合成生物学 ›› 2024, Vol. 5 ›› Issue (1): 126-143.DOI: 10.12211/2096-8280.2023-065
微生物发酵法合成虾青素的研究进展
周强1, 周大伟1, 孙敬翔1, 王靖楠1, 姜万奎1, 章文明1,2, 蒋羽佳1,2, 信丰学1,2, 姜岷1,2
- 1.南京工业大学生物与制药工程学院,材料化学工程国家重点实验室,江苏 南京 211816
2.南京工业大学,江苏先进生物与化学制造协同创新中心(SICAM),江苏 南京 210009
-
收稿日期:2023-09-14修回日期:2023-11-20出版日期:2024-02-29发布日期:2024-03-20 -
通讯作者:蒋羽佳,信丰学 -
作者简介:周强 (2000—),男,硕士。研究方向为代谢工程及合成生物学。 E-mail:2278766279@qq.com蒋羽佳 (1991—),女,博士,副教授,硕士生导师。研究方向为可再生生物能源底盘细胞的开发与利用,低劣生物质高值化利用的人工多细胞体系构建及菌株间互作机制解析。 E-mail:jiangyujia@njtech.edu.cn信丰学 (1982—),男,博士,教授,博士生导师。研究方向为:①木质纤维素、厨余垃圾等废弃碳资源的生物降解与大宗化学品、生物燃料的合成;②CBP和精细化学品合成人工混菌体系的设计、构建与功能调控;③GRAS级安全酵母的开发和细胞工厂构建,尤其针对功能脂肪酸、胡萝卜素、虾青素等医药营养品。 E-mail:xinfengxue@njtech.edu.cn -
基金资助:国家自然科学基金面上项目(22178169);江苏省杰出青年基金(BK20220052);山东省泰山产业领军人才工程(202306155)
Research progress in synthesis of astaxanthin by microbial fermentation
ZHOU Qiang1, ZHOU Dawei1, SUN Jingxiang1, WANG Jingnan1, JIANG Wankui1, ZHANG Wenming1,2, JIANG Yujia1,2, XIN Fengxue1,2, JIANG Min1,2
- 1.State key Laboratory of Materials Chemical Engineering,College of Biological and Pharmaceutical Engineering,Nanjing Tech University,Nanjing 211816,Jiangsu,China
2.Jiangsu Advanced Biological and Chemical Manufacturing Collaborative Innovation Center (SICAM),Nanjing Tech University,Nanjing 210009,Jiangsu,China
-
Received:2023-09-14Revised:2023-11-20Online:2024-02-29Published:2024-03-20 -
Contact:JIANG Yujia, XIN Fengxue
摘要:
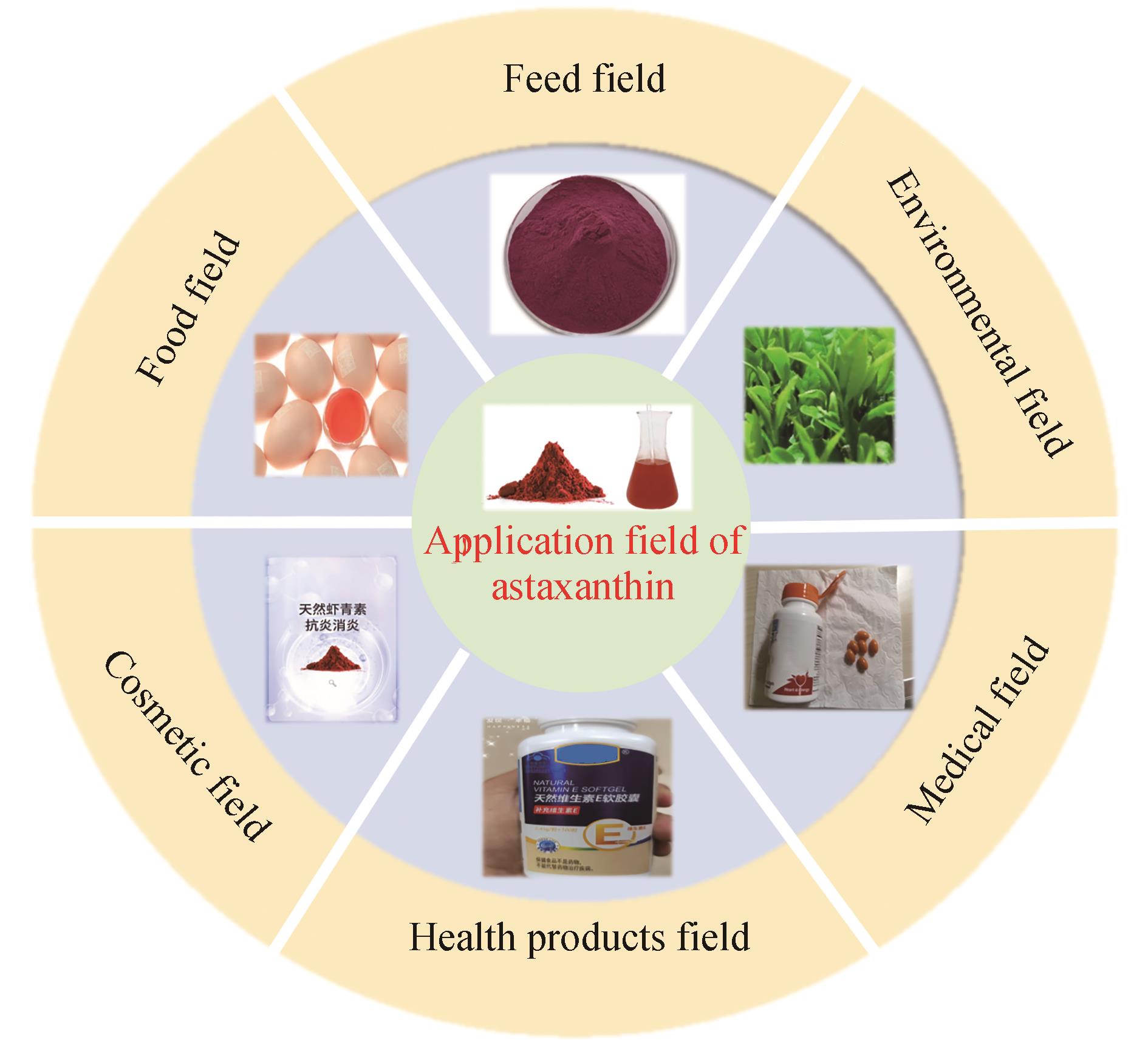
虾青素是一种高附加值的抗氧化萜类物质,具有很强的抗氧化活性,同时还具有抗癌、预防炎症、护眼等诸多功效。随着合成生物学技术的不断发展,利用微生物发酵法合成虾青素是实现虾青素工业化生产最有效的途径之一,也更能满足消费者对天然化合物的需求。目前,生产虾青素的微生物包括细菌、真菌、藻类等。本文系统介绍了虾青素的结构性质和生产方法,重点讲述了虾青素天然合成以及外源构建的合成路径,总结了不同微生物如雨生红球藻、酵母和大肠杆菌生产虾青素的最新进展,分析了利用基因工程和发酵过程调控手段提高虾青素产量的方法。未来,通过代谢工程等手段(如虾青素合成基因过表达、使用高强度启动子、代谢途径优化等)可提高虾青素产量,以进一步增加虾青素在食品、医疗、化妆品和饲料等产业的应用。
中图分类号:
引用本文
周强, 周大伟, 孙敬翔, 王靖楠, 姜万奎, 章文明, 蒋羽佳, 信丰学, 姜岷. 微生物发酵法合成虾青素的研究进展[J]. 合成生物学, 2024, 5(1): 126-143.
