合成生物学 ›› 2021, Vol. 2 ›› Issue (6): 1030-1045.DOI: 10.12211/2096-8280.2020-096
2-苯乙醇生物合成的研究进展
严伟1, 高豪1, 蒋羽佳1, 钱秀娟1, 周杰1,2, 董维亮1,2, 章文明1,2, 信丰学1,2, 姜岷1,2
- 1.南京工业大学生物与制药工程学院,材料化学工程国家重点实验室,江苏 南京 211816
2.南京工业大学江苏先进生物与化学制造协同创新中心(SICAM),江苏 南京 211816
-
收稿日期:2020-12-31修回日期:2021-03-10出版日期:2021-12-31发布日期:2022-01-21 -
通讯作者:信丰学,姜岷 -
作者简介:严伟 (1993—),男,博士。研究方向为代谢工程及合成生物学。E-mail:15051801815@163.com信丰学 (1982—),男,博士,教授。研究方向为生物化工与生物能源。E-mail:xinfengxue@njtech.edu.cn姜岷 (1972—),男,博士,教授。研究方向为生物转化与生物催化。E-mail:jiangmin@njtech.edu.cn -
基金资助:国家重点研发计划“合成生物学”重点专项(2018YFA0902200);国家自然科学基金(22178169);江苏省自然科学基金(BK20200683)
Research progress in 2-phenylethanol production through biological processes
YAN Wei1, GAO Hao1, JIANG Yujia1, QIAN Xiujuan1, ZHOU Jie1,2, DONG Weiliang1,2, ZHANG Wenming1,2, XIN Fengxue1,2, JIANG Min1,2
- 1.State Key Laboratory of Materials-Oriented Chemical Engineering,College of Biotechnology and Pharmaceutical Engineering,Nanjing Tech University,Nanjing 211816,Jiangsu,China
2.Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM),Nanjing Tech University,Nanjing 211816,Jiangsu,China
-
Received:2020-12-31Revised:2021-03-10Online:2021-12-31Published:2022-01-21 -
Contact:XIN Fengxue, JIANG Min
摘要:
2-苯乙醇(2-PE)是一种具有玫瑰香味的重要香料化合物,广泛应用于化妆品、香水、食品等行业。传统的2-PE生产主要是从植物原料中提取或化学合成。然而,这些方法无法满足消费者对天然香料日益增长的需求。以发酵法或酶法生产的L-苯丙氨酸为前体,利用酵母细胞将其转化为2-PE,产品既符合环境友好的要求,又满足“天然”产品的定义,可以取代从玫瑰或其他植物精油中提取的天然2-PE。因此,生物法合成2-PE已经引起人们的广泛关注。本文综述了生物法合成2-PE的现状和发展前景,指出相对于传统的化学合成和天然植物提取,生物转化比较具有优越性。并对2-PE的合成途径、全局调控机制、提高2-PE产量的策略以及农工废弃物作为原料的利用进行了系统的论述。此外,本文还讨论了原位产物分离技术在2-PE生物合成中的应用。
中图分类号:
引用本文
严伟, 高豪, 蒋羽佳, 钱秀娟, 周杰, 董维亮, 章文明, 信丰学, 姜岷. 2-苯乙醇生物合成的研究进展[J]. 合成生物学, 2021, 2(6): 1030-1045.
YAN Wei, GAO Hao, JIANG Yujia, QIAN Xiujuan, ZHOU Jie, DONG Weiliang, ZHANG Wenming, XIN Fengxue, JIANG Min. Research progress in 2-phenylethanol production through biological processes[J]. Synthetic Biology Journal, 2021, 2(6): 1030-1045.
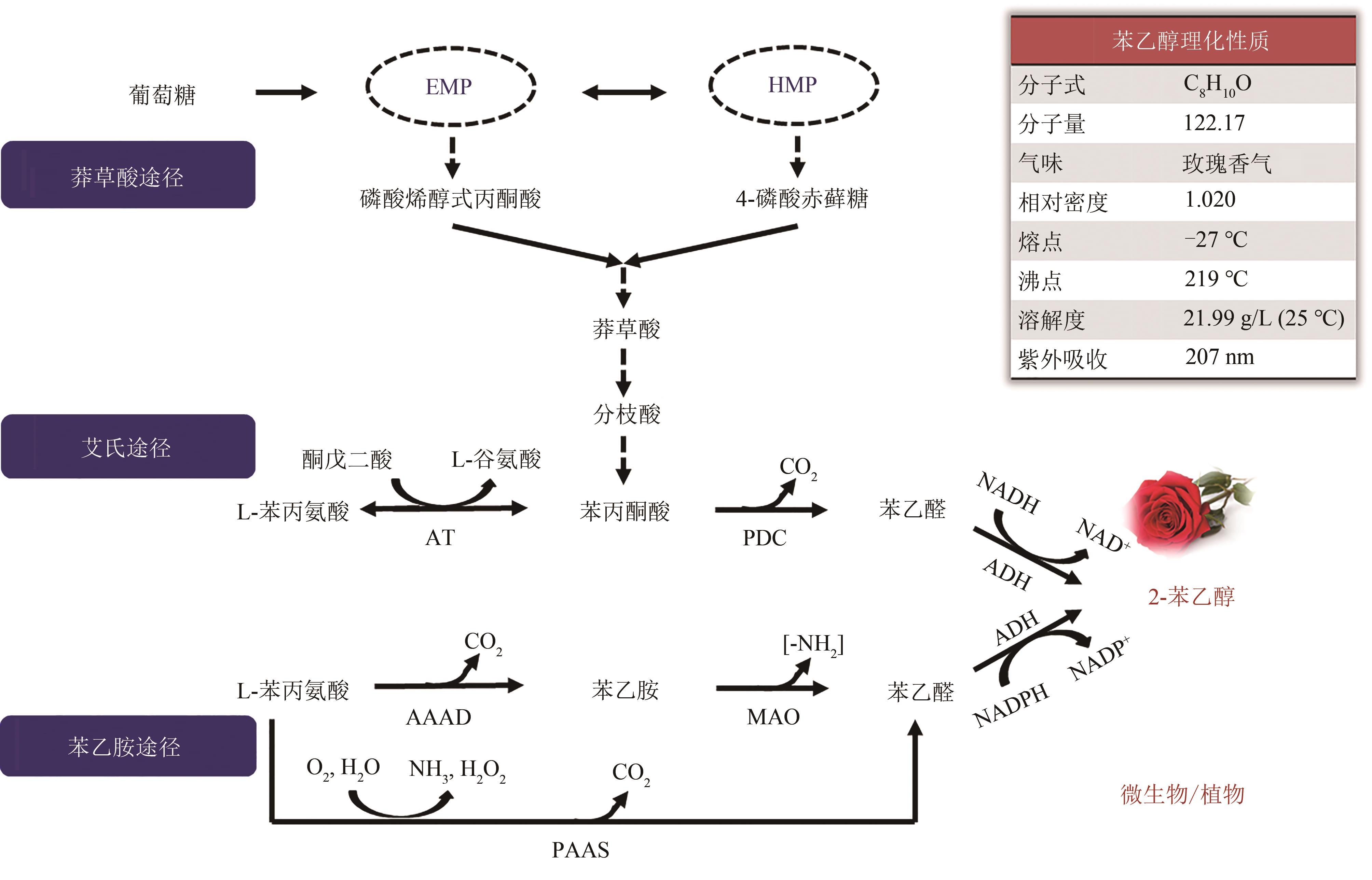
图1 2-苯乙醇的生物合成途径EMP—糖酵解途径;HMP—磷酸戊糖途径;AT—转氨酶;PDC—苯丙酮酸脱羧酶;ADH—醇脱氢酶;AAAD—芳香族氨基酸脱羧酶;MAO—单胺氧化酶;PAAS—苯乙醛合酶
Fig. 1 2-PE synthetic pathwaysEMP—glycolytic pathway; HMP—pentose phosphate pathway; AT—transaminase; PDC—phenylpyruvate decarboxylases; ADH—alcohol dehydrogenases; AAAD—aromatic amino acid decarboxylase; MAO—monoamine oxidase; PAAS—phenylacetaldehyde synthase

图2 苯乙醇生物合成的代谢网络调控(Gap1/Wap1/Agp1/Bap2/Bap3/Tat2—氨基酸通透酶;ARO9/ARO8/Bat1/Twt1/Bat2/TwT2—转氨酶;ARO10/PDC1/PDC5/PDC6/THI3—苯丙酮酸脱羧酶;Adh1/2/3/4/5—醇脱氢酶;Sfa1—醛脱氢酶)
Fig. 2 Regulatory network of 2-PE synthesis(Gap1/Wap1/Agp1/Bap2/Bap3/Tat2—amino acid permease; ARO9/ARO8/Bat1/Twt1/Bat2/TwT2—transaminase; ARO10/PDC1/PDC5/PDC6/THI3—phenylpyruvate decarboxylases; Adh1/2/3/4/5—alcohol dehydrogenases; Sfa1—formaldehyde dehydrogenase)
| 菌株 | 方法 | 条件优化 | 2-PE产量/(g/L) | 发酵体积 | 参考文献 |
|---|---|---|---|---|---|
| 产甘油假单胞菌WL2002-5 | 单因素设计 | 培养基成分、温度 | 5.0 | 3 L | [ |
| 马克思克鲁维酵母CBS600 | 遗传算法 | 培养基成分、温度 | 5.6 | 50 mL | [ |
| 酿酒酵母CWY132 | 单因素设计 | 培养基成分、接种量 | 3.52 | 2 L | [ |
| 酿酒酵母 | 单因素、正交设计、Box-Behnken 中心复合设计和响应面方法 | 培养基成分 | 4.82 | 30 mL | [ |
| 马克思克鲁维酵母 | 中心复合材料设计与响应面方法 | L-苯丙氨酸浓度、pH、温度 | 0.47 | 50 mL | [ |
| 酿酒酵母 | Plackett-Burman设计, steepest ascent 设计与Box-Behnken 设计 | 培养基成分、pH、发酵时间 | 1.55 | 50 mL | [ |
表1 基于统计设计的2-PE发酵工艺优化
Tab. 1 Statistics-based optimized fermentation processes for 2-PE production
| 菌株 | 方法 | 条件优化 | 2-PE产量/(g/L) | 发酵体积 | 参考文献 |
|---|---|---|---|---|---|
| 产甘油假单胞菌WL2002-5 | 单因素设计 | 培养基成分、温度 | 5.0 | 3 L | [ |
