|
||
|
Applications of protein engineering in pharmaceutical industry
Synthetic Biology Journal
2025, 6 (1):
65-86.
DOI: 10.12211/2096-8280.2024-061
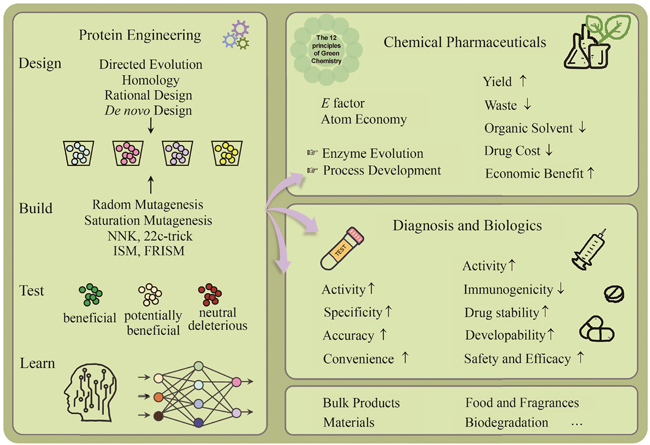
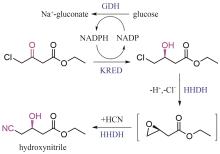
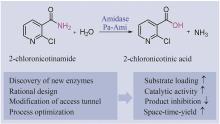
Protein engineering performs specific designs and modifications on proteins through directed evolution, semi-rational or rational design, computer-assisted design, and so on. The engineered proteins, with improved properties, have significant applications in food, medicine, fuel, and material industries. For the chemical and pharmaceutical industry, engineered enzymes can serve as efficient biocatalysts for the synthesis of active pharmaceutical ingredients (API) and their intermediates, aligning with the concepts and principles of green chemistry and manufacturing. For the biopharmaceutical industry, the engineering of peptide or protein modifying enzymes can boost the efficiency in preparing drug candidates, while engineered diagnostic enzymes can make detection more accurate and sensitive. Moreover, protein engineering can improve the bioactivities of biological drugs such as therapeutic enzymes and antibodies, increase stability, and mitigate immunogenic response for their safety and efficacy. Here, we review the tremendous progress in protein engineering, elucidate its importance in the research and development of chemically derived drugs and biologics, and provide examples of its applications. These examples encompass the discovery of enzymes or antibodies, the process of protein engineering, and the subsequent economic advantages. We aim to showcase the practical implementation of protein engineering in the pharmaceutical industry and facilitate technology transfer, thereby fostering seamless integration between research, development, and industrial production. Furthermore, we discuss challenges such as cost-effectiveness and market changes in the synthesis of API, and multi-target optimization, long cycle and high risk in the discovery and development of biopharmaceuticals. Finally, we look forward to the prospects of protein engineering in pharmaceutical industry. In the future, automated pipelines consisting artificial intelligence and self-driving laboratories will accelerate the design-build-test-learn cycle, leading to rapid progress in molecular design and discovery.

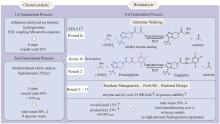
Fig. 5
Directed evolution of penicillin G acylase enables the site-selective bioconjugation of insulin
Extracts from the Article
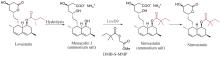
Merck和Codexis于2022年报道了一项开创性的工作,通过定向进化获得了一系列青霉素G酰化酶(penicillin G acylase,PGA)突变体,实现了胰岛素特定位置的修饰,可以方便地获得各种胰岛素类似物候选药物[113](图5)。大肠杆菌来源的PGA催化肽链上N-苯乙酰水解的活性较低,不能满足特定位点保护和解除保护的需求。团队通过筛选发现来源于Kluyvera cryocrescens的KcPGA对A1,B1,B29-苯乙酰基胰岛素的A1、B1、B29位苯乙酰基均有水解能力,当反应时间足够长时,3个苯乙酰基均会被水解。此外,KcPGA对3个位置表现出水解速率差异,B29>A1>B1,这说明通过酶改造实现胰岛素的位置特异性修饰具有可行性。之后通过定向进化获得的一套工具酶实现了胰岛素特定位置的选择性保护和脱保护,得到了A1、B1和B29三个位置分别具有苯乙酰基保护或未保护的共8种中间体。这个苯乙酰基胰岛素工具箱可以作为基础模块合成由胰岛素衍生的候选药物分子,缩短了合成路线,提高了产物的产量和纯度,加速了药物研发进程。此外,作者还证明了工程化的PGA可以耐受其他的酰基供体,也可以拓展至胰高血糖素等其他多肽的生物选择性偶联。
Other Images/Table from this Article
|