合成生物学 ›› 2023, Vol. 4 ›› Issue (3): 535-550.DOI: 10.12211/2096-8280.2022-066
数据驱动的酶反应预测与设计
曾涛, 巫瑞波
- 中山大学药学院,广东 广州 510006
-
收稿日期:2022-11-23修回日期:2022-12-27出版日期:2023-06-30发布日期:2023-07-05 -
通讯作者:巫瑞波 -
作者简介:曾涛 (1995—),男,博士研究生。研究方向为计算驱动的生物合成路线设计与优化。 E-mail:zengt28@mail2.sysu.edu.cn巫瑞波 (1984—),男,教授,博士生导师。研究方向为基于多尺度模拟的萜类天然产物生物智造与药效挖掘。 E-mail:wurb3@mail.sysu.edu.cn -
基金资助:广东省重点研发计划(2022B1111080005)
Data-driven prediction and design for enzymatic reactions
ZENG Tao, WU Ruibo
- School of Pharmaceutical Science,Sun Yat-Sen University,Guangzhou 510006,Guangdong,China
-
Received:2022-11-23Revised:2022-12-27Online:2023-06-30Published:2023-07-05 -
Contact:WU Ruibo
摘要:
酶催化已经在日用化学品、药物和功能材料等生产中得到越来越广泛的应用。酶,作为生物制造业的核心“芯片”,其催化反应的预测与设计是推动传统生物制造走向生物智造发展的核心驱动力之一。然而目前我们对大自然酶催化的了解仍然非常有限,这严重阻碍了我们对酶催化空间的探索和利用。随着大数据时代的到来,数据驱动的计算模拟已经成为酶催化新空间的挖掘及其功能优化设计的重要手段。各种计算工具和平台的开发正极大地加速并赋能于酶学相关领域的各类实验研究。本文针对酶催化过程中底物、产物和酶的预测及设计方法进行了综述,概述了近年来酶反应相关的数据库,汇总比较了数据驱动的酶反应设计工具,着重介绍了深度学习在该领域的应用,并从数据、模型、算法、平台等多方面展望和探讨了数据驱动型计算方法在酶反应预测与设计领域的发展前景。
中图分类号:
引用本文
曾涛, 巫瑞波. 数据驱动的酶反应预测与设计[J]. 合成生物学, 2023, 4(3): 535-550.
ZENG Tao, WU Ruibo. Data-driven prediction and design for enzymatic reactions[J]. Synthetic Biology Journal, 2023, 4(3): 535-550.
| 数据库 | 特点 | 网址 |
|---|---|---|
| KEGG[ | 具有物种、基因组、酶等多水平注释的合成(代谢)反应数据库 | https://www.kegg.jp/kegg |
| MetaCyc[ | 以全面的初级/次级代谢产物合成途径对反应进行注释 | https://metacyc.org |
| Rhea[ | 全面的生物酶反应数据库,与Uniprot高度关联 | https://www.rhea-db.org |
| BRENDA[ | 对酶的各项信息(如分类、反应、参数等)进行详细注释 | https://www.brenda-enzymes.org |
| SABIO-RK[ | 包含酶反应的动力学参数、反应条件等信息 | https://sabiork.h-its.org |
| Reactome[ | 综合的生物通路数据库,包括代谢、信号调控等通路数据 | https://reactome.org |
| PathBank[ | 以常见模式物种为基础的代谢、调控通路数据库 | http://www.pathbank.org |
| HMDB[ | 人体小分子代谢数据库,包含反应、MS、NMR谱图等信息 | https://hmdb.ca |
| MetaNetX[ | 整合了多个来源的生化反应数据库用于代谢网络模型构建 | https://www.metanetx.org |
| Reaxys[ | 从专利和文献搜集和整理的大量有机反应和酶反应路线(商业非开源) | https://www.reaxys.com |
表1 酶反应数据库
Table 1 Databases of enzymatic reactions
| 数据库 | 特点 | 网址 |
|---|---|---|
| KEGG[ | 具有物种、基因组、酶等多水平注释的合成(代谢)反应数据库 | https://www.kegg.jp/kegg |
| MetaCyc[ | 以全面的初级/次级代谢产物合成途径对反应进行注释 | https://metacyc.org |
| Rhea[ | 全面的生物酶反应数据库,与Uniprot高度关联 | https://www.rhea-db.org |
| BRENDA[ | 对酶的各项信息(如分类、反应、参数等)进行详细注释 | https://www.brenda-enzymes.org |
| SABIO-RK[ | 包含酶反应的动力学参数、反应条件等信息 | https://sabiork.h-its.org |
| Reactome[ | 综合的生物通路数据库,包括代谢、信号调控等通路数据 | https://reactome.org |
| PathBank[ | 以常见模式物种为基础的代谢、调控通路数据库 | http://www.pathbank.org |
| HMDB[ | 人体小分子代谢数据库,包含反应、MS、NMR谱图等信息 | https://hmdb.ca |
| MetaNetX[ | 整合了多个来源的生化反应数据库用于代谢网络模型构建 | https://www.metanetx.org |
| Reaxys[ | 从专利和文献搜集和整理的大量有机反应和酶反应路线(商业非开源) | https://www.reaxys.com |

图2 正向和逆向反应预测[正向和逆向反应预测都是从一个分子(绿色)出发预测其潜在底物或产物(黄色),箭头表示两者之间能够通过反应进行转化,在(a)中箭头从反应物指向产物,(b)中则相反。经过多次迭代能够获得一个反应网络,网络中既能采样到已知的分子(实心)又能获得全新的结构(空心)。但不同的是正向反应预测每一次迭代方向都是随机的,而逆合成预测通常有一个终点条件(蓝色,如特定的原料分子),且会采取算法使得迭代过程朝着终点的方向进行]
Fig. 2 Prediction of forward and backward enzymatic reactions[Prediction starts with an enzyme molecule (green node) to deduce its substrate or product (yellow nodes), the lines represent transformation reactions between two molecules, with arrow from substrate (enzyme) to product (a) and the reverse (b). A reaction network is developed after the iterative prediction in which both known (solid nodes) and unknown (hollow nodes) molecules are included. The forward prediction is generally random while a target (blue node, such as a building block) is specified in the backward prediction, and the exploration will lead to the target with the help of iterative algorithms.]