| 马克思克鲁维酵母CBS600 | 遗传算法 | 培养基成分、温度 | 5.6 | 50 mL | [ |
| 酿酒酵母CWY132 | 单因素设计 | 培养基成分、接种量 | 3.52 | 2 L | [ |
| 酿酒酵母 | 单因素、正交设计、Box-Behnken 中心复合设计和响应面方法 | 培养基成分 | 4.82 | 30 mL | [ |
| 马克思克鲁维酵母 | 中心复合材料设计与响应面方法 | L-苯丙氨酸浓度、pH、温度 | 0.47 | 50 mL | [ |
| 酿酒酵母 | Plackett-Burman设计, steepest ascent 设计与Box-Behnken 设计 | 培养基成分、pH、发酵时间 | 1.55 | 50 mL | [ |
| 技术 | 优势 | 劣势 | 材料 | 2-PE产量 | 参考文献 |
|---|---|---|---|---|---|
| 超临界CO2萃取 | 高效萃取力 | 剧毒,需要先去除细胞 | 超临界二氧化碳 | 可以提取90%以上的2-PE | [ |
| 液-液萃取 | 低成本,简单,高效,易扩展 | 有毒性,形成乳状液难以蒸发和去除 | 聚丙二醇1500 | 共7.5 g/L,有机相22 g/L | [ |
| 聚丙二醇1200 | 共10.2 g/L,有机相26.5 g/L | [ | |||
| 油酸 | 共12.6 g/L,有机相24 g/L | [ | |||
| 油醇 | 共3.0 g/L | [ | |||
| 菜籽油 | 共9.79 g/L,有机相18.5 g/L | [ | |||
| OMA[Tf2N], BMIM[Tf2N]和MPPyr[Tf2N] | 对照组1 g/L,OMA[Tf2N] 3 g/L,BMIM[Tf2N] 5 g/L,MPPyr[Tf2N] 3 g/L | [ | |||
| 液-固萃取 | 无乳状液,简单,高效 | 额外工作量,高成本,不易放大 | 将癸二酸二丁酯固定在聚乙烯聚合物基质中 | 共12.8 g/L,有机相20.5 g/L | [ |
| 将癸二酸二丁酯包埋于海藻酸盐基水凝胶中 | 共5.6 g/L,有机相47.2 g/L | [ | |||
| 疏水吸附 | 特异性强,操作简单,可放大,无毒,低成本,可再生 | 吸附容量低,缺乏吸附材料 | 大孔树脂 | 水相中2.7 g/L,每克水合树脂 含84 mg 2-PE | [ |
| 非极性大孔树脂D101 | 水相中3.15 g/L,每克树脂D101 含45.3 mg 2-PE | [ | |||
| 树脂HZ818 | 水相为1.6 g/L,共6.6 g/L | [ | |||
| HytrelV®8206 | 水相1.4 g/L,聚合物相97 g/L, 162 mg/g 2-PE | [ | |||
| 聚甲基丙烯酸甲酯(PMMA)微球 | 水相为1.65 g/L,聚合物相为5.4 g/L | [ | |||
| 颗粒活性炭 | 每克吸附剂182.06 mg 2-PE | [ | |||
| 有机渗透汽化 | 特异性强 | 分离能力低,成本高 | 聚辛基甲基硅氧烷 (POMS)膜 | 5.23 g/L | [ |
表2 用于2-PE生产的不同的原位产物分离(ISPR)技术
Tab. 2 Different in situ product removal (ISPR) techniques for 2-PE production
| 技术 | 优势 | 劣势 | 材料 | 2-PE产量 | 参考文献 |
|---|---|---|---|---|---|
| 超临界CO2萃取 | 高效萃取力 | 剧毒,需要先去除细胞 | 超临界二氧化碳 | 可以提取90%以上的2-PE | [ |
| 液-液萃取 | 低成本,简单,高效,易扩展 | 有毒性,形成乳状液难以蒸发和去除 | 聚丙二醇1500 | 共7.5 g/L,有机相22 g/L | [ |
| 聚丙二醇1200 | 共10.2 g/L,有机相26.5 g/L | [ | |||
| 油酸 | 共12.6 g/L,有机相24 g/L | [ | |||
| 油醇 | 共3.0 g/L | [ | |||
| 菜籽油 | 共9.79 g/L,有机相18.5 g/L | [ | |||
| OMA[Tf2N], BMIM[Tf2N]和MPPyr[Tf2N] | 对照组1 g/L,OMA[Tf2N] 3 g/L,BMIM[Tf2N] 5 g/L,MPPyr[Tf2N] 3 g/L | [ | |||
| 液-固萃取 | 无乳状液,简单,高效 | 额外工作量,高成本,不易放大 | 将癸二酸二丁酯固定在聚乙烯聚合物基质中 | 共12.8 g/L,有机相20.5 g/L | [ |
| 将癸二酸二丁酯包埋于海藻酸盐基水凝胶中 | 共5.6 g/L,有机相47.2 g/L | [ | |||
| 疏水吸附 | 特异性强,操作简单,可放大,无毒,低成本,可再生 | 吸附容量低,缺乏吸附材料 | 大孔树脂 | 水相中2.7 g/L,每克水合树脂 含84 mg 2-PE | [ |
| 非极性大孔树脂D101 | 水相中3.15 g/L,每克树脂D101 含45.3 mg 2-PE | [ | |||
| 树脂HZ818 | 水相为1.6 g/L,共6.6 g/L | [ | |||
| HytrelV®8206 | 水相1.4 g/L,聚合物相97 g/L, 162 mg/g 2-PE | [ | |||
| 聚甲基丙烯酸甲酯(PMMA)微球 | 水相为1.65 g/L,聚合物相为5.4 g/L | [ | |||
| 颗粒活性炭 | 每克吸附剂182.06 mg 2-PE | [ | |||
| 有机渗透汽化 | 特异性强 | 分离能力低,成本高 | 聚辛基甲基硅氧烷 (POMS)膜 | 5.23 g/L | [ |
| 10 | CARROLL A L, DESAI S H, ATSUMI S. Microbial production of scent and flavor compounds[J]. Current Opinion in Biotechnology, 2016, 37: 8-15. |
| 11 | ETSCHMANN M M W, SELL D, SCHRADER J,et al. Medium optimization for the production of the aroma compound 2-phenylethanol using a genetic algorithm[J]. Journal of Molecular Catalysis B Enzymatic, 2004, 29:187-193. |
| 12 | CELIŃSKA E, KUBIAK P, BIAŁAS W, et al. Yarrowia lipolytica: the novel and promising 2-phenylethanol producer[J]. Journal of Industrial Microbiology & Biotechnology, 2013, 40(3/4): 389-392. |
| 13 | MASUO S, OSADA L, ZHOU S M,et al. Aspergillus oryzae pathways that convert phenylalanine into the flavor volatile 2-phenylethanol[J]. Fungal Genetics and Biology, 2015, 77:22-30. |
| 14 | HUANG C-J, LEE S-L, C-C CHOU,et al. Production of 2-phenylethanol, a flavor ingredient, by Pichia fermentans L-5 under various culture conditions[J]. Food Research International, 2001, 34:277-282. |
