can interact with the SARS-CoV-2 spike protein receptor human ACE2[J]. Virology, 2021, 552: 107-111. |
| 140 | KURPE S R, GRISHIN S Y, SURIN A K, et al. Antimicrobial and amyloidogenic activity of peptides. can antimicrobial peptides be used against SARS-CoV-2? [J]. International Journal of Molecular Sciences, 2020, 21(24): 9552. |
| 141 | PENG X L, CHENG J S, GONG H L, et al. Advances in the design and development of SARS-CoV-2 vaccines[J]. Military Medical Research, 2021, 8(1): 67. |
| 142 | JIN G, WEINBERG A. Human antimicrobial peptides and cancer[J]. Seminars in Cell & Developmental Biology, 2019, 88: 156-162. |
| 143 | YANG Y Q, ZHANG H D, WANYAN Y K, et al. Effect of hydrophobicity on the anticancer activity of fatty-acyl-conjugated CM4 in breast cancer cells[J]. ACS Omega, 2020, 5(34): 21513-21523. |
| 144 | MAIJAROEN S, JANGPROMMA N, DADUANG J, et al. KT2 and RT2 modified antimicrobial peptides derived from Crocodylus siamensis Leucrocin I show activity against human colon cancer HCT-116 cells[J]. Environmental Toxicology and Pharmacology, 2018, 62: 164-176. |
| 145 | WANG D M, JIAO X, PLOTNIKOFF N P, et al. Killing effect of methionine enkephalin on melanoma in vivo and in vitro [J]. Oncology Reports, 2017, 38(4): 2132-2140. |
| 146 | WEI D X, DAO J W, CHEN G Q. A micro-ark for cells: highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration[J]. Advanced Materials, 2018, 30(31): e1802273. |
| 147 | RAHMAN M, PENG X L, ZHAO X H, et al. 3D bioactive cell-free-scaffolds for in-vitro/in-vivo capture and directed osteoinduction of stem cells for bone tissue regeneration[J]. Bioactive Materials, 2021, 6(11): 4083-4095. |
| 148 | WEI D X, QIAO R R, DAO J W, et al. Soybean lecithin-mediated nanoporous PLGA microspheres with highly entrapped and controlled released BMP-2 as a stem cell platform[J]. Small, 2018, 14(22): e1800063. |
| 149 | FAN L, HE Z J, PENG X L, et al. Injectable, intrinsically antibacterial conductive hydrogels with self-healing and pH stimulus responsiveness for epidermal sensors and wound healing[J]. ACS Applied Materials & Interfaces, 2021, 13(45): 53541-53552. |
| 150 | FAN L, XIE J L, ZHENG Y P, et al. Antibacterial, self-adhesive, recyclable, and tough conductive composite hydrogels for ultrasensitive strain sensing[J]. ACS Applied Materials & Interfaces, 2020, 12(19): 22225-22236. |
| 151 | WEI D X, DAO J W, LIU H W, et al. Suspended polyhydroxyalkanoate microspheres as 3D carriers for mammalian cell growth[J]. Artificial Cells, Nanomedicine, and Biotechnology, 2018, 46(sup2): 473-483. |
| 152 | XU N, PENG X L, LI H R, et al. Marine-derived collagen as biomaterials for human health[J]. Frontiers in Nutrition, 2021, 8: 702108. |
| 153 | CHEN L, SHAO L P, WANG F P, et al. Enhancement in sustained release of antimicrobial peptide and BMP-2 from degradable three dimensional-printed PLGA scaffold for bone regeneration[J]. RSC Advances, 2019, 9(19): 10494-10507. |
| 154 | LIU Z P, YUAN X, LIU M, et al. Antimicrobial peptide combined with BMP2-modified mesenchymal stem cells promotes calvarial repair in an osteolytic model[J]. Molecular Therapy, 2018, 26(1): 199-207. |
| 155 | MARTIN-GÓMEZ H, OLIVER-CERVELLÓ L, BUXADERA-PALOMERO J, et al. Chemically diverse multifunctional peptide platforms with antimicrobial and cell adhesive properties[J]. Chembiochem: a European Journal of Chemical Biology, 2021, 22(5): 839-844. |
| 156 | LIN Z F, WU T T, WANG W S, et al. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound[J]. International Journal of Biological Macromolecules, 2019, 140: 330-342. |
| 157 | ITO A, TAKAHASHI T, KAWABE Y, et al. Human beta defensin-3 engineered keratinocyte sheets constructed by a magnetic force-based tissue engineering technique[J]. Journal of Bioscience and Bioengineering, 2009, 108(3): 244-247. |
| 158 | KITTAKA M, SHIBA H, KAJIYA M, et al. The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect[J]. Peptides, 2013, 46: 136-142. |
