合成生物学 ›› 2022, Vol. 3 ›› Issue (3): 545-566.DOI: 10.12211/2096-8280.2021-102
Fe/α-酮戊二酸依赖型卤化酶在绿色卤化反应中的研究进展
王汇滨, 车昌丽, 游松
- 沈阳药科大学生命科学与生物制药学院,辽宁 沈阳 110016
-
收稿日期:2021-11-10修回日期:2021-11-28出版日期:2022-06-30发布日期:2022-07-13 -
通讯作者:游松 -
作者简介:王汇滨 (1998—),男,硕士研究生。研究方向为天然产物生物合成机制。 E-mail:huibin0918@163.com游松 (1963—),男,博士,教授,博士生导师。研究方向为生物催化与生物转化。 E-mail:yousong206@aliyun.com -
基金资助:国家重点研发计划“绿色生物制造”项目(2021YFC2102004)
Recent advances of enzymatic synthesis of organohalogens catalyzed by Fe/αKG-dependent halogenases
WANG Huibin, CHE Changli, YOU Song
- School of Life Sciences and Biopharmaceutical Sciences,Shenyang Pharmaceutical University,Shenyang 110016,Liaoning,China
-
Received:2021-11-10Revised:2021-11-28Online:2022-06-30Published:2022-07-13 -
Contact:YOU Song
摘要:
将卤素原子引入药物、农药等小分子中可以有效提升其生物活性,并且碳卤键可用于有机合成后期功能化反应。Fe/α-酮戊二酸(α-ketoglutaric acid,αKG)依赖型卤化酶可以高立体选择性和区域选择性催化卤素原子引入到未经活化的sp3杂化碳中心。大自然是最伟大的化学家,本文遵循从学习自然到改造自然的逻辑顺序,首先介绍Fe/αKG依赖型卤化酶的发现历程,之后分别总结天然产物生物合成途径中的载体依赖型和独立型Fe/αKG依赖型卤化酶,进一步分析Fe/αKG依赖型卤化酶的结构特征以及基于蛋白质工程等方法改造扩展其底物谱并拓展新的反应类型,最后从新酶的挖掘与表征、酶催化活性的提升、酶区域选择性的控制、酶反应类型的拓展、人工生物合成途径的创建等5方面进行展望,丰富对Fe/αKG依赖型卤化酶的催化机制、底物范围和反应杂泛性的相关认识,为后续合成生物学的应用研究奠定酶学基础。