ZHOU Qiang, ZHOU Dawei, SUN Jingxiang, WANG Jingnan, JIANG Wankui, ZHANG Wenming, JIANG Yujia, XIN Fengxue, JIANG Min. Research progress in synthesis of astaxanthin by microbial fermentation[J]. Synthetic Biology Journal, 2024, 5(1): 126-143.
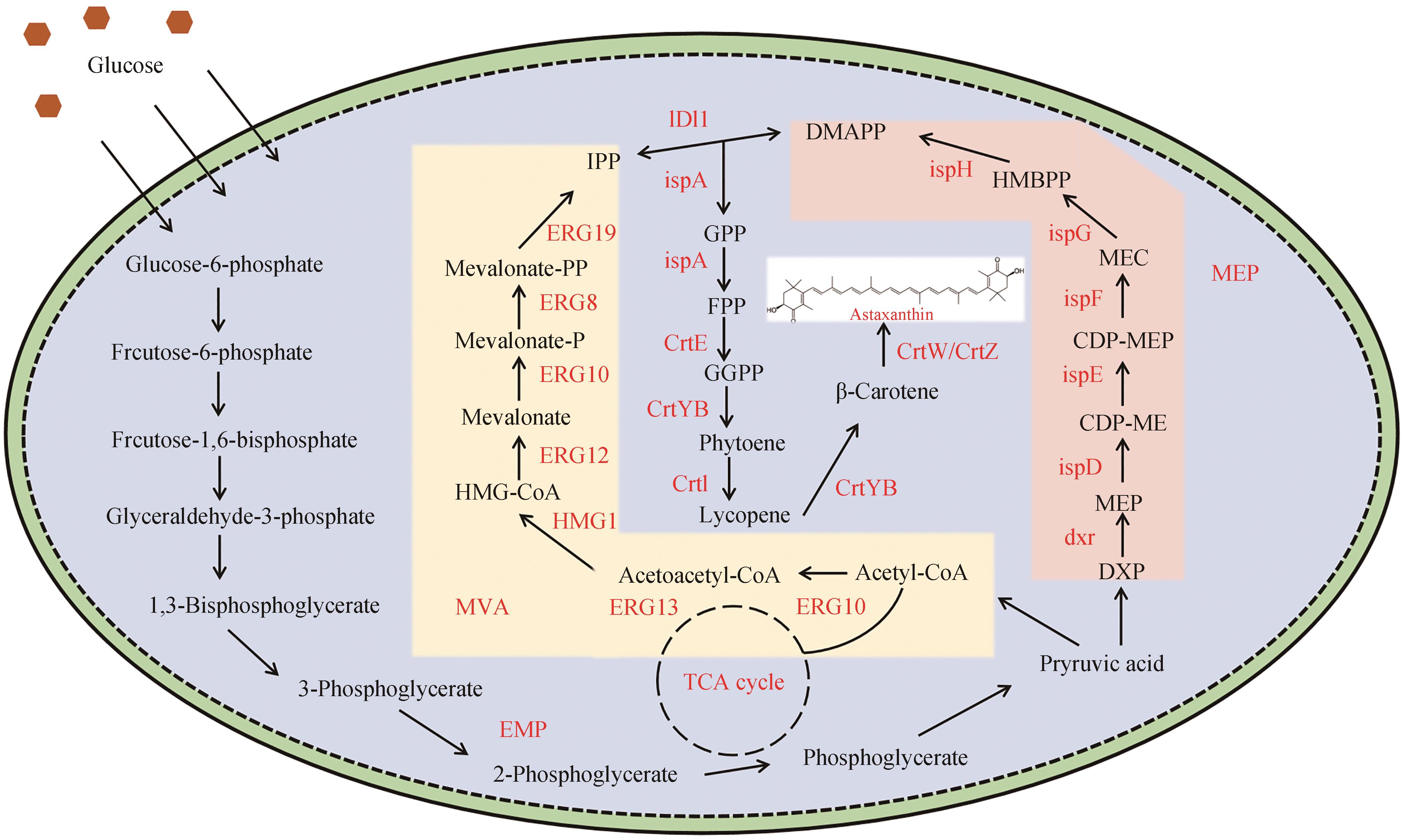
图3 微生物合成虾青素的代谢途径Glucose-6-phosphate—葡萄糖-6-磷酸;Frcutose-6-phosphate—果糖-6-磷酸;Frcutose-1,6-bisphosphate—1,6-二磷酸果糖;Glyceraldehyde-3-phosphate—三磷酸甘油醛;1,3-Bisphosphoglycerate—1,3-二磷酸甘油酸;3-Phosphoglycerate—三磷酸甘油酸;2-Phosphoglycerate—二磷酸甘油酸;Phosphoglycerate—磷酸甘油酸;Pryruvic acid—丙酮酸;Acetyl-CoA—乙酰辅酶A;Acetoacetyl-CoA—乙酰乙酰辅酶A;Mevalonate—甲羟戊酸;HMG-CoA—3-羟基-3-甲基戊二酸单酰辅酶A;IPP—焦磷酸异戊烯酯;DMAPP—焦磷酸二甲基烯丙酯;GPP—香叶基焦磷酸;FPP—法尼基焦磷酸;GGPP—香叶基二磷酸;Phytoene—八氢番茄红素;Lycopene—番茄红素;β-Carotene—β-胡萝卜素;Astaxanthin—虾青素;HMBPP —羟甲基丁烯基-4-磷;DXP—1-脱氧-D-木酮糖-5磷酸酯;ERG10—乙酰辅酶A硫解酶;ERG13—3-羟基-3-甲基戊二酰-CoA合酶;HMG1—3-羟基-3-甲基戊二酰辅酶A还原酶;ERG12—甲羟戊酸激酶;ERG8—磷酸甲羟戊酸激酶;ERG19—甲羟戊酸焦磷酸脱羧酶;IDI—异戊烯基焦磷酸异构酶;ispA—法尼基二磷酸合酶;CrtE—香叶基香叶基二磷酸合酶;CrtYB—八氢番茄红素合酶/环化酶;CrtI—八氢番茄红素去饱和酶;CrtW—β-胡萝卜素酮醇酶;CrtZ—β-胡萝卜素羟化酶;ispH—4-羟基-3-甲基乙烯-2-烯基-二磷酸还原酶;ispG—4-羟基-3-甲基乙烯基-2-磷酸合酶;ispF—2-C-甲基-D-赤藓醇激酶;ispE—4-胞啶-2-C-甲基-D-赤藓醇激酶;ispD—2-C-甲基-D-赤藓醇-4-磷酸胞苷酰转移酶;dxr—1-脱氧-D-木酮糖-5-磷酸还原异构酶
Fig. 3 Metabolic pathways for astaxanthin synthesis by microorganisms
| 菌株 | 碳源 | 菌株 类型 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| X. dendrorhous | 葡萄糖 | 野生菌 | 27.05 mg/L | 30L发酵罐 | [ |
| X. dendrorhous | 葡萄糖 | 诱变菌 | 85.02 mg/L | 5L发酵罐 | [ |
| X. dendrorhous | 甜高粱蔗渣 | 野生菌 | 48.9 mg/L | 分批补料发酵 | [ |
| X. dendrorhous | 葡萄糖 | 诱变菌 | 9.7 mg/g | 1.3L发酵罐 | [ |
| X. dendrorhous | 葡萄糖 | 野生菌 | 67.9 mg/L | 分批补料发酵 | [ |
表1 红法夫酵母产虾青素进展
Table 1 Progress in astaxanthin production by X. dendrorhous
| 菌株 | 碳源 | 菌株 类型 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| X. dendrorhous | 葡萄糖 | 野生菌 | 27.05 mg/L | 30L发酵罐 | [ |
| X. dendrorhous | 葡萄糖 | 诱变菌 | 85.02 mg/L | 5L发酵罐 | [ |
| X. dendrorhous | 甜高粱蔗渣 | 野生菌 | 48.9 mg/L | 分批补料发酵 | [ |
| X. dendrorhous | 葡萄糖 | 诱变菌 | 9.7 mg/g | 1.3L发酵罐 | [ |
| X. dendrorhous | 葡萄糖 | 野生菌 | 67.9 mg/L | 分批补料发酵 | [ |
| 菌株 | 碳源 | 菌株类型 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
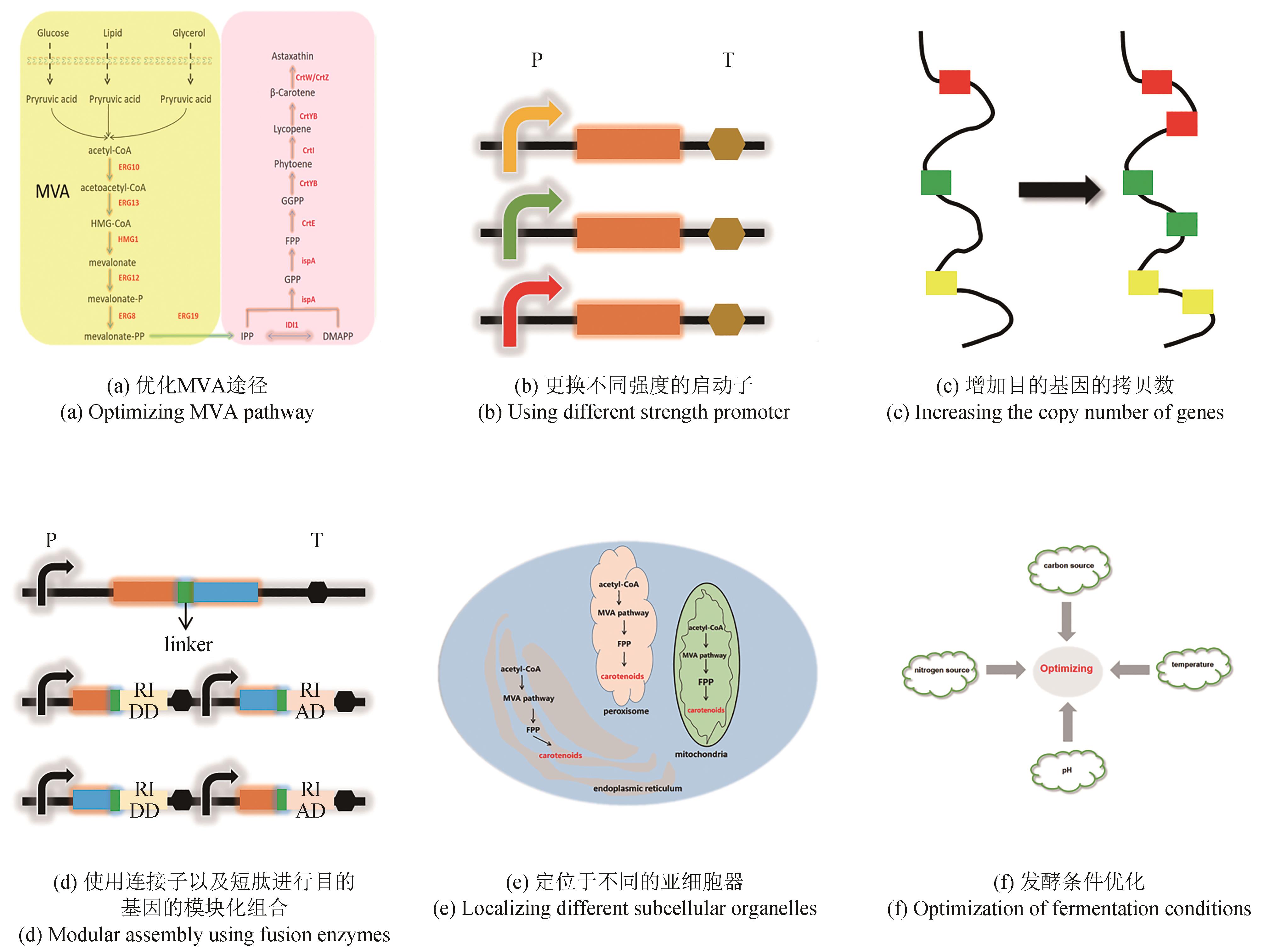
| S. cerevisiae | 葡萄糖 | 表达不同来源的crtW和crtZ,替换不同强度的启动子 | 81 mg/L | 5L发酵罐 | [ |
| S. cerevisiae | 葡萄糖 | 定向协同进化获得关键基因的突变体,借助温度调节响应系统 | 235 mg/L | 5L发酵罐 | [ |
| S. cerevisiae | 葡萄糖 | 适度调节脂质代谢的相关基因,平衡crtW和crtZ的表达 | 446.4 mg/L | 5L发酵罐 | [ |
| S. cerevisiae | 葡萄糖 | 组成型启动子表达crtW,诱导型启动子表达crtZ | 464.09 mg/L | [ |
表2 酿酒酵母产虾青素进展
Table 2 Progress in astaxanthin production by S. cerevisiae
| 菌株 | 碳源 | 菌株类型 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| S. cerevisiae | 葡萄糖 | 表达不同来源的crtW和crtZ,替换不同强度的启动子 | 81 mg/L | 5L发酵罐 | [ |
| S. cerevisiae | 葡萄糖 | 定向协同进化获得关键基因的突变体,借助温度调节响应系统 | 235 mg/L | 5L发酵罐 | [ |
| S. cerevisiae | 葡萄糖 | 适度调节脂质代谢的相关基因,平衡crtW和crtZ的表达 | 446.4 mg/L | 5L发酵罐 | [ |
| S. cerevisiae | 葡萄糖 | 组成型启动子表达crtW,诱导型启动子表达crtZ | 464.09 mg/L | [ |
| 菌株 | 碳源 | 改造策略 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| Y. lipolytica | 葡萄糖 | 表达不同来源的crtW和crtZ,增加关键基因拷贝数 | 54.6 mg/L | 96深孔板培养 | [ |
| Y. lipolytica | 葡萄糖 | 定位于不同的亚细胞器 | 858 mg/L | 3L发酵罐 | [ |
| Y. lipolytica | 蔗糖 | 加入外源油相,模块化工程途径融合关键基因 | 973.4 mg/L | 3L发酵罐 | [ |
| Y. lipolytica | 葡萄糖 | 表达来自雨生红球藻的关键基因,通过短肽连接,增加融合酶的拷贝数 | 3.3 g/L | 分批补料发酵 | [ |
| Y. lipolytica | 葡萄糖 | 表达来自夏侧金盏花的关键基因,引入crtX基因 | 3.46 mg/L | 摇瓶发酵 | [ |
| Y. lipolytica | 组合表达不同来源的crtW和crtZ | 99 mg/L | 摇瓶发酵 | [ |
表3 解脂耶氏酵母产虾青素进展
Table 3 Progress in astaxanthin production by Y. lipolytica
| 菌株 | 碳源 | 改造策略 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| Y. lipolytica | 葡萄糖 | 表达不同来源的crtW和crtZ,增加关键基因拷贝数 | 54.6 mg/L | 96深孔板培养 | [ |
| Y. lipolytica | 葡萄糖 | 定位于不同的亚细胞器 | 858 mg/L | 3L发酵罐 | [ |
| Y. lipolytica | 蔗糖 | 加入外源油相,模块化工程途径融合关键基因 | 973.4 mg/L | 3L发酵罐 | [ |
| Y. lipolytica | 葡萄糖 | 表达来自雨生红球藻的关键基因,通过短肽连接,增加融合酶的拷贝数 | 3.3 g/L | 分批补料发酵 | [ |
| Y. lipolytica | 葡萄糖 | 表达来自夏侧金盏花的关键基因,引入crtX基因 | 3.46 mg/L | 摇瓶发酵 | [ |
| Y. lipolytica | 组合表达不同来源的crtW和crtZ | 99 mg/L | 摇瓶发酵 | [ |