| 反应预测与酶设计工具 | ||||
|---|---|---|---|---|
| 基于相似性 | 基于反应规则 | 基于机器学习 | ||
| 正向反应预测 | BioSynther[ (http://www.rxnfinder.org/) | ATLASx[ (https://lcsb-databases.epfl.ch/Atlas2) BCSExplorer[ (http://www.rxnfinder.org/) | Reymond等[ (https://github.com/reymondgroup/OpenNMT-py) Kavraki等[ (https:// github.com/KavrakiLab/MetaTrans) | |
| 逆合成预测 | PrecursorFinder[ (http://www.rxnfinder.org/) | RetroPath[ (https://github.com/brsynth/RetroPathRL) RetroBioCat[ (https://retrobiocat.com) | BioNavi-NP[ (http://biopathnavi.qmclab.com/) Probst等[ (https://github.com/rxn4chemistry/biocatalysis-model) | |
| 酶搜索和设计 | EC-BLAST[ (https://www.ebi.ac.uk/) | Selenzyme[ (http://selenzyme.synbiochem.co.uk/) BridgIT[ (https://lcsb-databases.epfl.ch/Atlas2) E-zyme2[ (https://www.genome.jp/tools/e-zyme2/) | Faulon等[ (tool not available) Ranganathan等[ (https://github.com/ranganathanlab/bmDCA) | |
| 酶功能与性质预测工具 | ||||
| 酶功能预测 | 功能 分类 | DeepEC[ Araki等[ MTDNN[ | ||
| 功能 优化 | ECNet[ Gitter等[ | |||
| 酶反应性质预测 | Lercher等[ Palsson等[ DLKcat[ | |||
表2 酶反应预测与设计工具汇总
Table 2 Tools for the prediction and design of enzymatic reactions
| 反应预测与酶设计工具 | ||||
|---|---|---|---|---|
| 基于相似性 | 基于反应规则 | 基于机器学习 | ||
| 正向反应预测 | BioSynther[ (http://www.rxnfinder.org/) | ATLASx[ (https://lcsb-databases.epfl.ch/Atlas2) BCSExplorer[ (http://www.rxnfinder.org/) | Reymond等[ (https://github.com/reymondgroup/OpenNMT-py) Kavraki等[ (https:// github.com/KavrakiLab/MetaTrans) | |
| 逆合成预测 | PrecursorFinder[ (http://www.rxnfinder.org/) | RetroPath[ (https://github.com/brsynth/RetroPathRL) RetroBioCat[ (https://retrobiocat.com) | BioNavi-NP[ (http://biopathnavi.qmclab.com/) Probst等[ (https://github.com/rxn4chemistry/biocatalysis-model) | |