中图分类号:
引用本文
王汇滨, 车昌丽, 游松. Fe/α-酮戊二酸依赖型卤化酶在绿色卤化反应中的研究进展[J]. 合成生物学, 2022, 3(3): 545-566.
WANG Huibin, CHE Changli, YOU Song. Recent advances of enzymatic synthesis of organohalogens catalyzed by Fe/αKG-dependent halogenases[J]. Synthetic Biology Journal, 2022, 3(3): 545-566.

图2 Fe/αKG依赖型卤化酶序列一致性比对结果(利用MEGAX Version 10.2.4软件进行多重序列比对并通过Clustal 2.1程序分析序列一致性)
Fig. 2 Sequence identity matrix of Fe/αKG-dependent halogenases (Multiple sequence alignments were created by MEGAX Version 10.2.4 and the similarity percentage of the sequence identity matrix was analyzed by Clustal 2.1).

图3 载体依赖型Fe/αKG依赖型卤化酶参与含卤天然产物的生物合成途径
Fig. 3 Carrier-dependent Fe/αKG-dependent halogenases involved in the biosynthetic pathway of halogen-containing natural products

图4 载体依赖型Fe/αKG依赖型卤化酶参与催化含有环丙烷结构天然产物的生物合成途径
Fig. 4 Carrier-dependent Fe/αKG-dependent halogenases involved in the biosynthetic pathway of halogen-containing natural products featuring cyclopropane scaffold