| 159 | SCHWEIZER F. Cationic amphiphilic peptides with cancer-selective toxicity[J]. European Journal of Pharmacology, 2009, 625(1/2/3): 190-194. |
| 160 | HANCOCK R E W, SAHL H G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies[J]. Nature Biotechnology, 2006, 24(12): 1551-1557. |
| 161 | CHEN R, YU J M, GONG H L, et al. An easy long-acting BMP7 release system based on biopolymer nanoparticles for inducing osteogenic differentiation of adipose mesenchymal stem cells[J]. Journal of Tissue Engineering and Regenerative Medicine, 2020, 14(7): 964-972. |
| 162 | HU J, WANG M, XIAO X Y, et al. A novel long-acting azathioprine polyhydroxyalkanoate nanoparticle enhances treatment efficacy for systemic lupus erythematosus with reduced side effects[J]. Nanoscale, 2020, 12(19): 10799-10808. |
| 163 | ZHAO X H, PENG X L, GONG H L, et al. Osteogenic differentiation system based on biopolymer nanoparticles for stem cells in simulated microgravity[J]. Biomedical Materials, 2021, 16(4): 044102. |
| 164 | RADAIC A, DE JESUS M B, KAPILA Y L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: the state of the art toward improved bacteriocins[J]. Journal of Controlled Release, 2020, 321: 100-118. |
| 165 | LAM S J, O'BRIEN-SIMPSON N M, PANTARAT N, et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers[J]. Nature Microbiology, 2016, 1(11): 16162. |
| 166 | NIEMIROWICZ K, PROKOP I, WILCZEWSKA A Z, et al. Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells[J]. International Journal of Nanomedicine, 2015, 10: 3843-3853. |
| 167 | CARRATALÁ J V, SERNA N, VILLAVERDE A, et al. Nanostructured antimicrobial peptides: the last push towards clinics[J]. Biotechnology Advances, 2020, 44: 107603. |
| 168 | FALCIANI C, ZEVOLINI F, BRUNETTI J, et al. Antimicrobial peptide-loaded nanoparticles as inhalation therapy for Pseudomonas aeruginosa infections[J]. International Journal of Nanomedicine, 2020, 15: 1117-1128. |
| 169 | NYSTRÖM L, STRÖMSTEDT A A, SCHMIDTCHEN A, et al. Peptide-loaded microgels as antimicrobial and anti-inflammatory surface coatings[J]. Biomacromolecules, 2018, 19(8): 3456-3466. |
| 170 | NIEMIROWICZ K, DURNAŚ B, TOKAJUK G, et al. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13[J]. Scientific Reports, 2017, 7: 4610. |
| 171 | FINDLAY F, POHL J, SVOBODA P, et al. Carbon nanoparticles inhibit the antimicrobial activities of the human cathelicidin LL-37 through structural alteration[J]. Journal of Immunology, 2017, 199(7): 2483-2490. |
| 172 | WU J, YANG J, WANG X F, et al. A frog cathelicidin peptide effectively promotes cutaneous wound healing in mice[J]. The Biochemical Journal, 2018, 475(17): 2785-2799. |
| 173 | THAPA R K, DIEP D B, TØNNESEN H H. Topical antimicrobial peptide formulations for wound healing: current developments and future prospects[J]. Acta Biomaterialia, 2020, 103: 52-67. |
| 174 | YOO J H, HO S, TRAN D H Y, et al. Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis[J]. Cellular and Molecular Gastroenterology and Hepatology, 2015, 1(1): 55-74.e1. |
| 175 | RAMOS R, SILVA J P, RODRIGUES A C, et al. Wound healing activity of the human antimicrobial peptide LL37[J]. Peptides, 2011, 32(7): 1469-1476. |
| 176 | GAUGLITZ G G, BUREIK D, ZWICKER S, et al. The antimicrobial peptides psoriasin (S100A7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts[J]. Skin Pharmacology and Physiology, 2015, 28(3): 115-123. |
| 177 | KANAZAWA K, OKUMURA K, OGAWA H, et al. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-κB pathways[J]. Immunologic Research, 2016, 64(2): 594-603. |
| 178 | ROY S, GANGULY A, HAQUE M, et al. Angiogenic host defense peptide AG-30/5C and bradykinin B2 receptor antagonist icatibant are G protein biased agonists for MRGPRX2 in mast cells[J]. Journal of Immunology, 2019, 202(4): 1229-1238. |