| 酶搜索和设计 | EC-BLAST[ (https://www.ebi.ac.uk/) | Selenzyme[ (http://selenzyme.synbiochem.co.uk/) BridgIT[ (https://lcsb-databases.epfl.ch/Atlas2) E-zyme2[ (https://www.genome.jp/tools/e-zyme2/) | Faulon等[ (tool not available) Ranganathan等[ (https://github.com/ranganathanlab/bmDCA) | |
| 酶功能与性质预测工具 | ||||
| 酶功能预测 | 功能 分类 | DeepEC[ Araki等[ MTDNN[ | ||
| 功能 优化 | ECNet[ Gitter等[ | |||
| 酶反应性质预测 | Lercher等[ Palsson等[ DLKcat[ | |||
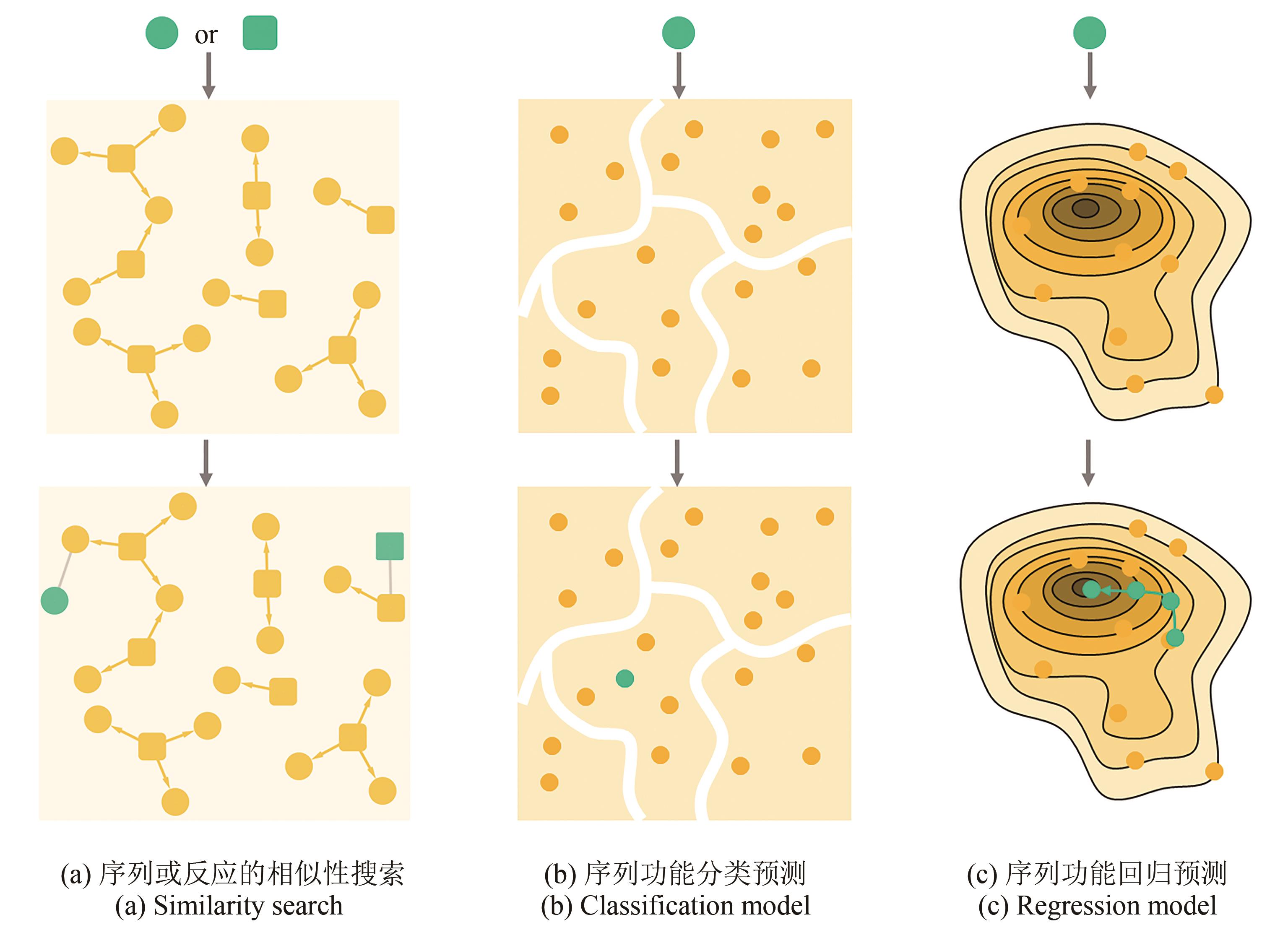
图3 不同类型的酶搜索和功能预测模型。[圆形代表酶,方形代表反应,黄色节点代表已知数据,绿色代表待预测对象。基于相似性的预测方法(a)是从已知的酶-反应数据对中(图中相连的两个节点)寻找与目标对象相似的样本,从而对其反应(或酶)进行预测。功能分类模型(b)是将已知功能(通常是离散变量)的酶序列作为训练集,寻找其潜在分类规律(白色分界线),从而对目标序列进行预测。回归模型(c)则是对活性或稳定性强弱等连续变量进行建模预测,绘制适应度景观图从而对目标序列的功能进行预测,并用于酶设计]
Fig. 3 Models for searching and predicting enzymes[Circular and square nodes represent sequences and reactions, respectively, and yellow filling indicates known data while green filling mean objects to be predicted. Similarity search (a) is to find a similar object in known enzyme-reaction pairs (connected nodes) to predict reactions (or enzymes) for target object. Classification model (b) is trained by enzymes with known function (usually discrete), in which the classification rule (white boundary) is clarified, and then the model can be used to classify an enzyme with unknown function. Regression model (c) is adapted to draw fitness landscape to predict continues variables such as the activity or stability of enzymes, which can then be used for enzyme design.]