| 菌株 | 碳源 | 改造策略 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| E. coli | 酵母粉 | 表达伴侣基因ApcpnA和ApcpnB | 890 μg/g | 摇瓶发酵 | [ |
| E. coli | 酵母粉 | 表达MEP途径关键基因与MVA途径共表达 | 1100 μg/g | 摇瓶发酵 | [ |
| E. coli | 酵母粉 | 表达类异戊二烯途径关键基因与MVA途径共表达 | 1200 μg/g | 摇瓶发酵 | [ |
| E. coli | 葡萄糖 | 通过γ-Red重组技术将虾青素合成途径的基因整合到大肠杆菌染色体中 | 1.4 mg/g | 摇瓶发酵 | [ |
| E. coli | 蔗糖 | 表达不同来源的crtW和crtZ,平衡相关基因的活性 | 7.4 mg/g | 摇瓶发酵 | [ |
| E. coli | 葡萄糖 | 表达不同来源的crtW和crtZ,通过短肽连接关键基因 | 5.18 mg/g | 摇瓶发酵 | [ |
| E. coli | 葡萄糖 | 表达不同来源的crtW和crtZ,表达不同强度的启动子 | 4.3 mg/g | 摇瓶发酵 | [ |
| E. coli | 酵母粉 | 删除形态、膜、氧化应激相关基因,建立温度敏感质粒互补表达系统 | 11.92 mg/g | 摇瓶发酵 | [ |
| E. coli | 甘油 | 对crtW进行随机突变,通过Cre-loxP平衡增加基因拷贝数 | 5.88 mg/g | 7L发酵罐 | [ |
| E. coli | 甘油 | 增加crtYB的拷贝数,调节操纵子的表达水平 | 6.17 mg/g | 5L发酵罐 | [ |
| E. coli | 甘油 | 筛选不同来源的crtZ并对这些不同底物偏好的酶进行联合利用 | 11.5 mg/g | 5L发酵罐 | [ |
表4 大肠杆菌产虾青素进展
Table 4 Progress in astaxanthin production by E. coli
| 菌株 | 碳源 | 改造策略 | 浓度/产量 | 发酵模式 | 参考文献 |
|---|---|---|---|---|---|
| E. coli | 酵母粉 | 表达伴侣基因ApcpnA和ApcpnB | 890 μg/g | 摇瓶发酵 | [ |
| E. coli | 酵母粉 | 表达MEP途径关键基因与MVA途径共表达 | 1100 μg/g | 摇瓶发酵 | [ |
| E. coli | 酵母粉 | 表达类异戊二烯途径关键基因与MVA途径共表达 | 1200 μg/g | 摇瓶发酵 | [ |
| E. coli | 葡萄糖 | 通过γ-Red重组技术将虾青素合成途径的基因整合到大肠杆菌染色体中 | 1.4 mg/g | 摇瓶发酵 | [ |
| E. coli | 蔗糖 | 表达不同来源的crtW和crtZ,平衡相关基因的活性 | 7.4 mg/g | 摇瓶发酵 | [ |
| E. coli | 葡萄糖 | 表达不同来源的crtW和crtZ,通过短肽连接关键基因 | 5.18 mg/g | 摇瓶发酵 | [ |
| E. coli | 葡萄糖 | 表达不同来源的crtW和crtZ,表达不同强度的启动子 | 4.3 mg/g | 摇瓶发酵 | [ |
| E. coli | 酵母粉 | 删除形态、膜、氧化应激相关基因,建立温度敏感质粒互补表达系统 | 11.92 mg/g | 摇瓶发酵 | [ |
| E. coli | 甘油 | 对crtW进行随机突变,通过Cre-loxP平衡增加基因拷贝数 | 5.88 mg/g | 7L发酵罐 | [ |
| E. coli | 甘油 | 增加crtYB的拷贝数,调节操纵子的表达水平 | 6.17 mg/g | 5L发酵罐 | [ |
| E. coli | 甘油 | 筛选不同来源的crtZ并对这些不同底物偏好的酶进行联合利用 | 11.5 mg/g | 5L发酵罐 | [ |
| 1 | RIZZARDI N, PEZZOLESI L, SAMORÌ C, et al. Natural astaxanthin is a green antioxidant able to counteract lipid peroxidation and ferroptotic cell death[J]. International Journal of Molecular Sciences, 2022, 23(23): 15137. |
| 2 | TURUJMAN S A, WAMER W G, WEI R R, et al. Rapid liquid chromatographic method to distinguish wild salmon from aquacultured salmon fed synthetic astaxanthin[J]. Journal of AOAC INTERNATIONAL, 1997, 80(3): 622-632. |
| 3 | 蔡俊, 游智能. 发酵法生产虾青素的研究进展[J]. 食品科学, 2015, 36(23): 358-366. |
| CAI J, YOU Z N. Current status of fermentative production of astaxanthin[J]. Food Science, 2015, 36(23): 358-366. | |
| 4 | 姜思, 佟少明. 雨生红球藻虾青素合成研究进展[J]. 生物工程学报, 2019, 35(6): 988-997. |
| JIANG S, TONG S M. Advances in astaxanthin biosynthesis in Haematococcus pluvialis [J]. Chinese Journal of Biotechnology, 2019, 35(6): 988-997. | |
| 5 | YUAN J P, CHEN F. Chromatographic separation and purification of trans-astaxanthin from the extracts of Haematococcus pluvialis [J]. Journal of Agricultural and Food Chemistry, 1998, 46(8): 3371-3375. |
| 6 | 王军, 张晴龙, 李曦月, 等. 高产虾青素红法夫酵母的代谢工程育种研究进展[J]. 化学与生物工程, 2022, 39(10): 1-5. |
| WANG J, ZHANG Q L, LI X Y, et al. Research progress in metabolic engineering breeding of high-yield astaxanthin-producing Xanthophyllomyces dendrorhous [J]. Chemistry & Bioengineering, 2022, 39(10): 1-5. | |
| 7 | KOHANDEL Z, FARKHONDEH T, ASCHNER M, et al. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases[J]. Biomedicine & Pharmacotherapy, 2022, 145: 112179. |
| 8 | JING Y W, WANG Y X, ZHOU D W, et al. Advances in the synthesis of three typical tetraterpenoids including β-carotene, lycopene and astaxanthin[J]. Biotechnology Advances, 2022, 61: 108033. |
| 9 | SCHMIDT I, SCHEWE H, GASSEL S, et al. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous [J]. Applied Microbiology and Biotechnology, 2011, 89(3): 555-571. |
| 10 | 苗丽青, 马旭辉, 李素贞, 等. 虾青素的生物合成与产业化应用[J]. 中国农业科技导报, 2023, 25(3): 21-29. |
| MIAO L Q, MA X H, LI S Z, et al. Biosynthesis and industrial application of astaxanthin[J]. Journal of Agricultural Science and Technology, 2023, 25(3): 21-29. | |