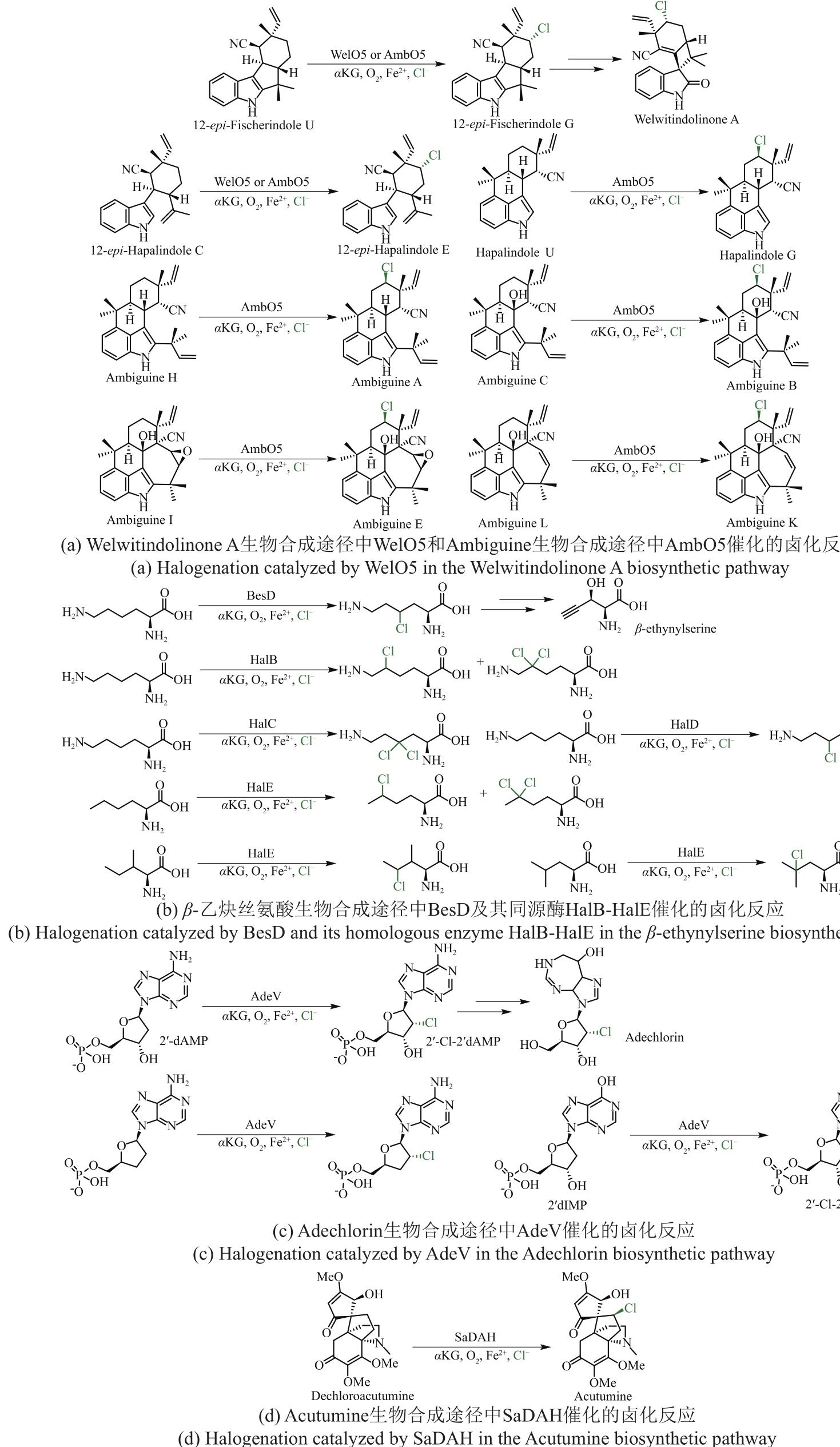
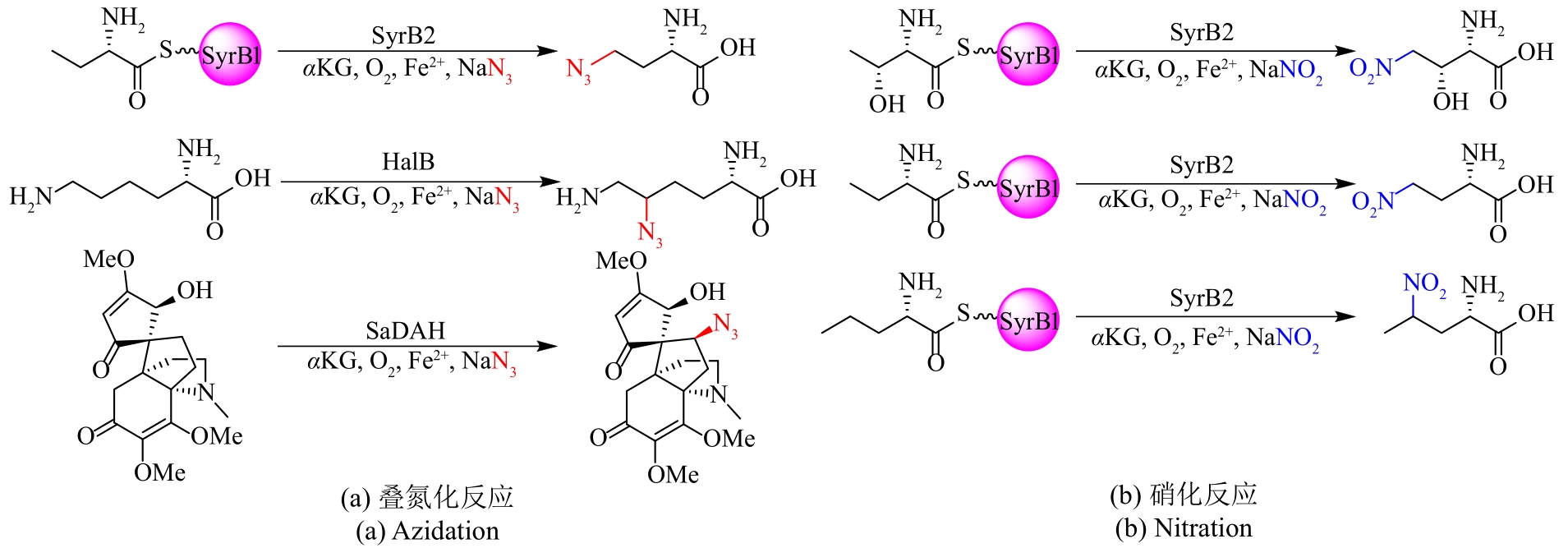
图5 独立型Fe/αKG依赖型卤化酶参与含卤天然产物的生物合成途径
Fig. 5 Non-carrier-dependent Fe/αKG-dependent halogenases involved in the biosynthetic pathway of halogen-containing natural products
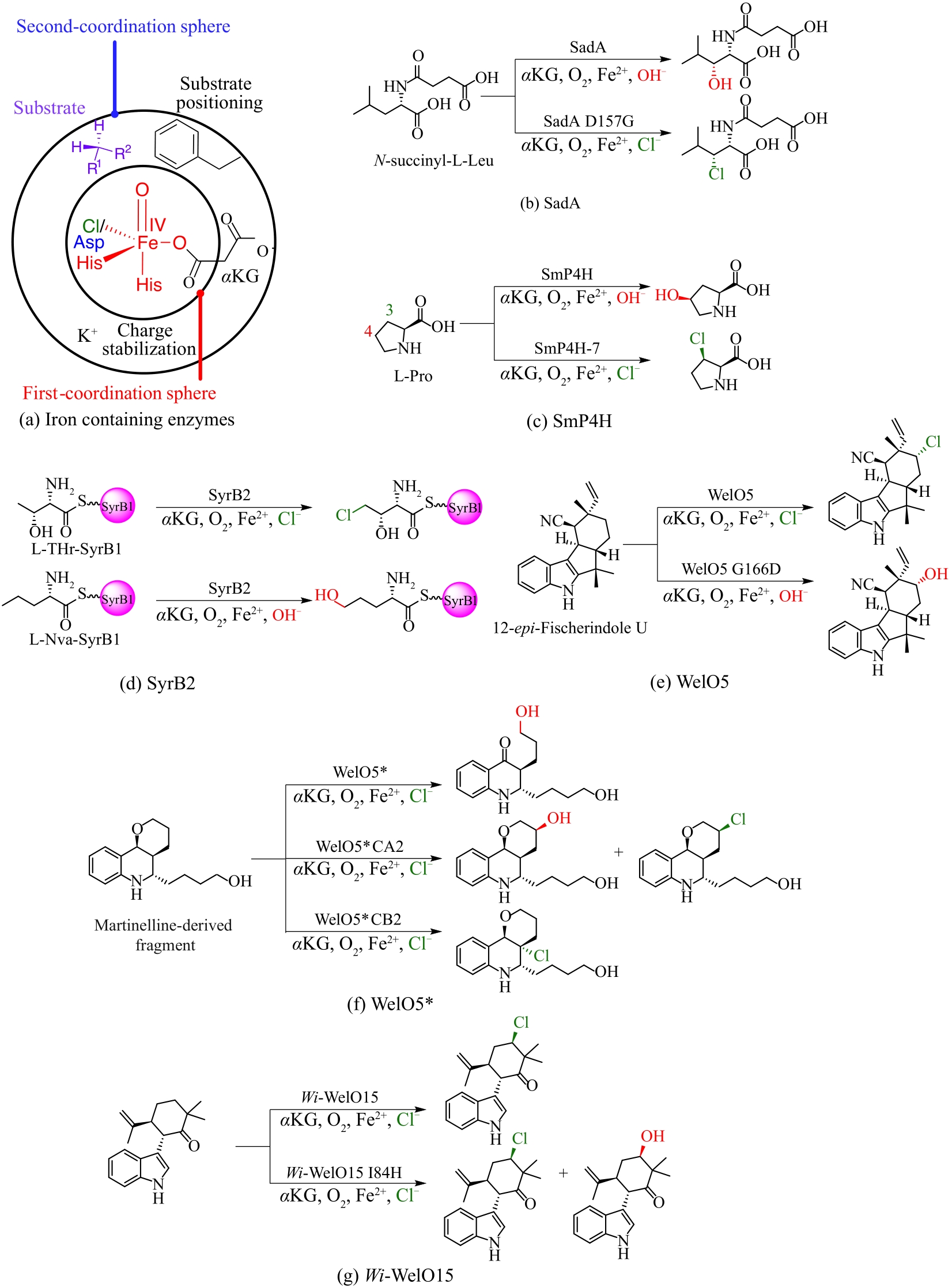
图10 Fe/αKG依赖型卤化酶和羟化酶功能互换实例(a)重塑Fe/αKG依赖型酶二级配位球的策略;(b)工程化改造羟化酶SadA催化卤化反应;(c)工程化改造羟化酶SmP4H催化卤化反应;(d)卤化酶SyrB2分别催化卤化反应和羟化反应;(e)工程化改造具有杂泛性的卤化酶WelO5*分别区域选择性催化羟化和卤化反应;(f)工程化改造卤化酶WelO5催化羟化反应;(g)工程化改造卤化酶Wi-WelO15催化羟化反应
Fig. 10 Function swap examples of Fe/αKG-dependent halogenases and hydroxylases(a) Strategies to reshape the second sphere region of Fe/αKG-dependent enzymes; (b) Engineering the hydroxylase SadA for halogenation; (c) Engineering the hydroxylase SmP4H for halogenation; (d) Halogenation reaction and hydroxylation catalyzed by halogenase SyrB2, respectively; (e) Engineering the promiscuous hydroxylase WelO5* for hydroxylation and halogenation, respectively; (f) Engineering the halogenase WelO5 for hydroxylation; (g) Engineering the halogenase Wi-WelO15 for hydroxylation.