| 1 | BENKOVIC S J, HAMMES-SCHIFFER S. A perspective on enzyme catalysis[J]. Science, 2003, 301(5637): 1196-1202. |
| 2 | BORNSCHEUER U T, BUCHHOLZ K. Highlights in biocatalysis-historical landmarks and current trends[J]. Engineering in Life Sciences, 2005, 5(4): 309-323. |
| 3 | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2): 801-838. |
| 4 | ZIMMERMAN J B, ANASTAS P T, ERYTHROPEL H C, et al. Designing for a green chemistry future[J]. Science, 2020, 367(6476): 397-400. |
| 5 | WINKLER C K, SCHRITTWIESER J H, KROUTIL W. Power of biocatalysis for organic synthesis[J]. ACS Central Science, 2021, 7(1): 55-71. |
| 6 | LIN G M, WARDEN-ROTHMAN R, VOIGT C A. Retrosynthetic design of metabolic pathways to chemicals not found in nature[J]. Current Opinion in Systems Biology, 2019, 14: 82-107. |
| 7 | SENN H M, THIEL W. QM/MM methods for biomolecular systems[J]. Angewandte Chemie International Edition, 2009, 48(7): 1198-1229. |
| 8 | ZHA W L, ZHANG F, SHAO J Q, et al. Rationally engineering santalene synthase to readjust the component ratio of sandalwood oil[J]. Nature Communications, 2022, 13: 2508. |
| 9 | WANG Y, MALACO MOROTTI A L, XIAO Y R, et al. Decoding the cytochrome P450 catalytic activity in divergence of benzophenone and xanthone biosynthetic pathways[J]. ACS Catalysis, 2022, 12(21): 13630-13637. |
| 10 | LIANG M M, ZHANG F, XU J X, et al. A conserved mechanism affecting hydride shifting and deprotonation in the synthesis of hopane triterpenes as compositions of wax in oat[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(12): e2118709119. |
| 11 | VAN DIJK E L, AUGER H, JASZCZYSZYN Y, et al. Ten years of next-generation sequencing technology[J]. Trends in Genetics, 2014, 30(9): 418-426. |
| 12 | WANG H Y, GUO H, WANG N, et al. Toward the heterologous biosynthesis of plant natural products: gene discovery and characterization[J]. ACS Synthetic Biology, 2021, 10(11): 2784-2795. |
| 13 | JUMPER J, EVANS R, PRITZEL A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589. |
| 14 | PEARCE R, ZHANG Y. Deep learning techniques have significantly impacted protein structure prediction and protein design[J]. Current Opinion in Structural Biology, 2021, 68: 194-207. |
| 15 | CUI Y L, SUN J Y, WU B. Computational enzyme redesign: large jumps in function[J]. Trends in Chemistry, 2022, 4(5): 409-419. |
| 16 | SHELDON R A, PEREIRA P C. Biocatalysis engineering: the big picture[J]. Chemical Society Reviews, 2017, 46(10): 2678-2691. |
| 17 | JANG W D, KIM G B, KIM Y J, et al. Applications of artificial intelligence to enzyme and pathway design for metabolic engineering[J]. Current Opinion in Biotechnology, 2022, 73: 101-107. |
| 18 | HADADI N, HATZIMANIKATIS V Design of computational retrobiosynthesis tools for the design of de novo synthetic pathways[J]. Current Opinion in Chemical Biology, 2015, 28: 99-104. |
| 19 | 周静茹, 刘鹏, 夏建业, 等. 基于约束的基因组规模代谢网络模型构建方法研究进展[J]. 生物工程学报, 2021, 37(5): 1526-1540. |
| ZHOU J R, LIU P, XIA J Y, et al. Advances in the development of constraint-based genome-scale metabolic network models[J]. Chinese Journal of Biotechnology, 2021, 37(5): 1526-1540. | |
| 20 | MACKLIN D N, RUGGERO N A, COVERT M W. The future of whole-cell modeling[J]. Current Opinion in Biotechnology, 2014, 28: 111-115. |
| 21 | MAZURENKO S, PROKOP Z, DAMBORSKY J. Machine learning in enzyme engineering[J]. ACS Catalysis, 2020, 10(2): 1210-1223. |
| 22 | LIAO X P, MA H W, TANG Y J. Artificial intelligence: a solution to involution of design-build-test-learn cycle[J]. Current Opinion in Biotechnology, 2022, 75: 102712. |
| 23 | KANEHISA M, GOTO S. KEGG: Kyoto encyclopedia of genes and genomes[J]. Nucleic Acids Research, 2000, 28(1): 27-30. |
| 24 | CASPI R, BILLINGTON R, KESELER I M, et al. The MetaCyc database of metabolic pathways and enzymes-a 2019 update[J]. Nucleic Acids Research, 2020, 48(D1): D445-D453. |
| 25 | BANSAL P, MORGAT A, AXELSEN K B, et al. Rhea, the reaction knowledgebase in 2022[J]. Nucleic Acids Research, 2022, 50(D1): D693-D700. |
| 26 | CHANG A, JESKE L, ULBRICH S, et al. BRENDA, the ELIXIR core data resource in 2021: new developments and updates[J]. Nucleic Acids Research, 2021, 49(D1): D498-D508. |