| 11 | LIGNELL A. Medicament for improvement of duration of muscle function or treatment of muscle disorders or diseases: US6245818[P].2001-06-12. |
| 12 | 李新杰, 朱伟, 姜威, 等. 天然虾青素对鸭肉品质和脂质氧化稳定性的影响[J]. 粮食与饲料工业, 2012(6): 43-45. |
| LI X J, ZHU W, JIANG W, et al. Effects of natural astaxanthin on duck meat quality and lipid oxidative stability[J]. Cereal & Feed Industry, 2012(6): 43-45. | |
| 13 | MUSSAGY C U, PEREIRA J F B, DUFOSSÉ L, et al. Advances and trends in biotechnological production of natural astaxanthin by Phaffia rhodozyma yeast[J]. Critical Reviews in Food Science and Nutrition, 2023, 63(13): 1862-1876. |
| 14 | HUSSEIN G, SANKAWA U, GOTO H, et al. Astaxanthin, a carotenoid with potential in human health and nutrition[J]. Journal of Natural Products, 2006, 69(3): 443-449. |
| 15 | CICHOŃSKI J, CHRZANOWSKI G. Microalgae as a source of valuable phenolic compounds and carotenoids[J]. Molecules, 2022, 27(24): 8852. |
| 16 | PARK S Y, BINKLEY R M, KIM W J, et al. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity[J]. Metabolic Engineering, 2018, 49: 105-115. |
| 17 | KITAHARA T. Carotenoids in the Pacific salmon during the marine period[J]. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1984, 78(4): 859-862. |
| 18 | ZHANG C Q, CHEN X X, TOO H P. Microbial astaxanthin biosynthesis: recent achievements, challenges, and commercialization outlook[J]. Applied Microbiology and Biotechnology, 2020, 104(13): 5725-5737. |
| 19 | LI J, ZHU D L, NIU J F, et al. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis [J]. Biotechnology Advances, 2011, 29(6): 568-574. |
| 20 | GONG Z K, WANG H L, TANG J L, et al. Coordinated expression of astaxanthin biosynthesis genes for improved astaxanthin production in Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2020, 68(50): 14917-14927. |
| 21 | RANI A, SAINI K, BAST F, et al. Microorganisms: a potential source of bioactive molecules for antioxidant applications[J]. Molecules, 2021, 26(4): 1142. |
| 22 | SAINI R K, KEUM Y S. Progress in microbial carotenoids production[J]. Indian Journal of Microbiology, 2017, 57(1): 129-130. |
| 23 | YE V M, BHATIA S K. Pathway engineering strategies for production of beneficial carotenoids in microbial hosts[J]. Biotechnology Letters, 2012, 34(8): 1405-1414. |
| 24 | ZHU X Y, MENG C X, SUN F J, et al. Sustainable production of astaxanthin in microorganisms: the past, present, and future[J]. Critical Reviews in Food Science and Nutrition, 2022: 1-17. |
| 25 | ZHOU D W, YANG X Y, WANG H X, et al. Biosynthesis of astaxanthin by using industrial yeast[J]. Biofuels, Bioproducts & Biorefining, 2023, 17(3): 602-615. |
| 26 | 张辰, 徐慧, 朱坤福, 等. 微生物法生产虾青素的研究进展[J]. 中国酿造, 2021, 40(10): 29-35. |
| ZHANG C, XU H, ZHU K F, et al. Research progress in the production of astaxanthin by microbial method[J]. China Brewing, 2021, 40(10): 29-35. | |
| 27 | GUERIN M, HUNTLEY M E, OLAIZOLA M. Haematococcus astaxanthin: applications for human health and nutrition[J]. Trends in Biotechnology, 2003, 21(5): 210-216. |
| 28 | CHENG X, RIORDON J, NGUYEN B, et al. Hydrothermal disruption of algae cells for astaxanthin extraction[J]. Green Chemistry, 2017, 19(1): 106-111. |
| 29 | RANJBAR R, INOUE R, KATSUDA T, et al. High efficiency production of astaxanthin in an airlift photobioreactor[J]. Journal of Bioscience and Bioengineering, 2008, 106(2): 204-207. |
| 30 | WANG X, MOU J H, QIN Z H, et al. Supplementation with rac-GR24 facilitates the accumulation of biomass and astaxanthin in two successive stages of Haematococcus pluvialis cultivation[J]. Journal of Agricultural and Food Chemistry, 2022, 70(15): 4677-4689. |
| 31 | HUANG W C, LIU H, SUN W W, et al. Effective astaxanthin extraction from wet Haematococcus pluvialis using switchable hydrophilicity solvents[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 1560-1563. |
| 32 | REN Y Y, DENG J Q, HUANG J C, et al. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: advances and outlook[J]. Bioresource Technology, 2021, 340: 125736. |
