|
||
|
Studies on hydrogenases for hydrogen production using in vitro synthetic enzymatic biosystems
Synthetic Biology Journal
2024, 5 (6):
1461-1484.
DOI: 10.12211/2096-8280.2024-052
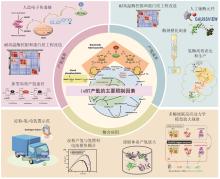
Hydrogenases are the most important enzymes in biological hydrogen production and hydrogen energy utilization. They are widely distributed, oxygen-sensitive, multiunit complexed metal enzymes. In vitro synthetic enzymatic biosystems (ivSEB) is a type of in vitro biotransformation (ivBT) technology, which is an emerging biomanufacturing powerhouse that combines microbial fermentation with enzymatic biocatalysis, allowing for novel and efficient hydrogen production, also breaking the Thauer limit and achieving a yield of hydrogen close to the theoretical value of chemistry (1 mole of glucose to produce 12 moles of hydrogen in maximum). It represents the future direction of biological hydrogen production. However, the recombinant expression of hydrogenase is the main bottleneck limiting the wide application of ivSEB for hydrogen production technology. Hydrogenases are widely distributed in all life domains, but are oxygen-sensitive and mostly consist of metalloproteins with multi-subunits, bearing [Fe] only, [NiFe] or [FeFe] dinuclear core in their catalytic center. Oxygen not only inhibits the activity of hydrogenase, but also affects the transcription of the enzyme-encoding gene and post-translational process of the enzymes. As a result, the levels of recombinant hydrogenase are usually