| 15 | AOKI T. Enhanced formation of 2-phenylethanol in Zygosaccharomyces rouxii due to prephenate dehydrogenase deficiency[J]. Agricultural and Biological Chemistry, 1990, 54:273-274. |
| 16 | ESHKOL N, SENDOVSKI M, BAHALUL M, et al. Production of 2-phenylethanol from L-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain[J]. Journal of Applied Microbiology, 2010, 106:534-542. |
| 17 | CHREPTOWICZ K, WIELECHOWSKA M, RYBAK E, et al. Production of natural 2-phenylethanol: from biotransformation to purified product[J]. Food & Bioproducts Processing, 2016, 100:275-281. |
| 18 | YAN W, ZHANG X Y, QIAN X J, et al. Comprehensive investigations of 2-phenylethanol production by high 2-phenylethanol tolerating Meyerozyma sp. strain YLG18[J]. Enzyme and Microbial Technology, 2020,140:109629. |
| 19 | YAN W, GAO H, QIAN X J, et al. Biotechnological applications of the non-conventional yeast Meyerozyma guilliermondii [J]. Biotechnology Advances, 2021, 46:107674. |
| 20 | SERRA S, FUGANTI C, BRENNA E. Biocatalytic preparation of natural flavours and fragrances[J]. Trends in Biotechnology, 2005, 23(4): 193-198. |
| 21 | DICKINSON J R. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae [J]. Journal of Biological Chemistry, 2003, 278:8028-8034. |
| 22 | EHRLICH F. Über die bedingungen der fuselölbildung und über ihren zusammenhang mit dem eiweißaufbau der Hefe [J]. European Journal of Inorganic Chemistry, 1907, 40:1027-1047. |
| 1 | SCOGNAMIGLIO J, JONES L, LETIZIA C S, et al. Fragrance material review on phenylethyl alcohol[J]. Food and Chemical Toxicology, 2012, 50:S224-S239. |
| 2 | BIAECKA F E, KRZYCZKOWSKA J, STOLARZEWICZ I, et al. Synthesis of 2-phenylethyl acetate in the presence of Yarrowia lipolytica KKP 379 biomass[J]. Journal of Molecular Catalysis:B, Enzymatic, 2012, 74:241-245. |
| 3 | ÇELIK D, BAYRAKTAR E. Biotransformation of 2-phenylethanol to phenylacetaldehyde in a two-phase fed-batch system[J]. Biochemical Engineering Journal, 2004, 17:5-13. |
| 4 | AUBERT C, BAUMANN S, ARGUEL H. Optimization of the analysis of flavor volatile compounds by liquid-liquid microextraction (LLME). Application to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes[J]. Journal of Agricultural and Food Chemistry, 2005, 53(23):8881-8895. |
| 5 | HORBOWICZ M, WICZKOWSKI W, SAWICKI T, et al. Methyl jasmonate stimulates biosynthesis of 2-phenylethylamine, phenylacetic acid and 2-phenylethanol in seedlings of common buckwheat[J]. Acta Biochimica Polonica, 2015, 62(2): 235-240. |
| 6 | KOVACHEVA N, RUSANOV K, ATANASSOV I. Industrial cultivation of oil bearing rose and rose oil production in Bulgaria during 21st century, directions and challenges[J]. Biotechnology & Biotechnological Equipment, 2010, 24(2): 1793-1798. |
| 7 | KIM B, CHO B-R, J-S HAHN, et al. Biotechnology, bioengineering, metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway[J]. Biotechnology and Bioengineering, 2013, 111:115-124. |
| 8 | ME J F. Production of β-phenylethanol by bioconversion[J]. Microbiology, 2005, 32:114-118. |
| 9 | KIRM I, MEDINA F, SUEIRAS J E, et al. Hydrogenation of styrene oxide in the presence of supported platinum catalysts to produce 2-phenylethanol[J]. Journal of Molecular Catalysis. A, Chemical, 2007, 261(1):98-103. |
| 23 | IRAQUI I, VISSERS S, Bruno André, et al. Transcriptional induction by aromatic amino acids in Saccharomyces cerevisiae [J]. Molecular Cell Biology, 1999, 19:3360-3371. |