| 33 | GASSEL S, BREITENBACH J, SANDMANN G. Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high-yield mutant[J]. Applied Microbiology and Biotechnology, 2014, 98(1): 345-350. |
| 34 | CHI S, HE Y F, REN J, et al. Overexpression of a bifunctional enzyme, CrtS, enhances astaxanthin synthesis through two pathways in Phaffia rhodozyma [J]. Microbial Cell Factories, 2015, 14: 90. |
| 35 | GERVASI T, SANTINI A, DALIU P, et al. Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate[J]. Agroforestry Systems, 2020, 94(4): 1229-1234. |
| 36 | STOKLOSA R J, JOHNSTON D B, NGHIEM N P. Utilization of sweet sorghum juice for the production of astaxanthin as a biorefinery co-product by Phaffia rhodozyma [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3124-3134. |
| 37 | HU Z C, ZHENG Y G, WANG Z, et al. pH control strategy in astaxanthin fermentation bioprocess by Xanthophyllomyces dendrorhous [J]. Enzyme and Microbial Technology, 2006, 39(4): 586-590. |
| 38 | ZHUANG Y, JIANG G L, ZHU M J. Atmospheric and room temperature plasma mutagenesis and astaxanthin production from sugarcane bagasse hydrolysate by Phaffia rhodozyma mutant Y1[J]. Process Biochemistry, 2020, 91: 330-338. |
| 39 | STOKLOSA R J, JOHNSTON D B, NGHIEM N P. Phaffia rhodozyma cultivation on structural and non-structural sugars from sweet sorghum for astaxanthin generation[J]. Process Biochemistry, 2019, 83: 9-17. |
| 40 | GASSEL S, SCHEWE H, SCHMIDT I, et al. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering[J]. Biotechnology Letters, 2013, 35(4): 565-569. |
| 41 | 茹毅, 沈宁燕, 倪辉, 等. 乙醇促进法夫酵母虾青素合成的机理及其代谢调控[J]. 中国食品学报, 2019, 19(8): 41-48. |
| RU Y, SHEN N Y, NI H, et al. Mechanism of ethanol promoting the synthesis of astaxanthin and its metabolic regulation by Phaffia rhodozyma [J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(8): 41-48. | |
| 42 | YANG H Y, YANG L, DU X P, et al. Metabolomics of astaxanthin biosynthesis and corresponding regulation strategies in Phaffia rhodozyma [J]. Yeast, 2023, 40(7): 254-264. |
| 43 | YAMAMOTO K, HARA K Y, MORITA T, et al. Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes[J]. Microbial Cell Factories, 2016, 15(1): 155. |
| 44 | HONG K K, NIELSEN J. Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries[J]. Cellular and Molecular Life Sciences, 2012, 69(16): 2671-2690. |
| 45 | WANG R Z, GU X L, YAO M D, et al. Engineering of β-carotene hydroxylase and ketolase for astaxanthin overproduction in Saccharomyces cerevisiae [J]. Frontiers of Chemical Science and Engineering, 2017, 11(1): 89-99. |
| 46 | ZHOU P P, LI M, SHEN B, et al. Directed coevolution of β-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin[J]. Journal of Agricultural and Food Chemistry, 2019, 67(4): 1072-1080. |
| 47 | LI M, ZHOU P P, CHEN M K, et al. Spatiotemporal regulation of astaxanthin synthesis in S. cerevisiae [J]. ACS Synthetic Biology, 2022, 11(8): 2636-2649. |
| 48 | 杨祖明, 王竞辉, 张雅萍, 等. 一株生产虾青素的重组酿酒酵母及其应用: CN113699052B[P]. 2023-08-11. |
| YANG Z M, WANG J H, ZHANG Y P, et al. Recombinant Saccharomyces cerevisiae for producing astaxanthin and its application: CN113699052B[P]. 2023-08-11. | |
| 49 | DING Y W, LU C Z, ZHENG Y, et al. Directed evolution of the fusion enzyme for improving astaxanthin biosynthesis in Saccharomyces cerevisiae [J]. Synthetic and Systems Biotechnology, 2023, 8(1): 46-53. |
| 50 | CHEN X Y, ZARO J L, SHEN W C. Fusion protein linkers: property, design and functionality[J]. Advanced Drug Delivery Reviews, 2013, 65(10): 1357-1369. |
| 51 | ZHOU P P, XIE W P, YAO Z, et al. Development of a temperature-responsive yeast cell factory using engineered Gal4 as a protein switch[J]. Biotechnology and Bioengineering, 2018, 115(5): 1321-1330. |
| 52 | CHEN G Q, WANG B B, HAN D X, et al. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae)[J]. The Plant Journal: for Cell and Molecular Biology, 2015, 81(1): 95-107. |