| 27 | WITTIG U, REY M, WEIDEMANN A, et al. SABIO-RK: an updated resource for manually curated biochemical reaction kinetics[J]. Nucleic Acids Research, 2018, 46(D1): D656-D660. |
| 28 | GILLESPIE M, JASSAL B, STEPHAN R, et al. The reactome pathway knowledgebase 2022 [J]. Nucleic Acids Research, 2022, 50(D1): D687-D692. |
| 29 | WISHART D S, LI C, MARCU A, et al. PathBank: a comprehensive pathway database for model organisms[J]. Nucleic Acids Research, 2020, 48(D1): D470-D478. |
| 30 | WISHART D S, GUO A C, OLER E, et al. HMDB 5.0: the human metabolome database for 2022[J]. Nucleic Acids Research, 2022, 50(D1): D622-D631. |
| 31 | MORETTI S, TRAN V D T, MEHL F, et al. MetaNetX/MNXref: unified namespace for metabolites and biochemical reactions in the context of metabolic models[J]. Nucleic Acids Research, 2021, 49(D1): D570-D574. |
| 32 | LAWSON A J, SWIENTY-BUSCH J, GÉOUI T, et al. The making of reaxys—towards unobstructed access to relevant chemistry information[M]//ACS Symposium Series: The Future of the History of Chemical Information. Washington, DC: American Chemical Society, 2014: 127-148. |
| 33 | CONSORTIUM T U. UniProt: a worldwide hub of protein knowledge[J]. Nucleic Acids Research, 2019, 47(D1): D506-D515. |
| 34 | XU Y J, LIN K J, WANG S W, et al. Deep learning for molecular generation[J]. Future Medicinal Chemistry, 2019, 11(6): 567-597. |
| 35 | ELTON D C, BOUKOUVALAS Z, FUGE M D, et al. Deep learning for molecular design—a review of the state of the art[J]. Molecular Systems Design & Engineering, 2019, 4(4): 828-849. |
| 36 | HAGHIGHATLARI M, LI J, HEIDAR-ZADEH F, et al. Learning to make chemical predictions: the interplay of feature representation, data, and machine learning methods[J]. Chem, 2020, 6(7): 1527-1542. |
| 37 | SENIOR A W, EVANS R, JUMPER J, et al. Improved protein structure prediction using potentials from deep learning[J]. Nature, 2020, 577(7792): 706-710. |
| 38 | LANDRUM G. RDKit: Open-source cheminformatics software[EB/OL][2022-12-01]. . |
| 39 | The Gene Ontology Consortium. The gene ontology resource: enriching a GOld mine[J]. Nucleic Acids Research, 2021, 49(D1): D325-D334. |
| 40 | MOHAMMADIPEYHANI H, HAFNER J, SVESHNIKOVA A, et al. Expanding biochemical knowledge and illuminating metabolic dark matter with ATLASx[J]. Nature Communications, 2022, 13: 1560. |
| 41 | HATZIMANIKATIS V, LI C H, IONITA J A, et al. Exploring the diversity of complex metabolic networks[J]. Bioinformatics, 2005, 21(8): 1603-1609. |
| 42 | HAFNER J, PAYNE J, MOHAMMADIPEYHANI H, et al. A computational workflow for the expansion of heterologous biosynthetic pathways to natural product derivatives[J]. Nature Communications, 2021, 12: 1760. |
| 43 | TIAN Y, WU L, YUAN L, et al. BCSExplorer: a customized biosynthetic chemical space explorer with multifunctional objective function analysis[J]. Bioinformatics, 2020, 36(5): 1642-1643. |
| 44 | TU W Z, ZHANG H R, LIU J, et al. BioSynther: a customized biosynthetic potential explorer[J]. Bioinformatics, 2016, 32(3): 472-473. |
| 45 | KREUTTER D, SCHWALLER P, REYMOND J L. Predicting enzymatic reactions with a molecular transformer[J]. Chemical Science, 2021, 12(25): 8648-8659. |
| 46 | LITSA E E, DAS P, KAVRAKI L E. Prediction of drug metabolites using neural machine translation[J]. Chemical Science, 2020, 11(47): 12777-12788. |
| 47 | YUAN L, TIAN Y, DING S Z, et al. PrecursorFinder: a customized biosynthetic precursor explorer[J]. Bioinformatics, 2019, 35(9): 1603-1604. |
| 48 | KOCH M, DUIGOU T, FAULON J L. Reinforcement learning for bioretrosynthesis[J]. ACS Synthetic Biology, 2020, 9(1): 157-168. |
| 49 | FINNIGAN W, HEPWORTH L J, FLITSCH S L, et al. RetroBioCat as a computer-aided synthesis planning tool for biocatalytic reactions and cascades[J]. Nature Catalysis, 2021, 4(2): 98-104. |
| 50 | ZHENG S J, ZENG T, LI C T, et al. Deep learning driven biosynthetic pathways navigation for natural products with BioNavi-NP[J]. Nature Communications, 2022, 13: 3342. |
| 51 | PROBST D, MANICA M, NANA TEUKAM Y G, et al. Biocatalysed synthesis planning using data-driven learning[J]. Nature Communications, 2022, 13: 964. |