图12 合成生物学理念指导下的Fe/αKG依赖型卤化酶工程化改造并整合至微生物细胞工厂
Fig. 12 Engineering of Fe/αKG-dependent halogenases and their adaption in microbial chemical factories under the guidance of the theory of synthetic biology
| 1 | NEUMANN C S, FUJIMORI D G, WALSH C T. Halogenation strategies in natural product biosynthesis[J]. Chemistry & Biology, 2008, 15(2): 99-109. |
| 2 | JESCHKE P. The unique role of halogen substituents in the design of modern agrochemicals[J]. Pest Management Science, 2010, 66(1): 10-27. |
| 3 | SMITH B R, EASTMAN C M, NJARDARSON J T. Beyond C, H, O, and N! Analysis of the elemental composition of US FDA approved drug architectures[J]. Journal of Medicinal Chemistry, 2014, 57(23): 9764-9773. |
| 4 | XU Z J, YANG Z, LIU Y T, et al. Halogen bond: its role beyond drug-target binding affinity for drug discovery and development[J]. Journal of Chemical Information and Modeling, 2014, 54(1): 69-78. |
| 5 | WILCKEN R, ZIMMERMANN M O, LANGE A, et al. Principles and applications of halogen bonding in medicinal chemistry and chemical biology[J]. Journal of Medicinal Chemistry, 2013, 56(4): 1363-1388. |
| 6 | CHUNG W J, VANDERWAL C D. Stereoselective halogenation in natural product synthesis[J]. Angewandte Chemie International Edition, 2016, 55(14): 4396-4434. |
| 7 | BLAKEMORE D C, CASTRO L, CHURCHER I, et al. Organic synthesis provides opportunities to transform drug discovery[J]. Nature Chemistry, 2018, 10(4): 383-394. |
| 8 | BERGER G, SOUBHYE J, MEYER F. Halogen bonding in polymer science: from crystal engineering to functional supramolecular polymers and materials[J]. Polymer Chemistry, 2015, 6(19): 3559-3580. |
| 9 | BERGER G, FRANGVILLE P, MEYER F. Halogen bonding for molecular recognition: new developments in materials and biological sciences[J]. Chemical Communications, 2020, 56(37): 4970-4981. |
| 10 | LIU X, GUO Z F, CHE Y, et al. Pillared metal-organic framework initiating intermolecular atom-transfer radical addition via visible-light-induced electron transfer activation of haloalkanes[J]. ACS Applied Materials & Interfaces, 2021, 13(29): 34114-34123. |
| 11 | ALONSO F, BELETSKAYA I P, YUS M. Metal-mediated reductive hydrodehalogenation of organic halides[J]. Chemical Reviews, 2002, 102(11): 4009-4091. |
| 12 | SCHMIDT R, STOLLE A, ONDRUSCHKA B. Aromatic substitution in ball mills: formation of aryl chlorides and bromides using potassium peroxomonosulfate and NaX[J]. Green Chemistry, 2012, 14(6): 1673. |
| 13 | FAWCETT A, KELLER M J, HERRERA Z, et al. Site selective chlorination of C(sp3)–H bonds suitable for late-stage functionalization[J]. Angewandte Chemie International Edition, 2021, 60(15): 8276-8283. |
| 14 | FRALEY A E, SHERMAN D H. Halogenase engineering and its utility in medicinal chemistry[J]. Bioorganic & Medicinal Chemistry Letters, 2018, 28(11): 1992-1999. |
| 15 | GKOTSI D S, DHALIWAL J, MCLACHLAN M M, et al. Halogenases: powerful tools for biocatalysis (mechanisms applications and scope)[J]. Current Opinion in Chemical Biology, 2018, 43: 119-126. |
| 16 | LATHAM J, BRANDENBURGER E, SHEPHERD S A, et al. Development of halogenase enzymes for use in synthesis[J]. Chemical Reviews, 2018, 118(1): 232-269. |
| 17 | AGARWAL V, MILES Z D, WINTER J M, et al. Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse[J]. Chemical Reviews, 2017, 117(8): 5619-5674. |
| 18 | CROWE C, MOLYNEUX S, SHARMA S V, et al. Halogenases: a palette of emerging opportunities for synthetic biology-synthetic chemistry and C—H functionalisation[J]. Chemical Society Reviews, 2021, 50(17): 9443-9481. |
| 19 | MENON B R K, RICHMOND D, MENON N. Halogenases for biosynthetic pathway engineering: toward new routes to naturals and non-naturals[J]. Catalysis Reviews, 2020: 1-59. |
| 20 | BUTLER A, SANDY M. Mechanistic considerations of halogenating enzymes[J]. Nature, 2009, 460(7257): 848-854. |
| 21 | SENN H M. Insights into enzymatic halogenation from computational studies[J]. Frontiers in Chemistry, 2014, 2: 98. |
| 22 | LUDEWIG H, MOLYNEUX S, FERRINHO S, et al. Halogenases: structures and functions[J]. Current Opinion in Structural Biology, 2020, 65: 51-60. |
| 23 | NAKANO Y, BIEGASIEWICZ K F, HYSTER T K. Biocatalytic hydrogen atom transfer: an invigorating approach to free-radical reactions[J]. Current Opinion in Chemical Biology, 2019, 49: 16-24. |
| 24 | VOSS M, HONDA MALCA S, BULLER R. Frontispiece: exploring the biocatalytic potential of Fe/α-ketoglutarate-dependent halogenases[J]. Chemistry-A European Journal, 2020, 26(33): chem.202083361. |
| 25 | SHILOV A E, SHUL'PIN G B. Activation of C-H bonds by metal complexes[J]. Chemical Reviews, 1997, 97(8): 2879-2932. |