| 53 | LIAN J Z, HAMEDIRAD M, HU S M, et al. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system[J]. Nature Communications, 2017, 8: 1688. |
| 54 | RODRIGUEZ-OCASIO E, KHALID A, TRUKA C J, et al. Survey of nonconventional yeasts for lipid and hydrocarbon biotechnology[J]. Journal of Industrial Microbiology and Biotechnology, 2022, 49(4): kuac010. |
| 55 | FRIEDLANDER J, TSAKRAKLIDES V, KAMINENI A, et al. Engineering of a high lipid producing Yarrowia lipolytica strain[J]. Biotechnology for Biofuels, 2016, 9(1): 77. |
| 56 | PARK Y K, DULERMO T, LEDESMA-AMARO R, et al. Optimization of odd chain fatty acid production by Yarrowia lipolytica [J]. Biotechnology for Biofuels, 2018, 11: 158. |
| 57 | WANG J P, LEDESMA-AMARO R, WEI Y J, et al. Metabolic engineering for increased lipid accumulation in Yarrowia lipolytica-a review[J]. Bioresource Technology, 2020, 313: 123707. |
| 58 | 赵禹, 刘士琦, 李建, 等. 解脂耶氏酵母作为微生物细胞工厂的应用研究进展[J]. 食品科学, 2021, 42(19): 388-400. |
| ZHAO Y, LIU S Q, LI J, et al. Advances in the application of Yarrowia lipolytica as a microbial cell factory[J]. Food Science, 2021, 42(19): 388-400. | |
| 59 | KILDEGAARD K R, ADIEGO-PÉREZ B, DOMÉNECH BELDA D, et al. Engineering of Yarrowia lipolytica for production of astaxanthin[J]. Synthetic and Systems Biotechnology, 2017, 2(4): 287-294. |
| 60 | MA Y S, LI J B, HUANG S W, et al. Targeting pathway expression to subcellular organelles improves astaxanthin synthesis in Yarrowia lipolytica [J]. Metabolic Engineering, 2021, 68: 152-161. |
| 61 | YUZBASHEVA E Y, TARATYNOVA M O, FEDYAEVA I M, et al. Large-scale bioproduction of natural astaxanthin in Yarrowia lipolytica [J]. Bioresource Technology Reports, 2023, 21: 101289. |
| 62 | KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux[J]. Nature Communications, 2019, 10: 4248. |
| 63 | CHEN J, ZHANG R L, ZHANG G L, et al. Heterologous expression of the plant-derived astaxanthin biosynthesis pathway in Yarrowia lipolytica for glycosylated astaxanthin production[J]. Journal of Agricultural and Food Chemistry, 2023, 71(6): 2943-2951. |
| 64 | 花强, 汪丹妮, 韦柳静, 等. 一种生产虾青素的解脂耶氏酵母基因工程菌的构建方法: CN116497052A[P]. 2023-07-28. |
| HUA Q, WANG D N, WEI L J, et al. A method for construction of genetic engineering strain of Yersinia lipolis for astaxanthin production: CN116497052A[P]. 2023-07-28. | |
| 65 | ZHU H Z, JIANG S, WU J J, et al. Production of high levels of 3S,3′S-astaxanthin in Yarrowia lipolytica via iterative metabolic engineering[J]. Journal of Agricultural and Food Chemistry, 2022, 70(8): 2673-2683. |
| 66 | HAMMER S K, AVALOS J L. Harnessing yeast organelles for metabolic engineering[J]. Nature Chemical Biology, 2017, 13(8): 823-832. |
| 67 | YOKOYAMA A, ADACHI K, SHIZURI Y. New carotenoid glucosides, astaxanthin glucoside and adonixanthin glucoside, isolated from the astaxanthin-producing marine bacterium, Agrobacterium aurantiacum [J]. Journal of Natural Products, 1995, 58(12): 1929-1933. |
| 68 | KIM J H, KIM S H, KIM K H, et al. Sphingomonas lacus sp. nov., an astaxanthin-dideoxyglycoside-producing species isolated from soil near a pond[J]. International Journal of Systematic and Evolutionary Microbiology, 2015, 65(Pt_9): 2824-2830. |
| 69 | CHANG J J, HO C Y, MAO C T, et al. A thermo- and toxin-tolerant kefir yeast for biorefinery and biofuel production[J]. Applied Energy, 2014, 132: 465-474. |
| 70 | BANAT I M, NIGAM P, MARCHANT R. Isolation of thermotolerant, fermentative yeasts growing at 52 ℃ and producing ethanol at 45 ℃ and 50 ℃[J]. World Journal of Microbiology and Biotechnology, 1992, 8(3): 259-263. |
| 71 | RAIMONDI S, ZANNI E, AMARETTI A, et al. Thermal adaptability of Kluyveromyces marxianus in recombinant protein production[J]. Microbial Cell Factories, 2013, 12: 34. |
| 72 | LIN Y J, CHANG J J, LIN H Y, et al. Metabolic engineering a yeast to produce astaxanthin[J]. Bioresource Technology, 2017, 245: 899-905. |