low and the enzymatic activities are also incomparable to the native enzymes, often leading to high production costs due to the strict anaerobic purification procedures. In order to meet the requirements of industrial hydrogen production, hydrogenases must possess excellent catalytic properties, such as a high catalytic turnover number, great thermal stability, and the ability to tolerate trace amounts of oxygen. This review summarizes the studies on the structural and catalytic characterizations of hydrogenases, including their classification, oxygen resistance mechanisms, and progress in recombinant expression. Additionally, the evolution of natural electron transfer chains and the design of artificial routes, which can improve hydrogen production efficiency and reduce costs, are briefly discussed. The review also discussed the progress in the studies on the mechanisms of hydrogenases’ tolerance toward oxygen, the strategies for microbial expression of recombinant hydrogenases as well as the optimization of the artificial electron transfer chains adapted for the production of hydrogen using ivSEB, in expectations of promoting the applications of hydrogenases involved ivSEB, from renewable energy storage, anaerobic artificial respiration, to clean hydrogenation or dehydrogenation in biocatalysis. 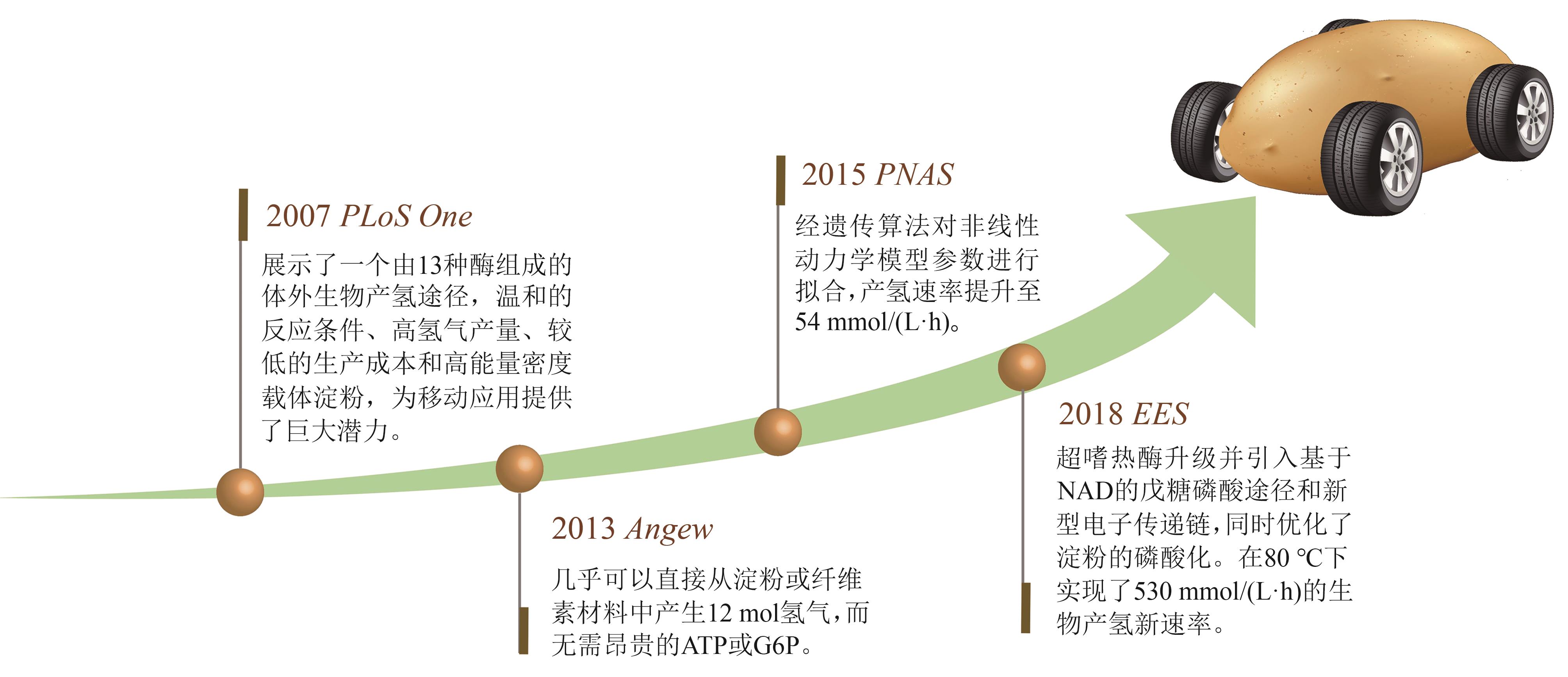
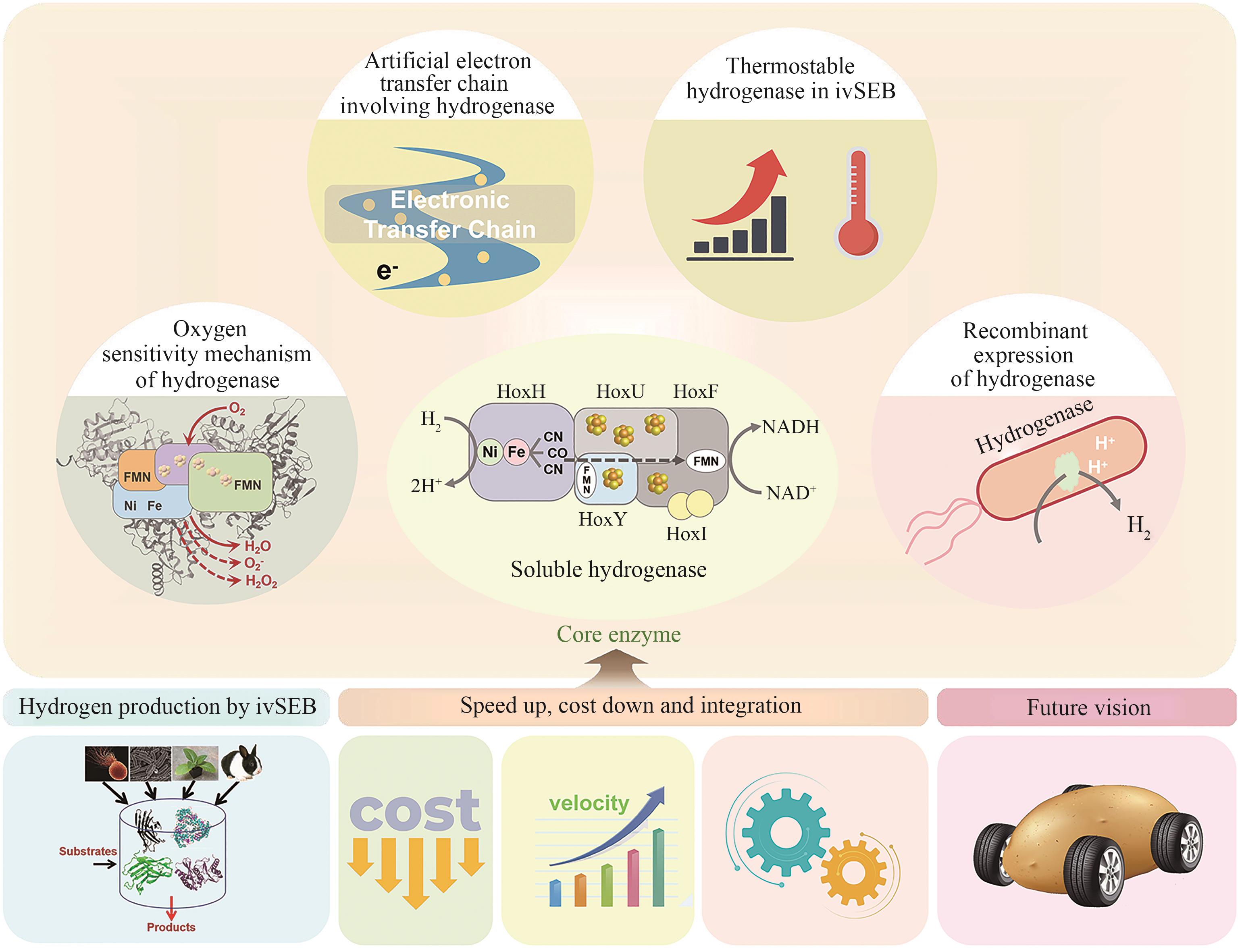
Fig. 4
Key breakthrough points of ivSEB hydrogen production
Extracts from the Article
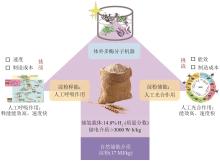