| 26 | FUJIMORI D G, WALSH C T. What's new in enzymatic halogenations[J]. Current Opinion in Chemical Biology, 2007, 11(5): 553-560. |
| 27 | CERNAK T, DYKSTRA K D, TYAGARAJAN S, et al. The medicinal chemist's toolbox for late stage functionalization of drug-like molecules[J]. Chemical Society Reviews, 2016, 45(3): 546-576. |
| 28 | BANERT K, BERNDT C, WEIGAND K. Synthesis of geminal azido-halo compounds and α-azidoalkyl esters from aldehydes via α-azido alcohols[J]. Organic Letters, 2017, 19(18): 4900-4903. |
| 29 | DENMARK S E, KUESTER W E, BURK M T. Catalytic, asymmetric halofunctionalization of alkenes-a critical perspective[J]. Angewandte Chemie International Edition, 2012, 51(44): 10938-10953. |
| 30 | SHIBUYA G M, KANADY J S, VANDERWAL C D. Stereoselective dichlorination of allylic alcohol derivatives to access key stereochemical arrays of the chlorosulfolipids[J]. Journal of the American Chemical Society, 2008, 130(37): 12514-12518. |
| 31 | PERRY G J P, QUIBELL J M, PANIGRAHI A, et al. Transition-metal-free decarboxylative iodination: new routes for decarboxylative oxidative cross-couplings[J]. Journal of the American Chemical Society, 2017, 139(33): 11527-11536. |
| 32 | HENDERSON S H, WEST R A, WARD S E, et al. Metal-free selective mono-halodecarboxylation of heteroarenes under mild conditions[J]. Royal Society Open Science, 2018, 5(6): 180333. |
| 33 | SCHMIDT V A, QUINN R K, BRUSOE A T, et al. Site-selective aliphatic C—H bromination using N-bromoamides and visible light[J]. Journal of the American Chemical Society, 2014, 136(41): 14389-14392. |
| 34 | WANG Y X, LI G X, YANG G H, et al. A visible-light-promoted radical reaction system for azidation and halogenation of tertiary aliphatic C—H bonds[J]. Chemical Science, 2016, 7(4): 2679-2683. |
| 35 | ZHAO M D, LU W J. Visible light-induced oxidative chlorination of alkyl sp3 C-H bonds with NaCl/oxone at room temperature[J]. Organic Letters, 2017, 19(17): 4560-4563. |
| 36 | LIU W, GROVES J T. Manganese porphyrins catalyze selective C-H bond halogenations[J]. Journal of the American Chemical Society, 2010, 132(37): 12847-12849. |
| 37 | LIU W, GROVES J T. Manganese catalyzed C-H halogenation[J]. Accounts of Chemical Research, 2015, 48(6): 1727-1735. |
| 38 | YANG X L, SUN Y H, SUN T Y, et al. Auxiliary-assisted palladium-catalyzed halogenation of unactivated C(sp3)-H bonds at room temperature[J]. Chemical Communications, 2016, 52(38): 6423-6426. |
| 39 | ZHU R Y, SAINT-DENIS T G, SHAO Y, et al. Ligand-enabled Pd(II)-catalyzed bromination and iodination of C(sp3)-H bonds[J]. Journal of the American Chemical Society, 2017, 139(16): 5724-5727. |
| 40 | OZAWA J, KANAI M. Silver-catalyzed C(sp3)-H chlorination[J]. Organic Letters, 2017, 19(6): 1430-1433. |
| 41 | GALONIĆ D P, VAILLANCOURT F H, WALSH C T. Halogenation of unactivated carbon centers in natural product biosynthesis: trichlorination of leucine during barbamide biosynthesis[J]. Journal of the American Chemical Society, 2006, 128(12): 3900-3901. |
| 42 | BLASIAK L C, VAILLANCOURT F H, WALSH C T, et al. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis[J]. Nature, 2006, 440(7082): 368-371. |
| 43 | WONG C, FUJIMORI D G, WALSH C T, et al. Structural analysis of an open active site conformation of nonheme iron halogenase CytC3[J]. Journal of the American Chemical Society, 2009, 131(13): 4872-4879. |
| 44 | PRATTER S M, IVKOVIC J, BIRNER-GRUENBERGER R, et al. More than just a halogenase: modification of fatty acyl moieties by a trifunctional metal enzyme[J]. Chembiochem, 2014, 15(4): 567-574. |
| 45 | VAILLANCOURT F H, YEH E, VOSBURG D A, et al. Cryptic chlorination by a non-haem iron enzyme during cyclopropyl amino acid biosynthesis[J]. Nature, 2005, 436(7054): 1191-1194. |
| 46 | GU L C, WANG B, KULKARNI A, et al. Metamorphic enzyme assembly in polyketide diversification[J]. Nature, 2009, 459(7247): 731-735. |
| 47 | MITCHELL A J, ZHU Q, MAGGIOLO A O, et al. Structural basis for halogenation by iron- and 2-oxo-glutarate-dependent enzyme WelO5[J]. Nature Chemical Biology, 2016, 12(8): 636-640. |
| 48 | ZHU Q, LIU X Y. Characterization of non-heme iron aliphatic halogenase WelO5* from Hapalosiphon welwitschii IC-52-3: identification of a minimal protein sequence motif that confers enzymatic chlorination specificity in the biosynthesis of welwitindolelinones[J]. Beilstein Journal of Organic Chemistry, 2017, 13: 1168-1173. |
| 49 | DUEWEL S, SCHMERMUND L, FABER T, et al. Directed evolution of an FeII-dependent halogenase for asymmetric C(sp3)-H chlorination [J]. ACS Catalysis, 2019, 10(2): 1272-1277. |
| 50 | HILLWIG M L, ZHU Q, ITTIAMORNKUL K, et al. Discovery of a promiscuous non-heme iron halogenase in ambiguine alkaloid biogenesis: implication for an evolvable enzyme family for late-stage halogenation of aliphatic carbons in small molecules[J]. Angewandte Chemie International Edition, 2016, 55(19): 5780-5784. |
