|
||
|
Studies on hydrogenases for hydrogen production using in vitro synthetic enzymatic biosystems
Synthetic Biology Journal
2024, 5 (6):
1461-1484.
DOI: 10.12211/2096-8280.2024-052
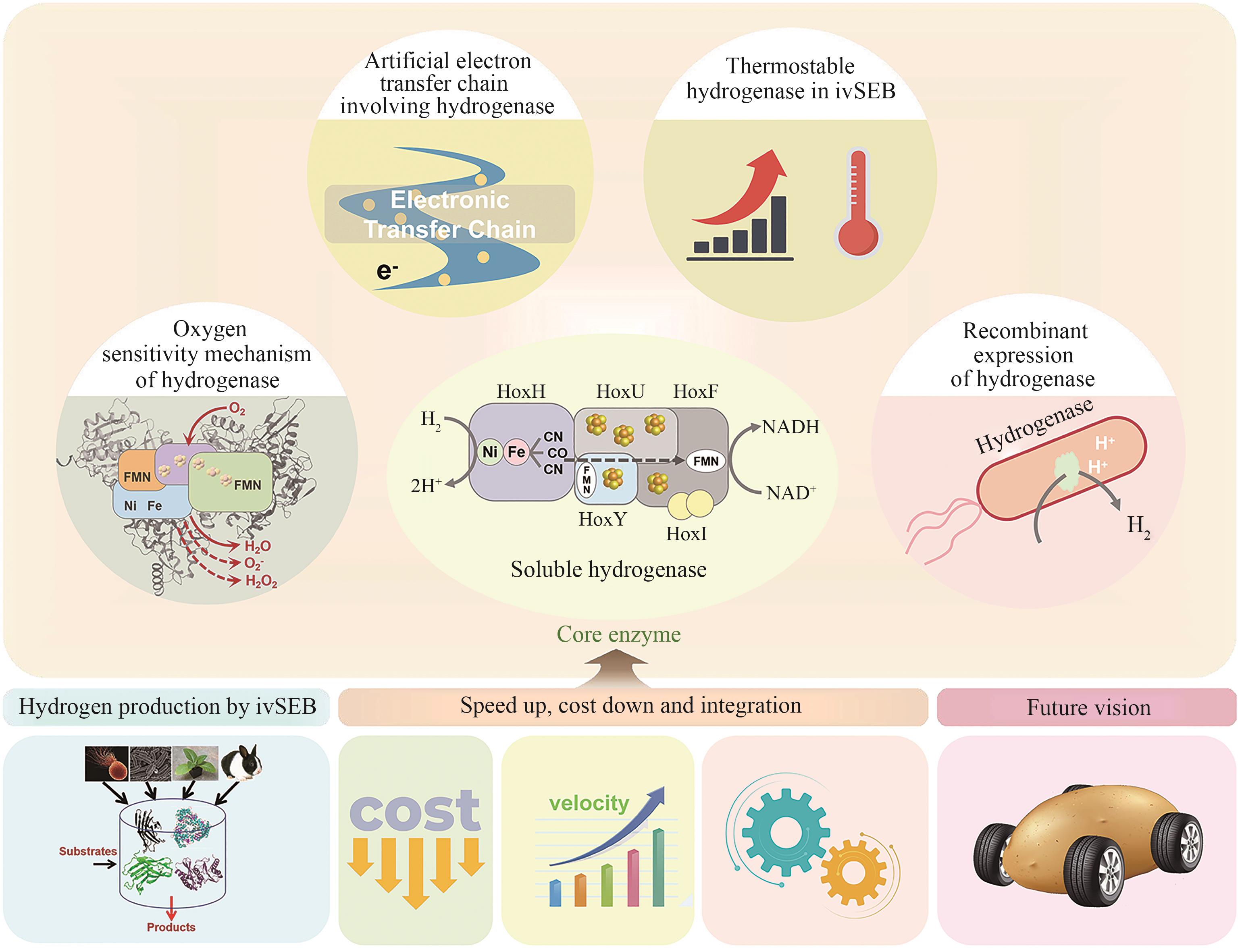
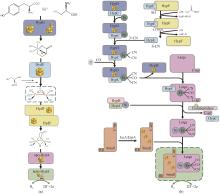
Hydrogenases are the most important enzymes in biological hydrogen production and hydrogen energy utilization. They are widely distributed, oxygen-sensitive, multiunit complexed metal enzymes. In vitro synthetic enzymatic biosystems (ivSEB) is a type of in vitro biotransformation (ivBT) technology, which is an emerging biomanufacturing powerhouse that combines microbial fermentation with enzymatic biocatalysis, allowing for novel and efficient hydrogen production, also breaking the Thauer limit and achieving a yield of hydrogen close to the theoretical value of chemistry (1 mole of glucose to produce 12 moles of hydrogen in maximum). It represents the future direction of biological hydrogen production. However, the recombinant expression of hydrogenase is the main bottleneck limiting the wide application of ivSEB for hydrogen production technology. Hydrogenases are widely distributed in all life domains, but are oxygen-sensitive and mostly consist of metalloproteins with multi-subunits, bearing [Fe] only, [NiFe] or [FeFe] dinuclear core in their catalytic center. Oxygen not only inhibits the activity of hydrogenase, but also affects the transcription of the enzyme-encoding gene and post-translational process of the enzymes. As a result, the levels of recombinant hydrogenase are usually low and the enzymatic activities are also incomparable to the native enzymes, often leading to high production costs due to the strict anaerobic purification procedures. In order to meet the requirements of industrial