| 52 | RAHMAN S A, CUESTA S M, FURNHAM N, et al. EC-BLAST: a tool to automatically search and compare enzyme reactions[J]. Nature Methods, 2014, 11(2): 171-174. |
| 53 | CARBONELL P, WONG J, SWAINSTON N, et al. Selenzyme: enzyme selection tool for pathway design[J]. Bioinformatics, 2018, 34(12): 2153-2154. |
| 54 | HADADI N, MOHAMMADIPEYHANI H, MISKOVIC L, et al. Enzyme annotation for orphan and novel reactions using knowledge of substrate reactive sites[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(15): 7298-7307. |
| 55 | MORIYA Y, YAMADA T, OKUDA S, et al. Identification of enzyme genes using chemical structure alignments of substrate-product pairs[J]. Journal of Chemical Information and Modeling, 2016, 56(3): 510-516. |
| 56 | MELLOR J, GRIGORAS I, CARBONELL P, et al. Semisupervised Gaussian process for automated enzyme search[J]. ACS Synthetic Biology, 2016, 5(6): 518-528. |
| 57 | RUSS W P, FIGLIUZZI M, STOCKER C, et al. An evolution-based model for designing chorismate mutase enzymes[J]. Science, 2020, 369(6502): 440-445. |
| 58 | RYU J Y, KIM H U, LEE S Y. Deep learning enables high-quality and high-throughput prediction of enzyme commission numbers[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(28): 13996-14001. |
| 59 | WATANABE N, MURATA M, OGAWA T, et al. Exploration and evaluation of machine learning-based models for predicting enzymatic reactions[J]. Journal of Chemical Information and Modeling, 2020, 60(3): 1833-1843. |
| 60 | FA R, COZZETTO D, WAN C, et al. Predicting human protein function with multi-task deep neural networks[J]. PLoS One, 2018, 13(6): e0198216. |
| 61 | LUO Y N, JIANG G D, YU T H, et al. ECNet is an evolutionary context-integrated deep learning framework for protein engineering[J]. Nature Communications, 2021, 12: 5743. |
| 62 | GELMAN S, FAHLBERG S A, HEINZELMAN P, et al. Neural networks to learn protein sequence-function relationships from deep mutational scanning data[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(48): e2104878118. |
| 63 | KROLL A, ENGQVIST M K M, HECKMANN D, et al. Deep learning allows genome-scale prediction of Michaelis constants from structural features[J]. PLoS Biology, 2021, 19(10): e3001402. |
| 64 | HECKMANN D, LLOYD C J, MIH N, et al. Machine learning applied to enzyme turnover numbers reveals protein structural correlates and improves metabolic models[J]. Nature Communications, 2018, 9: 5252. |
| 65 | LI F R, YUAN L, LU H Z, et al. Deep learning-based kcat prediction enables improved enzyme-constrained model reconstruction[J]. Nature Catalysis, 2022, 5(8): 662-672. |
| 66 | VASWANI A, SHAZEER N, PARMAR N, et al. Attention is all you need[C]//Proceedings of the 31st International Conference on Neural Information Processing Systems. December 4-9, 2017, Long Beach, California, USA. New York: ACM, 2017: 6000-6010. |
| 67 | COREY E J. General methods for the construction of complex molecules[J]. Pure and Applied Chemistry, 1967, 14(1): 19-38. |
| 68 | COREY E J, WIPKE W T. Computer-assisted design of complex organic syntheses[J]. Science, 1969, 166(3902): 178-192. |
| 69 | DELÉPINE B, DUIGOU T, CARBONELL P, et al. RetroPath2.0: a retrosynthesis workflow for metabolic engineers[J]. Metabolic Engineering, 2018, 45: 158-170. |
| 70 | SEGLER M H S, PREUSS M, WALLER M P. Planning chemical syntheses with deep neural networks and symbolic AI[J]. Nature, 2018, 555(7698): 604-610. |
| 71 | LIU B W, RAMSUNDAR B, KAWTHEKAR P, et al. Retrosynthetic reaction prediction using neural sequence-to-sequence models[J]. ACS Central Science, 2017, 3(10): 1103-1113. |
| 72 | CHEN B H, LI C T, DAI H J, et al. Retro*: learning retrosynthetic planning with neural guided a* search[C]//Proceedings of the 37th International Conference on Machine Learning. New York: ACM, 2020: 1608-1616. |
| 73 | 张建志, 付立豪, 唐婷, 等. 基于合成生物学策略的酶蛋白元件规模化挖掘[J]. 合成生物学, 2020, 1(3): 319-336. |
| ZHANG J Z, FU L H, TANG T, et al. Scalable mining of proteins for biocatalysis via synthetic biology[J]. Synthetic Biology Journal, 2020, 1(3): 319-336. | |
| 74 | FIGLIUZZI M, BARRAT-CHARLAIX P, WEIGT M. How pairwise coevolutionary models capture the collective residue variability in proteins?[J]. Molecular Biology and Evolution, 2018, 35(4): 1018-1027. |