| 51 | ZHAO C H, YAN S, LI Q, et al. An Fe2+ - and α-ketoglutarate-dependent halogenase acts on nucleotide substrates[J]. Angewandte Chemie International Edition, 2020, 59(24): 9478-9484. |
| 52 | KIM C Y, MITCHELL A J, GLINKERMAN C M, et al. The chloroalkaloid (-)-acutumine is biosynthesized via a Fe(II)- and 2-oxoglutarate-dependent halogenase in Menispermaceae plants [J]. Nature Communications, 2020, 11: 1867. |
| 53 | NEUGEBAUER M E, SUMIDA K H, PELTON J G, et al. A family of radical halogenases for the engineering of amino-acid-based products[J]. Nature Chemical Biology, 2019, 15(10): 1009-1016. |
| 54 | SITACHITTA N, ROSSI J, ROBERTS M A, et al. Biosynthesis of the marine cyanobacterial metabolite barbamide (I): Origin of the trichloromethyl group[J]. Journal of the American Chemical Society, 1998, 120(28): 7131-7132. |
| 55 | HILLWIG M L, LIU X Y. A new family of iron-dependent halogenases acts on freestanding substrates[J]. Nature Chemical Biology, 2014, 10(11): 921-923. |
| 56 | VAILLANCOURT F H, YIN J, WALSH C T. SyrB2 in syringomycin E biosynthesis is a nonheme FeII α-ketoglutarate- and O2-dependent halogenase[J]. Proceedings of the National Academy of Sciences of the United States, 2005, 102(29): 10111-10116. |
| 57 | UEKI M, GALONIĆ D P, VAILLANCOURT F H, et al. Enzymatic generation of the antimetabolite γ,γ-dichloroaminobutyrate by NRPS and mononuclear iron halogenase action in a streptomycete[J]. Chemistry & Biology, 2006, 13(11): 1183-1191. |
| 58 | JIANG W, HEEMSTRA J R JR, FORSETH R R, et al. Biosynthetic chlorination of the piperazate residue in kutzneride biosynthesis by KthP[J]. Biochemistry, 2011, 50(27): 6063-6072. |
| 59 | ADAK S, MOORE B S. Cryptic halogenation reactions in natural product biosynthesis[J]. Natural Product Reports, 2021, 38(10): 1760-1774. |
| 60 | MAREK I, SIMAAN S, MASARWA A. Enantiomerically enriched cyclopropene derivatives: versatile building blocks in asymmetric synthesis[J]. Angewandte Chemie International Edition, 2007, 46(39): 7364-7376. |
| 61 | YUEN T Y, BROWN C J, TAN Y S, et al. Synthesis of chiral alkenyl cyclopropane amino acids for incorporation into stapled peptides[J]. The Journal of Organic Chemistry, 2019, 85(3): 1556-1566. |
| 62 | NEUMANN C S, WALSH C T. Biosynthesis of (-)-(1S, 2R)-allocoronamic acyl thioester by an FeII-dependent halogenase and a cyclopropane-forming flavoprotein[J]. Journal of the American Chemical Society, 2008, 130(43): 14022-14023. |
| 63 | FISCHBACH M A, CLARDY J. One pathway, many products[J]. Nature Chemical Biology, 2007, 3(7): 353-355. |
| 64 | AUSTIN M B, O'MAILLE P E, NOEL J P. Evolving biosynthetic tangos negotiate mechanistic landscapes[J]. Nature Chemical Biology, 2008, 4(4): 217-222. |
| 65 | EDWARDS D J, MARQUEZ B L, NOGLE L M, et al. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula [J]. Chemistry & Biology, 2004, 11(6): 817-833. |
| 66 | FUJIMORI D G, HRVATIN S, NEUMANN C S, et al. Cloning and characterization of the biosynthetic gene cluster for kutznerides[J]. Proceedings of the National Academy of Sciences of the United States, 2007, 104(42): 16498-16503. |
| 67 | LIU X Y. In vitro analysis of cyanobacterial nonheme iron-dependent aliphatic halogenases WelO5 and AmbO5[J]. Methods in Enzymology, 2018, 604: 389-404. |
| 68 | MARCHAND J A, NEUGEBAUER M E, ING M C, et al. Discovery of a pathway for terminal-alkyne amino acid biosynthesis[J]. Nature, 2019, 567(7748): 420-424. |
| 69 | GAO S S, NAOWAROJNA N, CHENG R H, et al. Recent examples of α-ketoglutarate-dependent mononuclear non-haem iron enzymes in natural product biosyntheses[J]. Natural Product Reports, 2018, 35(8): 792-837. |
| 70 | KHARE D, WANG B, GU L C, et al. Conformational switch triggered by alpha-ketoglutarate in a halogenase of curacin A biosynthesis[J]. Proceedings of the National Academy of Sciences of the United States, 2010, 107(32): 14099-14104. |
| 71 | AIK W S, CHOWDHURY R, CLIFTON I J, et al. CHAPTER 2. introduction to structural studies on 2-oxoglutarate-dependent oxygenases and related enzymes[M]// 2-Oxoglutarate-Dependent Oxygenases. Cambridge: Royal Society of Chemistry, 2015: 59-94. |
| 72 | HUANG X Y, GROVES J T. Oxygen activation and radical transformations in heme proteins and metalloporphyrins[J]. Chemical Reviews, 2018, 118(5): 2491-2553. |
| 73 | OSWALD V F, LEE J L, BISWAS S, et al. Effects of noncovalent interactions on high-spin Fe(IV)-oxido complexes[J]. Journal of the American Chemical Society, 2020, 142(27): 11804-11817. |