| 24 | LEE K, HAHN J S. Interplay of Aro80 and GATA activators in regulation of genes for catabolism of aromatic amino acids in Saccharomyces cerevisiae [J]. Molecular Microbiology, 2013, 88(6):1120-1134. |
| 25 | WUSTER A, BABU M M. Transcriptional control of the quorum sensing response in yeast[J]. Molecular BioSystems, 2010, 6(1):134-141. |
| 26 | SCHÜLLER H J. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae [J]. Current Genetics, 2003, 43(3):139-160. |
| 27 | WALTHER K, SCHÜLLER H J. Adr1 and Cat8 synergistically activate the glucose-regulated alcohol dehydrogenase gene ADH2 of the yeast Saccharomyces cerevisiae [J]. Microbiology and Molecular Biology Reviews, 2001, 147:2037-2044. |
| 28 | WANG Z Y, BAI X J, GUO X N, et al. Regulation of crucial enzymes and transcription factors on 2-phenylethanol biosynthesis via Ehrlich pathway in Saccharomyces cerevisiae [J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(1):129-139. |
| 29 | RAI R, TATE J J, GEORIS I, et al. Constitutive and nitrogen catabolite repression-sensitive production of Gat1 isoforms[J]. The Journal of Biological Chemistry, 2014, 289(5): 2918-2933.[PubMed] |
| 30 | LJUNGDAHL P O, DAIGNAN-FORNIER B. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae [J]. Genetics, 2012, 190(3): 885-929. |
| 31 | SALMON J M, BARRE P. Improvement of nitrogen assimilation and fermentation kinetics under enological conditions by derepression of alternative nitrogen-assimilatory pathways in an industrial Saccharomyces cerevisiae strain[J]. Applied & Environmental Microbiology, 1998, 64(10):3831-3837. |
| 32 | CHEN H, FINK G R. Feedback control of morphogenesis in fungi by aromatic alcohols[J]. Genes & Development, 2006, 20(9): 1150-1161. |
| 33 | SHEN L, NISHIMURA Y, MATSUDA F, et al. Overexpressing enzymes of the Ehrlich pathway and deleting genes of the competing pathway in Saccharomyces cerevisiae for increasing 2-phenylethanol production from glucose[J]. Journal of Bioscience and Bioengineering, 2016, 122(1):34-39. |
| 34 | HERRMANN K M. The shikimate pathway as an entry to aromatic secondary metabolism[J]. Plant Physiology, 1995, 107(1): 7-12. |
| 35 | TIEMAN D, TAYLOR M, SCHAUER N, et al. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(21): 8287-8292. |
| 36 | YIN S, ZHOU H, XIAO X, et al. Improving 2-phenylethanol production via Ehrlich pathway using genetic engineered Saccharomyces cerevisiae strains[J]. Current Microbiology, 2015, 70(5):762-767. |
| 37 | CHEN X, WANG Z, GUO X, et al. Regulation of general amino acid permeases Gap1p, GATA transcription factors Gln3p and Gat1p on 2-phenylethanol biosynthesis via Ehrlich pathway[J]. Journal of Biotechnology, 2017, 242: 83-91. |
| 38 | WANG Z, JIANG M, GUO X, et al. Reconstruction of metabolic module with improved promoter strength increases the productivity of 2-phenylethanol in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2018, 17(1):60. |
| 39 | KIM T Y, LEE S W, OH M K. Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus [J]. Enzyme & Microbial Technology, 2014, 61/62: 44-47. |
| 40 | HASSING E J, DE GROOT P A, MARQUENIE V R, et al. Connecting central carbon and aromatic amino acid metabolisms to improve de novo 2-phenylethanol production in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2019, 56: 165-180. |
| 41 | GU Y, MA J B, ZHU Y L, et al. Engineering Yarrowia lipolytica as a chassis for de novo synthesis of five aromatic-derived natural products and chemicals[J]. ACS Synthetic Biology, 2020, 9(8): 2096-2106. |