| 73 | YANG X X, WANG D M, HONG J. Carotenoid production from nondetoxified xylose mother liquid or corncob hydrolysate with engineered Kluyveromyces marxianus [J]. Bioresource Technology, 2022, 364: 128080. |
| 74 | 서용배, 이종규, 정태혁, 등. 대장균에서 고세균 샤페론을 이용한 아스타잔틴 생산능 향상을 위한 연구[J/OL]. 생명과학회지, 2015, 25(12): 1339-1346[2023-09-01]. . |
| SEO Y B, LEE J K, JEONG T H, et al. Enhanced production of astaxanthin by Archaea chaperonin in Escherichia coli [J/OL]. Journal of Life Science, 2015, 25(12): 1339-1346[2023-09-01]. . | |
| 75 | JEONG T H, CHO Y S, CHOI S S, et al. Enhanced production of astaxanthin by metabolically engineered non-mevalonate pathway in Escherichia coli [J/OL]. Microbiology and Biotechnology Letters, 2018, 46(2): 114-119[2023-09-01]. . |
| 76 | 이재형, 서용배, 김영태. 대장균에서 이소프레노이드 생합성 경로의 대사공학적 개량에 의한 아스타잔틴의 생산성 향상[J/OL]. 생명과학회지, 2008, 18(12): 1764-1770[2023-09-01]. . |
| LEE J H, SEO Y B, KIM Y T. Enhanced production of astaxanthin by metabolic engineered isoprenoid pathway in Escherichia coli [J]. Journal of Life Science, 2008, 18(12): 1764-1770[2023-09-01]. . | |
| 77 | LEMUTH K, STEUER K, ALBERMANN C. Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin[J]. Microbial Cell Factories, 2011, 10: 29. |
| 78 | LU Q, BU Y F, LIU J Z. Metabolic engineering of Escherichia coli for producing astaxanthin as the predominant carotenoid[J]. Marine Drugs, 2017, 15(10): 296. |
| 79 | WU Y Q, YAN P P, LIU X W, et al. Combinatorial expression of different β-carotene hydroxylases and ketolases in Escherichia coli for increased astaxanthin production[J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(11): 1505-1516. |
| 80 | LI S, HUANG J C. Assessment of expression cassettes and culture media for different Escherichia coli strains to produce astaxanthin[J]. Natural Products and Bioprospecting, 2018, 8(5): 397-403. |
| 81 | LU Q, LIU J Z. Enhanced astaxanthin production in Escherichia coli via morphology and oxidative stress engineering[J]. Journal of Agricultural and Food Chemistry, 2019, 67(42): 11703-11709. |
| 82 | LI D, LI Y, XU J Y, et al. Engineering CrtW and CrtZ for improving biosynthesis of astaxanthin in Escherichia coli [J]. Chinese Journal of Natural Medicines, 2020, 18(9): 666-676. |
| 83 | ZHANG M, GONG Z K, TANG J L, et al. Improving astaxanthin production in Escherichia coli by co-utilizing CrtZ enzymes with different substrate preference[J]. Microbial Cell Factories, 2022, 21(1): 71. |
| 84 | LV Y K, EDWARDS H, ZHOU J W, et al. Combining 26S rDNA and the cre-IoxP system for iterative gene integration and efficient marker curation in yarrowia lipolytica[J]. ACS Synthetic Biology, 2019, 8(3): 568-576. |
| 85 | DATSENKO K A, WANNER B L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(12): 6640-6645. |
| 86 | YE L J, ZHU X N, WU T, et al. Optimizing the localization of astaxanthin enzymes for improved productivity[J]. Biotechnology for Biofuels, 2018, 11: 278. |
| 87 | FU D X, LIBSON A, MIERCKE L J W, et al. Structure of a glycerol-conducting channel and the basis for its selectivity[J]. Science, 2000, 290(5491): 481-486. |
| 88 | NEOPHYTOU I, HARVEY R, LAWRENCE J, et al. Eukaryotic integral membrane protein expression utilizing the Escherichia coli glycerol-conducting channel protein (GlpF)[J]. Applied Microbiology and Biotechnology, 2007, 77(2): 375-381. |
| 89 | KATSUMATA T, ISHIBASHI T, KYLE D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats[J]. Toxicology Reports, 2014, 1: 582-588. |
| 90 | HONDA M, KAGEYAMA H, HIBINO T, et al. Efficient and environmentally friendly method for carotenoid extraction from Paracoccus carotinifaciens utilizing naturally occurring Z-isomerization-accelerating catalysts[J]. Process Biochemistry, 2020, 89: 146-154. |
| 91 | HONDA M, KAGEYAMA H, HIBINO T, et al. Improved carotenoid processing with sustainable solvents utilizing Z-isomerization-induced alteration in physicochemical properties: a review and future directions[J]. Molecules, 2019, 24(11): 2149. |
