|
||
|
Applications of protein engineering in pharmaceutical industry
Synthetic Biology Journal
2025, 6 (1):
65-86.
DOI: 10.12211/2096-8280.2024-061
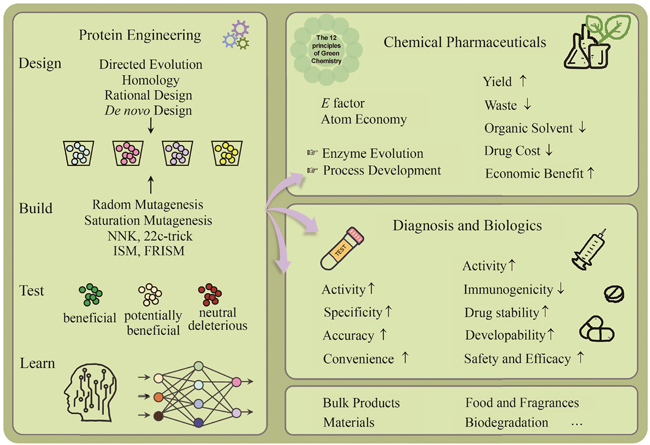
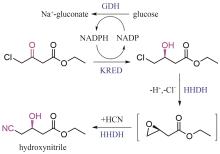
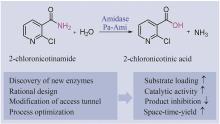
Protein engineering performs specific designs and modifications on proteins through directed evolution, semi-rational or rational design, computer-assisted design, and so on. The engineered proteins, with improved properties, have significant applications in food, medicine, fuel, and material industries. For the chemical and pharmaceutical industry, engineered enzymes can serve as efficient biocatalysts for the synthesis of active pharmaceutical ingredients (API) and their intermediates, aligning with the concepts and principles of green chemistry and manufacturing. For the biopharmaceutical industry, the engineering of peptide or protein modifying enzymes can boost the efficiency in preparing drug candidates, while engineered diagnostic enzymes can make detection more accurate and sensitive. Moreover, protein engineering can improve the bioactivities of biological drugs such as therapeutic enzymes and antibodies, increase stability, and mitigate immunogenic response for their safety and efficacy. Here, we review the tremendous progress in protein engineering, elucidate its importance in the research and development of chemically derived drugs and biologics, and provide examples of its applications. These examples encompass the discovery of enzymes or antibodies, the process of protein engineering, and the subsequent economic advantages. We aim to showcase the practical implementation of protein engineering in the pharmaceutical industry and facilitate technology transfer, thereby fostering seamless integration between research, development, and industrial production. Furthermore, we discuss challenges such as cost-effectiveness and market changes in the synthesis of API, and multi-target optimization, long cycle and high risk in the discovery and development of biopharmaceuticals. Finally, we look forward to the prospects of protein engineering in pharmaceutical industry. In the future, automated pipelines consisting artificial intelligence and self-driving laboratories will accelerate the design-build-test-learn cycle, leading to rapid progress in molecular design and discovery.

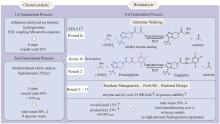
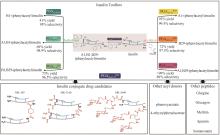
Fig. 2
LovD9 catalyzing the acylation of monacolin J to produce simvastatin with the non-natural acyl donor DMB-S-MMP
Extracts from the Article
酰基转移酶(acyltransferase,EC 2.3.1.X)催化酰基从供体分子转移到受体分子的羟基、氨基或巯基上,从而产生酰基酯衍生物。2006年,唐奕课题组[67]发现洛伐他汀生物合成基因簇编码的LovD对酰基载体、酰基底物和十氢萘酰基受体有一定的杂泛性。以DMB-S-NAC和DMB-S-MMP等多种硫酯作为酰基供体,用全细胞催化对莫那可林J的C-8位羟基实现了区域选择性的酰基化[66](图2)。这意味着从洛伐他汀到辛伐他汀的合成过程被极大简化。2009年,该课题组通过有限的突变将LovD催化辛伐他汀合成这一非天然反应的kcat提高了5倍[68]。Codexis研究团队获得了工艺许可之后进一步对酶和工艺进行了优化,基于ProSAR对LovD进行了9轮定向进化[69-71],建库216个,筛选突变体61 779个,最终获得了含有29个突变的LovD9。LovD9催化辛伐他汀合成的kcat/Km是野生型LovD的330倍,产生辛伐他汀的效率是LovD的1000倍。一些企业使用该酶和工艺实现了辛伐他汀的大规模生产。Codexis公司和唐奕教授因此获得EPA颁发的“总统绿色化学挑战:2012年更绿色合成途径奖”[72]。LovD9对天然底物α-甲基丁基-酰基载体蛋白(acyl carrier protein,ACP)的活性完全丧失。LovD被改造为了一个能够接受小的游离酰基硫酯,而不再需要ACP的酶。分析突变体晶体结构发现,在进化过程中底物通道逐渐变窄使得LovF的ACP结构域无法进入活性位点。通过较长时间尺度(1~1.5 μs)的分子动力学模拟发现,突变显著改变了催化残基的构象动力学[71]。此外该研究团队还从理性设计的角度对LovD9定向进化的路径进行了阐释[73],为这类酶的改造提供了宝贵经验。
活性↑,稳定性↑ ... Improved Lov-D acyltransferase mediated acylation 1 ... 酰基转移酶(acyltransferase,EC 2.3.1.X)催化酰基从供体分子转移到受体分子的羟基、氨基或巯基上,从而产生酰基酯衍生物.2006年,唐奕课题组[ Variant LovD polypeptides and their uses 0 The role of distant mutations and allosteric regulation on LovD active site dynamics 2 2014 ... 酰基转移酶(acyltransferase,EC 2.3.1.X)催化酰基从供体分子转移到受体分子的羟基、氨基或巯基上,从而产生酰基酯衍生物.2006年,唐奕课题组[
Other Images/Table from this Article
|