| 42 | WANG Y Q, ZHANG H, LU X Y, et al. Advances in 2-phenylethanol production from engineered microorganisms[J]. Biotechnology Advances, 2019, 37(3):403-409. |
| 43 | GUO D, ZHANG L, PAN H, et al. Metabolic engineering of Escherichia coli for production of 2-phenylethylacetate from L-phenylalanine[J]. Microbiology, 2017, 6 (4): 1-5. |
| 44 | ATSUMI S, HANAI T, LIAO J C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels[J]. Nature, 2008, 451(7174): 86-89. |
| 45 | HWANG J Y, PARK J, SEO J H, et al. Simultaneous synthesis of 2-phenylethanol and L-homophenylalanine using aromatic transaminase with yeast Ehrlich pathway[J]. Biotechnology and Bioengineering, 2009, 102(5):1323–1329. |
| 46 | WANG P C, YANG X W, LIN B X, et al. Cofactor self-sufficient whole-cell biocatalysts for the production of 2-phenylethanol[J]. Metabolic Engineering, 2017, 44:143-149. |
| 47 | GUO D Y, ZHANG L H, KONG S J, et al. Metabolic engineering of Escherichia coli for production of 2-phenylethanol and 2-phenylethyl acetate from glucose[J]. Journal of Agricultural and Food Chemistry,2018, 66(23):5886-5891. |
| 48 | KOMA D, YAMANAKA H, MORIYOSHI K, et al. Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway[J]. Applied & Environmental Microbiology, 2012, 78(17):6203-6216. |
| 49 | KANG Z, ZHANG C, DU G, et al. Biotechnology, metabolic engineering of Escherichia coli for production of 2-phenylethanol from renewable glucose[J]. Applied Biochemistry and Biotechnology, 2014, 172:2012-2021. |
| 50 | WANG H, DONG Q, GUAN A, et al. Synergistic inhibition effect of 2-phenylethanol and ethanol on bioproduction of natural 2-phenylethanol by Saccharomyces cerevisiae and process enhancement[J]. Journal of Bioscience and Bioengineering, 2011, 112(1): 26-31. |
| 51 | LU X Y, WANG Y Q, ZONG H, et al. Bioconversion of L-phenylalanine to 2-phenylethanol by the novel stress-tolerant yeast Candida glycerinogenes WL2002-5[J]. Bioengineered, 2016, 7(6): 418-423. |
| 52 | 崔志峰,车智博,杨霄,等. 2-苯乙醇耐受性高产酵母菌株的选育[J]. 浙江工业大学学报,2008,36:427-431. |
| CUI Z F, CHE Z B, YANG X, et al. Screening of the Saccharomyces cerevisiae strain for resistance and higher production of 2-phenylethanol[J]. Journal of Zhejiang University of Technology, 2008, 36:427-431. | |
| 53 | MEI J F. Breeding of yeast strain for production of 2-phenylethanol by biotransformation[J]. Food and Fermentation Industries, 2007, 33:22-24. |
| 54 | WELKER S, RUDOLPH B, FRENZEL E, et al. Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function[J]. Molecular Cell, 2010, 39:507-520. |
| 55 | ETSCHMANN M, BLUEMKE W, SELL D, et al. Biotechnological production of 2-phenylethanol[J]. Applied Microbiology and Biotechnology, 2002, 59(1): 1-8. |
| 56 | CUI Z F, YANG X, SHEN Q J, et al. Optimisation of biotransformation conditions for production of 2-phenylethanol by a Saccharomyces cerevisiae CWY132 mutant[J]. Natural Product Research, 2011, 25(7): 754-759. |
| 57 | BARBOSA C, FALCO V, MENDES-FAIA A, et al. Nitrogen addition influences formation of aroma compounds, volatile acidity and ethanol in nitrogen deficient media fermented by Saccharomyces cerevisiae wine strains[J]. Journal of Bioscience & Bioengineering, 2009, 108:99-104. |
| 58 | GETHINS L, GUNESER O, DEMIRKOL A, et al. Influence of carbon and nitrogen source on production of volatile fragrance and flavour metabolites by the yeast Kluyveromyces marxianus [J]. Yeast, 2015, 32(1): 67-76. |