| 74 | SRNEC M, WONG S D, MATTHEWS M L, et al. Electronic structure of the ferryl intermediate in the α-ketoglutarate dependent non-heme iron halogenase SyrB2: contributions to H atom abstraction reactivity[J]. Journal of the American Chemical Society, 2016, 138(15): 5110-5122. |
| 75 | YE S F, NEESE F. Nonheme oxo-iron(IV) intermediates form an oxyl radical upon approaching the C-H bond activation transition state[J]. Proceedings of the National Academy of Sciences of the United States, 2011, 108(4): 1228-1233. |
| 76 | MARTINEZ S, HAUSINGER R P. Catalytic mechanisms of Fe(II)- and 2-oxoglutarate-dependent oxygenases[J]. Journal of Biological Chemistry, 2015, 290(34): 20702-20711. |
| 77 | GRIBBLE G W. The natural production of organobromine compounds[J]. Environmental Science and Pollution Research International, 2000, 7(1): 37-47. |
| 78 | GRIBBLE G W. The diversity of naturally produced organohalogens[J]. Chemosphere, 2003, 52(2): 289-297. |
| 79 | CABRITA M T, VALE C, RAUTER A P. Halogenated compounds from marine algae[J]. Marine Drugs, 2010, 8(8): 2301-2317. |
| 80 | LOHMAN D C, EDWARDS D R, WOLFENDEN R. Catalysis by desolvation: the catalytic prowess of SAM-dependent halide-alkylating enzymes[J]. Journal of the American Chemical Society, 2013, 135(39): 14473-14475. |
| 81 | VAILLANCOURT F H, VOSBURG D A, WALSH C T. Dichlorination and bromination of a threonyl-S-carrier protein by the non-heme Fe(II) halogenase SyrB2[J]. Chembiochem: a European Journal of Chemical Biology, 2006, 7(5): 748-752. |
| 82 | GALONIĆ FUJIMORI D, BARR E W, MATTHEWS M L, et al. Spectroscopic evidence for a high-spin br-Fe(IV)-oxo intermediate in the α-ketoglutarate-dependent halogenase CytC3 from Streptomyces [J]. Journal of the American Chemical Society, 2007, 129(44): 13408-13409. |
| 83 | ZHU Q, HILLWIG M L, DOI Y, et al. Aliphatic halogenase enables late-stage C-H functionalization: selective synthesis of a brominated fischerindole alkaloid with enhanced antibacterial activity[J]. Chembiochem: a European Journal of Chemical Biology, 2016, 17(6): 466-470. |
| 84 | REN X K, FASAN R D. Engineered and artificial metalloenzymes for selective C-H functionalization[J]. Current Opinion in Green and Sustainable Chemistry, 2021, 31: 100494. |
| 85 | FREY R, HAYASHI T, BULLER R M. Directed evolution of carbon-hydrogen bond activating enzymes[J]. Current Opinion in Biotechnology, 2019, 60: 29-38. |
| 86 | MINGES H, SEWALD N. Recent advances in synthetic application and engineering of halogenases[J]. ChemCatChem, 2020, 12(18): 4450-4470. |
| 87 | HAYASHI T, LIGIBEL M, SAGER E, et al. Evolved aliphatic halogenases enable regiocomplementary C-H functionalization of a pharmaceutically relevant compound[J]. Angewandte Chemie International Edition, 2019, 58(51): 18535-18539. |
| 88 | DE V S P. Second-coordination sphere effects on selectivity and specificity of heme and nonheme iron enzymes[J]. Chemistry, 2020, 26(24): 5308-5327. |
| 89 | GRZYSKA P K, MÜLLER T A, CAMPBELL M G, et al. Metal ligand substitution and evidence for quinone formation in taurine/α-ketoglutarate dioxygenase[J]. Journal of Inorganic Biochemistry, 2007, 101(5): 797-808. |
| 90 | GORRES K L, PUA K H, RAINES R T. Stringency of the 2-His-1-Asp active-site motif in prolyl 4-hydroxylase[J]. PLoS One, 2009, 4(11): e7635. |
| 91 | MITCHELL A J, DUNHAM N P, BERGMAN J A, et al. Structure-guided reprogramming of a hydroxylase to halogenate its small molecule substrate[J]. Biochemistry, 2017, 56(3): 441-444. |
| 92 | HARA R, KINO K. Characterization of novel 2-oxoglutarate dependent dioxygenases converting L-proline to cis-4-hydroxy-L-proline[J]. Biochemical and Biophysical Research Communications, 2009, 379(4): 882-886. |
| 93 | PAPADOPOULOU A, MEIERHOFER J, MEYER F, et al. Re-programming and optimization of a L-proline cis-4-hydroxylase for the cis-3-halogenation of its native substrate[J]. ChemCatChem, 2021, 13(18): 3914-3919. |
| 94 | KULIK H J, DRENNAN C L. Substrate placement influences reactivity in non-heme Fe(II) halogenases and hydroxylases[J]. Journal of Biological Chemistry, 2013, 288(16): 11233-11241. |
| 95 | MATTHEWS M L, KREST C M, BARR E W, et al. Substrate-triggered formation and remarkable stability of the C-H bond-cleaving chloroferryl intermediate in the aliphatic halogenase, SyrB2[J]. Biochemistry, 2009, 48(20): 4331-4343. |
| 96 | MEHMOOD R, QI H W, STEEVES A H, et al. The protein's role in substrate positioning and reactivity for biosynthetic enzyme complexes: the case of SyrB2/SyrB1[J]. ACS Catalysis, 2019, 9(6): 4930-43. |
| 97 | PANDIAN S, VINCENT M A, HILLIER I H, et al. Why does the enzyme SyrB2 chlorinate, but does not hydroxylate, saturated hydrocarbons? A density functional theory (DFT) study[J]. Dalton Transactions, 2009(31): 6201-6207. |