| 179 | CHUNG E M C, DEAN S N, PROPST C N, et al. Komodo dragon-inspired synthetic peptide DRGN-1 promotes wound-healing of a mixed-biofilm infected wound[J]. Npj Biofilms and Microbiomes, 2017, 3: 9. |
| 180 | KIM D H, HWANG J S, LEE I H, et al. The insect peptide CopA3 increases colonic epithelial cell proliferation and mucosal barrier function to prevent inflammatory responses in the gut[J]. Journal of Biological Chemistry, 2016, 291(7): 3209-3223. |
| 181 | LUONG H X, THANH T T, TRAN T H. Antimicrobial peptides-advances in development of therapeutic applications[J]. Life Sciences, 2020, 260: 118407. |
| 182 | SEO M D, WON H S, KIM J H, et al. Antimicrobial peptides for therapeutic applications: a review[J]. Molecules, 2012, 17(10): 12276-12286. |
| 183 | KANG H K, KIM C, SEO C H, et al. The therapeutic applications of antimicrobial peptides (AMPs): a patent review[J]. Journal of Microbiology, 2017, 55(1): 1-12. |
| 184 | ZHANG G L, SUNKARA L T. Avian antimicrobial host defense peptides: from biology to therapeutic applications[J]. Pharmaceuticals, 2014, 7(3): 220-247. |
| 185 | SPALLER B L, TRIEU J M, ALMEIDA P F. Hemolytic activity of membrane-active peptides correlates with the thermodynamics of binding to 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine bilayers[J]. The Journal of Membrane Biology, 2013, 246(3): 257-262. |
| 186 | WEI H Q, XIE Z P, TAN X C, et al. Temporin-like peptides show antimicrobial and anti-biofilm activities against Streptococcus mutans with reduced hemolysis[J]. Molecules, 2020, 25(23): 5724. |
| 187 | TOROPOVA A P, TOROPOV A A, BEEG M, et al. Utilization of the Monte Carlo method to build up QSAR models for hemolysis and cytotoxicity of antimicrobial peptides[J]. Current Drug Discovery Technologies, 2017, 14(4): 229-243. |
| 188 | SON M, LEE Y R, HWANG H, et al. Disruption of interactions between hydrophobic residues on nonpolar faces is a key determinant in decreasing hemolysis and increasing antimicrobial activities of α-helical amphipathic peptides[J]. ChemMedChem, 2013, 8(10): 1638-1642. |
| 189 | PINI A, GIULIANI A, FALCIANI C, et al. Characterization of the branched antimicrobial peptide M6 by analyzing its mechanism of action and in vivo toxicity[J]. Journal of Peptide Science: an Official Publication of the European Peptide Society, 2007, 13(6): 393-399. |
| 190 | ZHANG Q H, XU Y Z, WANG Q, et al. Potential of novel antimicrobial peptide P3 from bovine erythrocytes and its analogs to disrupt bacterial membranes in vitro and display activity against drug-resistant bacteria in a mouse model[J]. Antimicrobial Agents and Chemotherapy, 2015, 59(5): 2835-2841. |
| 191 | LI Z J, XU X B, MENG L X, et al. Hp1404, a new antimicrobial peptide from the scorpion Heterometrus petersii [J]. PLoS One, 2014, 9(5): e97539. |
| 192 | EDWARDS I A, ELLIOTT A G, KAVANAGH A M, et al. Structure-activity and-toxicity relationships of the antimicrobial peptide tachyplesin-1[J]. ACS Infectious Diseases, 2017, 3(12): 917-926. |
| 193 | XU D, LIAO C B, ZHANG B, et al. Human enteric α-defensin 5 promotes shigella infection by enhancing bacterial adhesion and invasion[J]. Immunity, 2018, 48(6): 1233-1244.e6. |
| 194 | XU D, LIAO C B, XIAO J, et al. Human enteric defensin 5 promotes shigella infection of macrophages[J]. Infection and Immunity, 2019, 88(1): e00769-e00719. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | LI Shikai, ZENG Dong′ao, DU Fangzhou, ZHANG Jingzhong, YU Shuang. The construction approaches and biomaterials for vascularized organoids [J]. Synthetic Biology Journal, 2024, 5(4): 851-866. |
| [13] | HAN Yizhao, GUO Jia, SHAO Yue. Stem cell-based synthetic development: cellular components, embryonic models, and engineering approaches [J]. Synthetic Biology Journal, 2024, 5(4): 734-753. |
| [14] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [15] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||