| 59 | CHUNG H, LEE S L, CHOU C C. Production and molar yield of 2-phenylethanol by Pichia fermentans L-5 as affected by some medium components[J]. Journal of Bioscience and Bioengineering, 2000, 90:142-148. |
| 60 | ETSCHMANN M M W, SCHRADER J. Production of 2-phenylethanol and 2-phenylethylacetate from L-phenylalanine by coupling whole-cell biocatalysis with organophilic pervaporation[J]. Biotechnology & Bioengineering, 2010, 92:624-634. |
| 61 | MEI J F, LU Z. Biocatalytic synthesis of 2-phenylethanol by yeast cells[J]. Chinese Journal of Catalysis, 2007, 28:993-998. |
| 62 | SAERENS S M G, VERBELEN P J, VANBENEDEN N, et al. Monitoring the influence of high-gravity brewing and fermentation temperature on flavour formation by analysis of gene expression levels in brewing yeast[J]. Applied Microbiology & Biotechnology, 2008, 80(6): 1039-1051. |
| 63 | MU L, LIU X. Production of 2-phenylethanol by microbial mixed cultures allows resource recovery of cane molasses wastewater[J]. Fresenius Environmental Bulletin, 2014, 23:1356-1366. |
| 64 | GHOSH S, KEBAARA B W, ATKIN A L, et al. Regulation of aromatic alcohol production in Candida albicans [J]. Applied and Environmental Microbiology, 2008, 74(23):7211-7218. |
| 65 | MEI J, HANG M. Enhanced biotransformation of l-phenylalanine to 2-phenylethanol using an in situ product adsorption technique[J]. Process Biochemistry, 2009, 44:886-890. |
| 66 | BOER V M, TAI S L, VURALHAN Z, et al. Transcriptional responses of Saccharomyces cerevisiae to preferred and nonpreferred nitrogen sources in glucose-limited chemostat cultures[J]. FEMS Yeast Research, 2007, 7(4): 604-620.[LinkOut] |
| 67 | VURALHAN Z, MORAIS M A, TAI S L, et al. Identification and characterization of phenylpyruvate decarboxylase genes in Saccharomyces cerevisiae [J]. Applied and Environmental Microbiology, 2003, 69(8):4534-4541. |
| 68 | TIAN X, YE R, WANG J W, et al. Effects of aroma quality on the biotransformation of natural 2-phenylethanol produced using ascorbic acid[J]. Electronic Journal of Biotechnology, 2015, 18:286-290. |
| 69 | STARK D, MUNCH T, SONNLEITNER B, et al. Bioconversion of 2-phenylethanol from L-phenylalanine by Saccharomyces cerevisiae [J]. Biotechnology Progress, 2002, 18:514-523. |
| 70 | RONG S F, DING B M, ZHANG X L, et al. Enhanced biotransformation of 2-phenylethanol with ethanol oxidation in a solid-liquid two-phase system by active dry yeast[J]. Current Microbiology, 2011, 63(5): 503-509. |
| 71 | GARAVAGLIA J, FLÔRES S H, PIZZOLATO T M, et al. Bioconversion of L-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures[J]. World Journal of Microbiology & Biotechnology, 2007, 23(9): 1273-1279. |
| 72 | WANG Q, SONG Y F, JIN Y R, et al. Biosynthesis of 2-phenylethanol using tobacco waste as feedstock[J]. Biocatalysis and Biotransformation, 2013, 31(6): 292-298. |
| 73 | SARKAR N, GHOSH S K, BANNERJEE S, et al. Bioethanol production from agricultural wastes: An overview[J]. Renewable Energy, 2012, 37:19-27. |
| 74 | ETSCHMANN M M W, SELL D, SCHRADER J. Screening of yeasts for the production of the aroma compound 2-phenylethanol in a molasses-based medium[J]. Biotechnology Letters, 2003, 25(7): 531-536. |
| 75 | MARTÍNEZ O, SÁNCHEZ A, FONT X, et al. Bioproduction of 2-phenylethanol and 2-phenethyl acetate by Kluyveromyces marxianus through the solid-state fermentation of sugarcane bagasse[J]. Applied Microbiology & Biotechnology, 2018, 102(11): 4703-4716. |