| 75 | HUANG P S, BOYKEN S E, BAKER D. The coming of age of de novo protein design[J]. Nature, 2016, 537(7620): 320-327. |
| 76 | DAUPARAS J, ANISHCHENKO I, BENNETT N, et al. Robust deep learning-based protein sequence design using ProteinMPNN[J]. Science, 2022, 378(6615): 49-56. |
| 77 | LIU Y F, ZHANG L, WANG W L, et al. Rotamer-free protein sequence design based on deep learning and self-consistency[J]. Nature Computational Science, 2022, 2(7): 451-462. |
| 78 | JIANG L, ALTHOFF E A, CLEMENTE F R, et al. De novo computational design of Retro-Oldol enzymes[J]. Science, 2008, 319(5868): 1387-1391. |
| 79 | ALTSCHUL S F, GISH W, MILLER W, et al. Basic local alignment search tool[J]. Journal of Molecular Biology, 1990, 215(3): 403-410. |
| 80 | LI Z R, LIN H H, HAN L Y, et al. PROFEAT: a web server for computing structural and physicochemical features of proteins and peptides from amino acid sequence[J]. Nucleic Acids Research, 2006, 34(): W32-W37. |
| 81 | VAVRICKA C J, TAKAHASHI S, WATANABE N, et al. Machine learning discovery of missing links that mediate alternative branches to plant alkaloids[J]. Nature Communications, 2022, 13(1): 1405. |
| 82 | MISTRY J, CHUGURANSKY S, WILLIAMS L, et al. Pfam: the protein families database in 2021[J]. Nucleic Acids Research, 2021, 49(D1): D412-D419. |
| 83 | YANG K K, WU Z, ARNOLD F H. Machine-learning-guided directed evolution for protein engineering[J]. Nature Methods, 2019, 16(8): 687-694. |
| 84 | WITTMANN B J, JOHNSTON K E, WU Z, et al. Advances in machine learning for directed evolution[J]. Current Opinion in Structural Biology, 2021, 69: 11-18. |
| 85 | KLUMPP S, SCOTT M, PEDERSEN S, et al. Molecular crowding limits translation and cell growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(42): 16754-16759. |
| 86 | CHEN Y, NIELSEN J. Energy metabolism controls phenotypes by protein efficiency and allocation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(35): 17592-17597. |
| 87 | ALLEY E C, KHIMULYA G, BISWAS S, et al. Unified rational protein engineering with sequence-based deep representation learning[J]. Nature Methods, 2019, 16(12): 1315-1322. |
| 88 | BORGER S, LIEBERMEISTER W, KLIPP E. Prediction of enzyme kinetic parameters based on statistical learning[J]. Genome Informatics International Conference on Genome Informatics, 2006, 17(1): 80-87. |
| 89 | YAN S M, SHI D Q, NONG H, et al. Predicting Km values of beta-glucosidases using cellobiose as substrate[J]. Interdisciplinary Sciences: Computational Life Sciences, 2012, 4(1): 46-53. |
| 90 | LI F R, CHEN Y, ANTON M, et al. GotEnzymes: an extensive database of enzyme parameter predictions[J]. Nucleic Acids Research, 2023, 51(D1): D583-D586. |
| 91 | MACKLIN D N, AHN-HORST T A, CHOI H, et al. Simultaneous cross-evaluation of heterogeneous E. coli datasets via mechanistic simulation[J]. Science, 2020, 369(6502): eaav3751. |
| 92 | THORNBURG Z R, BIANCHI D M, BRIER T A, et al. Fundamental behaviors emerge from simulations of a living minimal cell[J]. Cell, 2022, 185(2): 345-360.e28. |
| 93 | ENGQVIST M K M. Correlating enzyme annotations with a large set of microbial growth temperatures reveals metabolic adaptations to growth at diverse temperatures[J]. BMC Microbiology, 2018, 18(1): 177. |
| 94 | LI G, HU Y T, ZRIMEC J, et al. Bayesian genome scale modelling identifies thermal determinants of yeast metabolism[J]. Nature Communications, 2021, 12: 190. |
| 95 | REMBEZA E, ENGQVIST M K M. Experimental and computational investigation of enzyme functional annotations uncovers misannotation in the EC 1.1.3.15 enzyme class[J]. PLoS Computational Biology, 2021, 17(9): e1009446. |
| 96 | RAO R, BHATTACHARYA N, THOMAS N, et al. Evaluating protein transfer learning with TAPE[J]. Advances in Neural Information Processing Systems, 2019, 32: 9689-9701. |
| 97 | RIVES A, MEIER J, SERCU T, et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(15): e2016239118. |
| 98 | JAEGER S, FULLE S, TURK S. Mol2vec: unsupervised machine learning approach with chemical intuition[J]. Journal of Chemical Information and Modeling, 2018, 58(1): 27-35. |