hydrogen production, hydrogenases must possess excellent catalytic properties, such as a high catalytic turnover number, great thermal stability, and the ability to tolerate trace amounts of oxygen. This review summarizes the studies on the structural and catalytic characterizations of hydrogenases, including their classification, oxygen resistance mechanisms, and progress in recombinant expression. Additionally, the evolution of natural electron transfer chains and the design of artificial routes, which can improve hydrogen production efficiency and reduce costs, are briefly discussed. The review also discussed the progress in the studies on the mechanisms of hydrogenases’ tolerance toward oxygen, the strategies for microbial expression of recombinant hydrogenases as well as the optimization of the artificial electron transfer chains adapted for the production of hydrogen using ivSEB, in expectations of promoting the applications of hydrogenases involved ivSEB, from renewable energy storage, anaerobic artificial respiration, to clean hydrogenation or dehydrogenation in biocatalysis. 
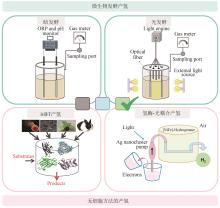
Fig. 5
The main limiting factors in ivSEB hydrogen production
Extracts from the Article
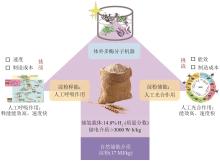
对比微生物发酵产氢,ivSEB产氢体系展现出多方面的优势:①可实现小型分布式碳中性产氢的新方法;②所产氢气的纯度高,副产物少;③即产(氢)即用(氢);④从碳水化合物到氢能的能量转换效率最高。这些技术优势为淀粉作为高密度储氢载体迈出了关键性的一步。尽管如此,现有ivSEB糖产氢技术依然难以实现工业应用的经济性,其主要限制因素是产氢反应速率及反应中加入的酶及辅酶的成本(图5)。对于产氢反应速率,有许多策略可以有效进行提升,如(超)热稳定酶替代、提高反应温度、优化关键酶比例、增加底物浓度、增加酶载量甚至代谢产物通道化等,这些方式可以将生物氢产生速率加快数个数量级。例如,微生物燃料电池的功率密度在过去十年中提高了大约106倍。近年来,宿主菌的系统优化、发酵控制技术的进步让重组蛋白生产的成本大大降低[151-152]。此外,通过使用(超)热稳定酶、酶固定化、低成本快速的酶纯化技术等,也让重组酶的成本进一步下降。例如,长期热稳定酶的固定化在工业上已成功应用。1 kg固定化葡萄糖异构酶可以在55 ℃左右转化至少15 000 kg果葡糖浆,并可在持续几个月后才进行更换[13]。
Other Images/Table from this Article
|