| 76 | CHREPTOWICZ K, STERNICKA M K, KOWALSKA P D, et al. Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media[J]. Letters in Applied Microbiology, 2018, 66(2): 153-160. |
| 77 | OLIVEIRA, GOMES S D, SENE L, et al. Production of 2-phenylethanol by Geotrichum fragrans, Saccharomyces cerevisiae and Kluyveromyces marxianus in cassava wastewater[J]. Journal of Food Agriculture and Environment, 2013, 11:158-164. |
| 78 | GÜNEŞER O, DEMIRKOL A, KARAGÜL YÜCEER Y, et al. Bioflavour production from tomato and pepper pomaces by Kluyveromyces marxianus and Debaryomyces hansenii [J]. Bioprocess & Biosystems Engineering, 2015, 38(6): 1143-1155. |
| 79 | OLIVEIRA S M M, GOMES S D, SENE L, et al. Production of natural aroma by yeast in wastewater of cassava starch industry[J]. Engenharia Agrícola, 2015, 35:721-732. |
| 80 | SIKKEMA J, DE BONT J A, POOLMAN B. Mechanisms of membrane toxicity of hydrocarbons[J]. Microbiological Reviews, 1995, 59(2): 201-222. |
| 81 | GAO F, DAUGULIS A J. Bioproduction of the aroma compound 2-phenylethanol in a solid-liquid two-phase partitioning bioreactor system by Kluyveromyces marxianus [J]. Biotechnology & Bioengineering, 2009, 104(2): 332-339. |
| 82 | HUA D L, LIANG X H, CHE C C, et al. Bioconversion of L-phenylalanine to 2-phenylethanol using polypropylene glycol 1500[J]. Asian Journal of Chemistry, 2013, 25:5951-5955. |
| 83 | SERP D, STOCKAR U V, MARISON J B. Bioengineering, Enhancement of 2-phenylethanol productivity by Saccharomyces cerevisiae in two-phase fed-batch fermentations using solvent immobilization[J]. Biotechnology & Bioengineering, 2010, 82:103-110. |
| 84 | CHREPTOWICZ K, MIERZEJEWSKA J. Enhanced bioproduction of 2-phenylethanol in a biphasic system with rapeseed oil[J]. New Biotechnology, 2018, 42: 56-61. |
| 85 | MIHAL M, MARKOS J. Investigation of 2-phenylethanol production in fed-batch hybrid bioreactor: membrane extraction and microfiltration[J]. Purification Technology, 2012, 95:126-135. |
| 86 | QUIJANO G, COUVERT A, AMRANE A. Ionic liquids: applications and future trends in bioreactor technology[J]. Bioresource Technology, 2010, 101(23): 8923-8930. |
| 87 | SENDOVSKI M, NIR N, FISHMAN A. Bioproduction of 2-phenylethanol in a biphasic ionic liquid aqueous system[J]. Journal of Agricultural and Food Chemistry, 2010, 58(4): 2260-2265. |
| 88 | KROLIKOWSKI M, PACHLA J, RAMJUGERNATH D, et al. Extraction of 2-phenylethanol (PEA) from aqueous phases using tetracyanoborate-based ionic liquids[J]. Journal of Molecular Liquids, 2016, 224:1124-1130. |
| 89 | OKUNIEESKA P, DOMANSKA U, WICKOWSKI M, et al. Recovery of 2-phenylethanol from aqueous solutions of biosynthesis using ionic liquids[J]. Separation and Purification Technology, 2017, 188:530-538. |
| 90 | STARK D, ZALA D, MUNCH T, et al. Inhibition aspects of the bioconversion of l-phenylalanine to 2-phenylethanol by Saccharomyces cerevisiae [J]. Enzyme and Microbial Technology, 2003, 32:212-223. |
| 91 | SOTO M L, MOURE A, DOMINGUEZ H, et al. Recovery, concentration and purification of phenolic compounds by adsorption: a review[J]. Journal of Food Engineering, 2011, 105:1-27. |
| 92 | HUA D L, LIN S, LI Y F, et al. Enhanced 2-phenylethanol production from L-phenylalanine via in situ product adsorption[J]. Biocatalysis and Biotransformation, 2010, 28(4): 259-266. |