| 98 | SRNEC M, WONG S D, ENGLAND J, et al. π-Frontier molecular orbitals in S=2 ferryl species and elucidation of their contributions to reactivity[J]. Proceedings of the National Academy of Sciences of the United States, 2012, 109(36): 14326-14331. |
| 99 | WONG S D, SRNEC M, MATTHEWS M L, et al. Elucidation of the Fe(IV)=O intermediate in the catalytic cycle of the halogenase SyrB2[J]. Nature, 2013, 499(7458): 320-323. |
| 100 | MATTHEWS M L, NEUMANN C S, MILES L A, et al. Substrate positioning controls the partition between halogenation and hydroxylation in the aliphatic halogenase, SyrB2[J]. Proceedings of the National Academy of Sciences of the United States, 2009, 106(42): 17723-17728. |
| 101 | MEHMOOD R, VENNELAKANTI V, KULIK H J. Spectroscopically guided simulations reveal distinct strategies for positioning substrates to achieve selectivity in nonheme Fe(II)/α-ketoglutarate-dependent halogenases[J]. ACS Catalysis, 2021, 11(19): 12394-12408. |
| 102 | ZHANG X, WANG Z K, GAO J, et al. Chlorination versus hydroxylation selectivity mediated by the non-heme iron halogenase WelO5[J]. Physical Chemistry Chemical Physics: PCCP, 2020, 22(16): 8699-8712. |
| 103 | MATTHEWS M L, CHANG W C, LAYNE A P, et al. Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase[J]. Nature Chemical Biology, 2014, 10(3): 209-215. |
| 104 | WALSH C. Molecular mechanisms that confer antibacterial drug resistance[J]. Nature, 2000, 406(6797): 775-781. |
| 105 | GRGURINA I, BARCA A, CERVIGNI S, et al. Relevance of chlorine-substituent for the antifungal activity of syringomycin and syringotoxin, metabolites of the phytopathogenic bacterium Pseudomonas syringae pv. syringae[J]. Experientia, 1994, 50(2): 130-133. |
| 106 | CLARK T, HENNEMANN M, MURRAY J S, et al. Halogen bonding: The sigma-hole. proceedings of “modeling interactions in biomolecules Ⅱ”, Prague, September 5th-9th, 2005[J]. Journal of Molecular Modeling, 2007, 13(2): 291-296. |
| 107 | ZALLOT R, OBERG N, GERLT J A. Discovery of new enzymatic functions and metabolic pathways using genomic enzymology web tools[J]. Current Opinion in Biotechnology, 2021, 69: 77-90. |
| 108 | PETERS C, BULLER R. Industrial application of 2-oxoglutarate-dependent oxygenases[J]. Catalysts, 2019, 9(3): 221. |
| 109 | HEDGES J B, RYAN K S. Biosynthetic pathways to nonproteinogenic α-amino acids[J]. Chemical Reviews, 2020, 120(6): 3161-3209. |
| 110 | DAVIDSON M, MCNAMEE M, FAN R X, et al. Repurposing nonheme iron hydroxylases to enable catalytic nitrile installation through an azido group assistance[J]. Journal of the American Chemical Society, 2019, 141(8): 3419-3423. |
| 111 | OLOO W N, QUE L. Bioinspired nonheme iron catalysts for C-H and C=C bond oxidation: insights into the nature of the metal-based oxidants[J]. Accounts of Chemical Research, 2015, 48(9): 2612-2621. |
| 112 | NASTRI F, CHINO M, MAGLIO O, et al. Design and engineering of artificial oxygen-activating metalloenzymes[J]. Chemical Society Reviews, 2016, 45(18): 5020-5054. |
| 113 | PIZZI A, PIGLIACELLI C, BERGAMASCHI G, et al. Biomimetic engineering of the molecular recognition and self-assembly of peptides and proteins via halogenation[J]. Coordination Chemistry Reviews, 2020, 411: 213242. |
| 114 | CHEN K, ARNOLD F H. Engineering new catalytic activities in enzymes[J]. Nature Catalysis, 2020, 3(3): 203-213. |
| 115 | DUNHAM N P, ARNOLD F H. Nature's machinery, repurposed: expanding the repertoire of iron-dependent oxygenases[J]. ACS Catalysis, 2020, 10(20): 12239-12255. |
| 116 | GOLDBERG N W, KNIGHT A M, ZHANG R K, et al. Nitrene transfer catalyzed by a non-heme iron enzyme and enhanced by non-native small-molecule ligands[J]. Journal of the American Chemical Society, 2019, 141(50): 19585-19588. |
| [1] | 温艳华, 刘合栋, 曹春来, 巫瑞波. 蛋白质工程在医药产业中的应用[J]. 合成生物学, 2025, 6(1): 65-86. |
| [2] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [3] | 王子渊, 杨立荣, 吴坚平, 郑文隆. 酶促合成手性氨基酸的研究进展[J]. 合成生物学, 2024, 5(6): 1319-1349. |
| [4] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [5] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [6] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [7] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [8] | 付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049. |
| [9] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [10] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [11] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [12] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [13] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [14] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [15] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||