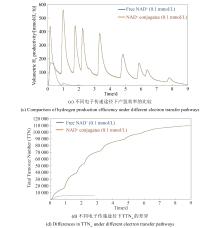
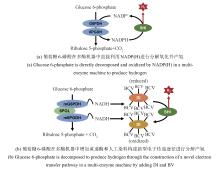
同其他工业制造技术一样,ivSEB若要实现特定产品的产业化,也必须要满足产品的经济性。在生物转化过程中,基于重量的总周转次数(weight-based total turnover number,TTNW)是经济性的一个重要影响因素,它是生产产物质量与所需要的生物催化剂的质量的比值[147]。微生物发酵的TTNw通常为0.001~20[148],例如,利用微生物可将发酵的糖底物的大约一半质量转化为乙醇,酵母细胞需要量大概占乙醇质量的1/6,因此其TTNw约为6;若酵母细胞被回收利用形成产品,则该生产过程的TTNw上升至10以上。但若是使用植物或动物或其培养的细胞进行生物制造,其TTNw值要低得多,大约为0.01,因此,利用动植物只适合生产高价值产品。而ivSEB只使用酶,针对底物进行直接的生物转化,因此其TTNw值会大幅提升,一般是50以上,甚至能达到100万。如果在ivSEB中结合酶固定化技术,其TTNw可比使用游离酶再高出两个数量级以上。例如纤维素酶水解纤维素生物质类生产乙醇,其TTNw可比使用游离酶再高出两个数量级以上;纤维素酶水解纤维素生物质类生产乙醇,其TTNw值在50~100之间,而淀粉酶水解淀粉生产乙醇,由于成熟的工业淀粉酶和葡萄糖淀粉酶活性很高,导致TTNw提高至5000。因此,利用玉米原料生产乙醇比利用木质纤维素生物质制造乙醇便宜很多。在生物制氢方面,张以恒团队设计的ivSEB途径在以淀粉为底物时,可以获得接近理论转化值的12 mol氢/mol葡萄糖,其产氢TTNw也是微生物发酵难以实现的。该多酶体途径主要包含五个子模块:①由磷酸化酶催化将多聚糖转化为葡萄糖-1-磷酸(G1P);②由磷酸葡糖异构酶催化从G1P生成葡萄糖-6-磷酸(G6P);③由两种脱氢酶所化产生NADPH和磷酸戊糖;④由磷酸戊糖途径(PPP)的八种酶催化从核糖-5-磷酸再生G6P;⑤由氢酶催化从NADPH生成氢气[20-21]。2015年,张以恒研究团队[149]继续展示了基于体外合成酶途径将植物生物质中的纤维多糖和木聚糖原料完全转化为H2和CO2的过程,同时将酶元件反应温度提升到50 ℃,并经遗传算法对非线性动力学模型的参数拟合,由全局敏感性分析确定了对反应速率和产量影响最大的酶。经模型对酶载荷优化后,H2产量增加了3倍,达到32 mmol/(L·h);再通过提高反应温度、底物和酶浓度后,产氢速率提升至54 mmol/(L·h),与初始研究相比提高了67倍。2018年,他们继续实现突破,以15种超热嗜热酶替代之前的中温酶元件,引入基于NAD的戊糖磷酸途径,设计了由黄递酶(DI),电子中介物苄基紫精(BV)和一种可溶性[NiFe]氢酶组成的新型电子传递链,并且优化了淀粉的磷酸化,因此在80 ℃下,该途径实现了530 mmol/(L·h)的生物产氢新速率[29]。在测试的9天持续产氢中,NAD的总周转次数超过10万次[29]。如图4所示,ivSEB产氢在近年来产氢速率因多尺度集成得到大幅提升,这种具有最高化学能效率和以超高反应速率为特征的糖水制氢技术有望解决与成本效益、氢气高密度储存相关的技术挑战,形成分布式氢气工业生产的技术基础。此外,利用碳材料、金属及半导体、高分子或MOF固定化氢酶有利于提高氢酶的稳定性,改善氢酶对于氧气的敏感性,增加反应体系对氢酶的负载率,从而提升产氢效率[150]。
Other Images/Table from this Article
|