| 93 | WANG H, DONG Q, MENG C, et al. A continuous and adsorptive bioprocess for efficient production of the natural aroma chemical 2-phenylethanol with yeast[J]. Enzyme & Microbial Technology, 2011, 48(4/5): 404-407. |
| 94 | FABRE, FLAVORIST. Extraction of 2-phenylethyl alcohol:by techniques such as adsorption, inclusion, supercritical CO2, liquid-liquid and membrane separations[J]. Perfumer & Flavorist, 1996, 21:27-40. |
| 95 | LIPNIZKI F, HAUSMANNS S, TEN P K, et al. Organophilic pervaporation: prospects and performance[J]. Chemical Engineering Journal, 1999, 73:113-129. |
| 96 | JI H B, LONG Q P, CHEN H Y, et al. β-Cyclodextrin inclusive interaction driven separation of organic compounds[J]. AIChE Journal, 2010, 57:2341-2352. |
| 97 | HERRERO M, MENDIOLA J A, CIFUENTES A, et al. Supercritical fluid extraction: recent advances and applications[J]. Journal of Chromatography A, 2010, 1217(16): 2495-2511. |
| 98 | TANIGUCHI M, TSUJI T, SHIBATA M, et al. Extraction of oils from wheat germ with supercritical carbon dioxide[J]. Journal of the Agricultural Chemical Society of Japan, 1985, 49:2367-2372. |
| 99 | REVERCHON E, PORTA G D, GORGOGLIONE D J F. Supercritical CO2 extraction of volatile oil from rose concrete[J]. Flavour and Fragrance Journal, 1997, 12:37-41. |
| 100 | FABRE C E, CONDORET J S, MARTY A J B. Bioengineering, extractive fermentation of aroma with supercritical CO2 [J]. Biotechnology & Bioengineering, 2015, 64:392-400. |
| 101 | ETSCHMANN M M W, SCHRADER J. An aqueous-organic two-phase bioprocess for efficient production of the natural aroma chemicals 2-phenylethanol and 2-phenylethylacetate with yeast[J]. Applied Microbiology and Biotechnology, 2006, 71(4): 440-443. |
| 102 | STARK D, KORNMANN H, MÜNCH T, et al. Novel type of in situ extraction: Use of solvent containing microcapsules for the bioconversion of 2-phenylethanol from L-phenylalanine by Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 2003, 83(4): 376-385. |
| 103 | ACHMON Y, GOLDSHTEIN J, MARGEL S, et al. Hydrophobic microspheres for in situ removal of 2-phenylethanol from yeast fermentation[J]. Journal of Microencapsulation, 2011, 28(7): 628-638. |
| 104 | CARPINE D, DAGOSTIN J L A, SILVA V R D, et al. Adsorption of volatile aroma compound 2-phenylethanol from synthetic solution onto granular activated carbon in batch and continuous modes[J]. Journal of Food Engineering, 2013, 117:370-377. |
| 105 | HUA D, XU P. Recent advances in biotechnological production of 2-phenylethanol[J]. Biotechnology Advances, 2011, 29(6): 654-660. |
| [1] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [2] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [3] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [4] | 赵亮, 李振帅, 付丽平, 吕明, 王士安, 张全, 刘立成, 李福利, 刘自勇. 生物转化一碳化合物原料产油脂与单细胞蛋白研究进展[J]. 合成生物学, 2024, 5(6): 1300-1318. |
| [5] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [6] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [7] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [8] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [9] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [10] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [11] | 禹伟, 高教琪, 周雍进. 一碳生物转化合成有机酸的研究进展[J]. 合成生物学, 2024, 5(5): 1169-1188. |
| [12] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [13] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [14] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [15] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

