|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Plant synthetic biology: new opportunities for large-scale culture of plant cells
Synthetic Biology Journal
2025, 6 (5):
1107-1125.
DOI: 10.12211/2096-8280.2024-095
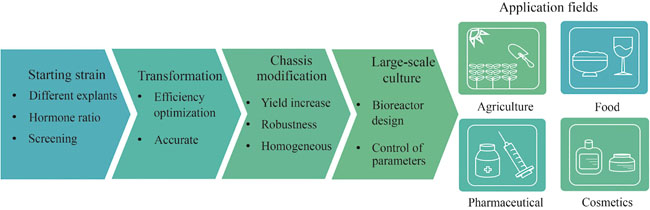
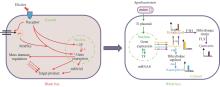
Plant Cell Culture (PCC) has emerged as a highly promising chassis for synthetic biology, offering a range of advantages such as short growth cycles, cost-effectiveness, absence of pathogenic risks, and abundant secondary metabolites. These features make PCC an attractive alternative for applications in medicine, food, and health. However, insufficient production efficiency due to difficulties in genetic transformation, complex regulatory networks, cell aggregation, and poor genetic stability remains a major obstacle that limits the commercialization of PCC. Synthetic biology, with its bottom-up engineering design approach, provides a powerful toolkit to address these challenges. By enabling the precise design and modification of native plant cells, synthetic biology offers innovative strategies to develop efficient and economically viable plant cell factories. In this paper, we first review the current status of PCC in synthesizing high-value compounds, particularly recombinant proteins and secondary metabolites. Recent advancements have demonstrated the potential of PCC to produce therapeutic proteins, vaccines, industrial enzymes and bioactive compounds such as alkaloids, flavonoids, and terpenoids. These successes underscore the versatility of PCC as a bioproduction platform. We then explore the role of synthetic biology in advancing PCC industrialization. Key developments include the creation of high-quality plant cell lines through genome editing tools like CRISPR/Cas9, enhancing genetic stability and metabolic efficiency. Additionally, synthetic biology has improved genetic transformation systems, overcoming a critical bottleneck in PCC. Enhanced expression systems, incorporating synthetic promoters and regulatory elements, have significantly boosted target compound yields. Furthermore, synthetic biology has expanded PCC applications by enabling the biosynthesis of heterologous compounds beyond their native metabolic pathways. Finally, we discuss future prospects, emphasizing the potential of synthetic biology to overcome current technical challenges. Emerging technologies including multi-omics integration, machine learning, and synthetic organelle development are anticipated to further enhance PCC’s scalability and efficiency. By addressing these challenges, synthetic biology will pave the way for large-scale plant cell cultivation, thereby facilitating its widespread adoption in industrial bioproduction. The convergence of PCC and synthetic biology holds immense potential for the sustainable, cost-effective, and scalable production of high-value compounds.
Table 4
Cases of secondary metabolites synthesized by plant cells
Extracts from the Article
由此可见,在植物合成生物学的助力下,植物细胞不仅可以提高自身高附加值产物的产量,还可借助细胞本身的优势高效生产异源产物。这些研究案例(表4)为解决全球面临的健康、环境及食品安全等挑战提供了创新思路和高效解决方案,证实了植物合成生物学及植物细胞大规模培养在推动农业、医药和化工等领域可持续发展中的应用潜力。
Other Images/Table from this Article
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||