| 99 | UNSAL S, ATAS H, ALBAYRAK M, et al. Learning functional properties of proteins with language models[J]. Nature Machine Intelligence, 2022, 4(3): 227-245. |
| 100 | COLEY C W, ROGERS L, GREEN W H, et al. Computer-assisted retrosynthesis based on molecular similarity[J]. ACS Central Science, 2017, 3(12): 1237-1245. |
| 101 | FERRUZ N, SCHMIDT S, HÖCKER B. ProtGPT2 is a deep unsupervised language model for protein design[J]. Nature Communications, 2022, 13(1): 4348. |
| 102 | SHIN J E, RIESSELMAN A J, KOLLASCH A W, et al. Protein design and variant prediction using autoregressive generative models[J]. Nature Communications, 2021, 12: 2403. |
| 103 | REPECKA D, JAUNISKIS V, KARPUS L, et al. Expanding functional protein sequence spaces using generative adversarial networks[J]. Nature Machine Intelligence, 2021, 3(4): 324-333. |
| 104 | BISWAS S, KHIMULYA G, ALLEY E C, et al. Low-N protein engineering with data-efficient deep learning[J]. Nature Methods, 2021, 18(4): 389-396. |
| 105 | LUO S T, SU Y F, PENG X G, et al. Antigen-specific antibody design and optimization with diffusion-based generative models for protein structures[EB/OL]. bioRxiv, 2022[2022-12-01]. . |
| 106 | WANG L, TITOV A, MCGIBBON R, et al. Discovering chemistry with an ab initio nanoreactor[J]. Nature Chemistry, 2014, 6(12): 1044-1048. |
| 107 | SIMM G N, VAUCHER A C, REIHER M. Exploration of reaction pathways and chemical transformation networks[J]. The Journal of Physical Chemistry A, 2019, 123(2): 385-399. |
| 108 | WANG Y H, XU H C, ZOU J, et al. Catalytic role of carbonyl oxygens and water in selinadiene synthase[J]. Nature Catalysis, 2022, 5(2): 128-135. |
| 109 | ZANGHELLINI A, JIANG L, WOLLACOTT A M, et al. New algorithms and an in silico benchmark for computational enzyme design[J]. Protein Science, 2006, 15(12): 2785-2794. |
| 110 | LEAVER-FAY A, TYKA M, LEWIS S M, et al. Chapter nineteen-Rosetta3: an object-oriented software suite for the simulation and design of macromolecules[M]// Methods in enzymology. Pittsburgh, PA, USA: Academic Press, 2011, 487: 545-574. |
| 111 | SIEGEL J B, SMITH A L, POUST S, et al. Computational protein design enables a novel one-carbon assimilation pathway[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(12): 3704-3709. |
| 112 | VERGES A, CAMBON E, BARBE S, et al. Computer-aided engineering of a transglycosylase for the glucosylation of an unnatural disaccharide of relevance for bacterial antigen synthesis[J]. ACS Catalysis, 2015, 5(2): 1186-1198. |
| 113 | CUI Y L, WANG Y H, TIAN W Y, et al. Development of a versatile and efficient C-N lyase platform for asymmetric hydroamination via computational enzyme redesign[J]. Nature Catalysis, 2021, 4(5): 364-373. |
| 114 | ZENG T, HESS B A, ZHANG F, et al. Bio-inspired chemical space exploration of terpenoids[J]. Briefings in Bioinformatics, 2022, 23(5): bbac197. |
| 115 | ZHANG L F, HAN J Q, WANG H, et al. Deep potential molecular dynamics: a scalable model with the accuracy of quantum mechanics[J]. Physical Review Letters, 2018, 120(14): 143001. |
| 116 | HU Q N, DENG Z, HU H N, et al. RxnFinder: biochemical reaction search engines using molecular structures, molecular fragments and reaction similarity[J]. Bioinformatics, 2011, 27(17): 2465-2467. |
| [1] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [2] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [3] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [4] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [5] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [6] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [7] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [8] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [9] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [10] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [11] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [12] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [13] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| [14] | 冯金, 潘海学, 唐功利. 近十年天然产物药物的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 408-446. |
| [15] | 奚萌宇, 胡逸灵, 顾玉诚, 戈惠明. 基因组挖掘指导天然药物分子的发现[J]. 合成生物学, 2024, 5(3): 447-473. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||