| 92 | YANG S, ZHOU Q X, YANG L, et al. Effect of thermal processing on astaxanthin and astaxanthin esters in Pacific white shrimp Litopenaeus vannamei [J]. Journal of Oleo Science, 2015, 64(3): 243-253. |
| 93 | YUAN J P, CHEN F. Isomerization of trans-astaxanthin to cis-isomers in organic solvents[J]. Journal of Agricultural and Food Chemistry, 1999, 47(9): 3656-3660. |
| 94 | HONDA M, KAGEYAMA H, MURAKAMI K, et al. Isomerization of Paracoccus carotinifaciens-derived carotenoids (astaxanthin, adonirubin, and adonixanthin) under subcritical water conditions[J]. ACS Food Science & Technology, 2021, 1(10): 1861-1868. |
| 95 | CHOUGLE J A, SINGHAL R S. Metabolic precursors and cofactors stimulate astaxanthin production in Paracoccus MBIC 01143[J]. Food Science and Biotechnology, 2012, 21(6): 1695-1700. |
| 96 | 蹇华丽, 朱明军, 吴振强, 等. 环状芽孢杆菌胞壁溶解酶用于红发夫酵母虾青素提取的研究[J]. 高校化学工程学报, 2006, 20(1): 147-151. |
| JIAN H L, ZHU M J, WU Z Q, et al. Extracting astaxanthin of Phaffia rhodozyma with lytic enzyme produced by Bacillus circulans A 1.383[J]. Journal of Chemical Engineering of Chinese Universities, 2006, 20(1): 147-151. | |
| 97 | NI H, CHEN Q H, HE G Q, et al. Optimization of acidic extraction of astaxanthin from Phaffia rhodozyma [J]. Journal of Zhejiang University Science B (Biomedicine & Biotechnology), 2008, 9(1): 51-59. |
| 98 | 欧阳琴, 陈兴才, 黄亚治. 雨生红球藻提取虾青素不同机械破壁方法的研究[J]. 福州大学学报(自然科学版), 2005, 33(1): 111-115. |
| OUYANG Q, CHEN X C, HUANG Y Z. Study on extracting astaxanthin from Haematococcus pluvialis by various mechanical methods[J]. Journal of Fuzhou University (Natural Sciences Edtion), 2005, 33(1): 111-115. | |
| 99 | 邢涛, 张慧, 祁琳琳, 等. 从雨生球藻中提取虾青素的工艺研究[J]. 中国食品添加剂, 2018(11): 169-174. |
| XING T, ZHANG H, QI L L, et al. Study on the extraction of astaxanthin from Haematcoccus pluvialis [J]. China Food Additives, 2018(11): 169-174. | |
| 100 | 高岩, 邢丽红, 孙伟红, 等. 不同来源虾青素提取、纯化及定量检测方法的研究进展[J]. 食品安全质量检测学报, 2020, 11(5): 1414-1423. |
| GAO Y, XING L H, SUN W H, et al. Research progress on extraction, purification and quantitative detection methods of astaxanthin from different sources[J]. Journal of Food Safety & Quality, 2020, 11(5): 1414-1423. | |
| 101 | ANG F S, KHAW S Y, FEW L L, et al. Isolation of a stable astaxanthin-hyperproducing mutant of Xanthophyllomyces dendrorhous through random mutagenesis[J]. Applied Biochemistry and Microbiology, 2019, 55(3): 255-263. |
| 102 | 周锦珂, 李金华, 葛发欢, 等. 酶法提取雨生红球藻中虾青素的新工艺研究[J]. 中药材, 2008, 31(9): 1423-1425. |
| ZHOU J K, LI J H, GE F H, et al. Study on the new technology of enzymatic extraction of astaxanthin from Haematococcus pluvialis [J]. Journal of Chinese Medicinal Materials, 2008, 31(9): 1423-1425. | |
| 103 | LARROUDE M, CELINSKA E, BACK A, et al. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene[J]. Biotechnology and Bioengineering, 2018, 115(2): 464-472. |
| 104 | GAO S L, TONG Y Y, ZHU L, et al. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production[J]. Metabolic Engineering, 2017, 41: 192-201. |
| [1] | 郭姝媛, 张倩楠, 姑丽克孜·买买提热夏提, 杨一群, 于涛. 液体生物燃料合成与炼制的研究进展[J]. 合成生物学, 2025, 6(1): 18-44. |
| [2] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [3] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [4] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [5] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [6] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [7] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [8] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [9] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [10] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [11] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [12] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [13] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [14] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [15] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||