合成生物学 ›› 2020, Vol. 1 ›› Issue (2): 226-246.DOI: 10.12211/2096-8280.2020-010
多酶催化串联策略在复杂天然产物合成中的应用
贺俊斌, 孟松, 潘海学, 唐功利
- 中国科学院上海有机化学研究所, 生命有机化学国家重点实验室, 上海 200032
-
收稿日期:2020-02-29修回日期:2020-03-17出版日期:2020-04-30发布日期:2020-08-04 -
作者简介:贺俊斌(1992—),男,博士,博士后,主要从事天然产物生物合成研究。E-mail:jbhe@sioc.ac.cn
唐功利(1971—),男,博士,研究员,主要从事天然产物生物合成和化学生物学研究。E-mail:gltang@sioc.ac.cn -
基金资助:上海市自然科学基金项目(18ZR1448500)
Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products
HE Junbin, MENG Song, PAN Haixue, TANG Gongli
- State Key Laboratory of Bio-organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China
-
Received:2020-02-29Revised:2020-03-17Online:2020-04-30Published:2020-08-04
摘要:
结构新颖、复杂多样的天然产物及其衍生物一直是新药发现的重要源泉。然而,传统的从动植物及微生物中提取分离天然产物的方法往往存在效率低、耗时长、成本高等问题,极大地制约了新型药物的研发。天然产物的全合成主要包括化学合成和生物合成,其中体外酶催化合成因其具有催化效率高、反应选择性强、目标产物专一、反应条件简单温和、绿色环保等优点,为复杂天然产物的合成提供了有效策略。本文总结了近年来采用体外酶催化方法合成复杂天然产物的研究进展,着重介绍了多酶催化串联方法在聚酮类、生物碱类、萜类、甾体类、大环内酰胺类、核苷类等复杂天然产物合成中的应用,并对其目前存在的问题以及解决对策进行了讨论,同时对其未来发展也进行了展望。
中图分类号:
引用本文
贺俊斌, 孟松, 潘海学, 唐功利. 多酶催化串联策略在复杂天然产物合成中的应用[J]. 合成生物学, 2020, 1(2): 226-246.
HE Junbin, MENG Song, PAN Haixue, TANG Gongli. Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products[J]. Synthetic Biology Journal, 2020, 1(2): 226-246.

图1 多酶催化串联法合成肠道菌素(1)和wailupemycins[SAM,S-腺苷蛋氨酸 (S-adenosyl methionine);NADPH,烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate);ATP,三磷酸腺苷(adenosine triphosphate);ferredoxin,铁氧还蛋白;ferredoxin-NADP+ reductase,铁氧还蛋白- NADP+还原酶;catalase,过氧化氢酶]
Fig. 1 Multienzyme-catalyzed tandem synthesis of enterocin (1) and wailupemycins
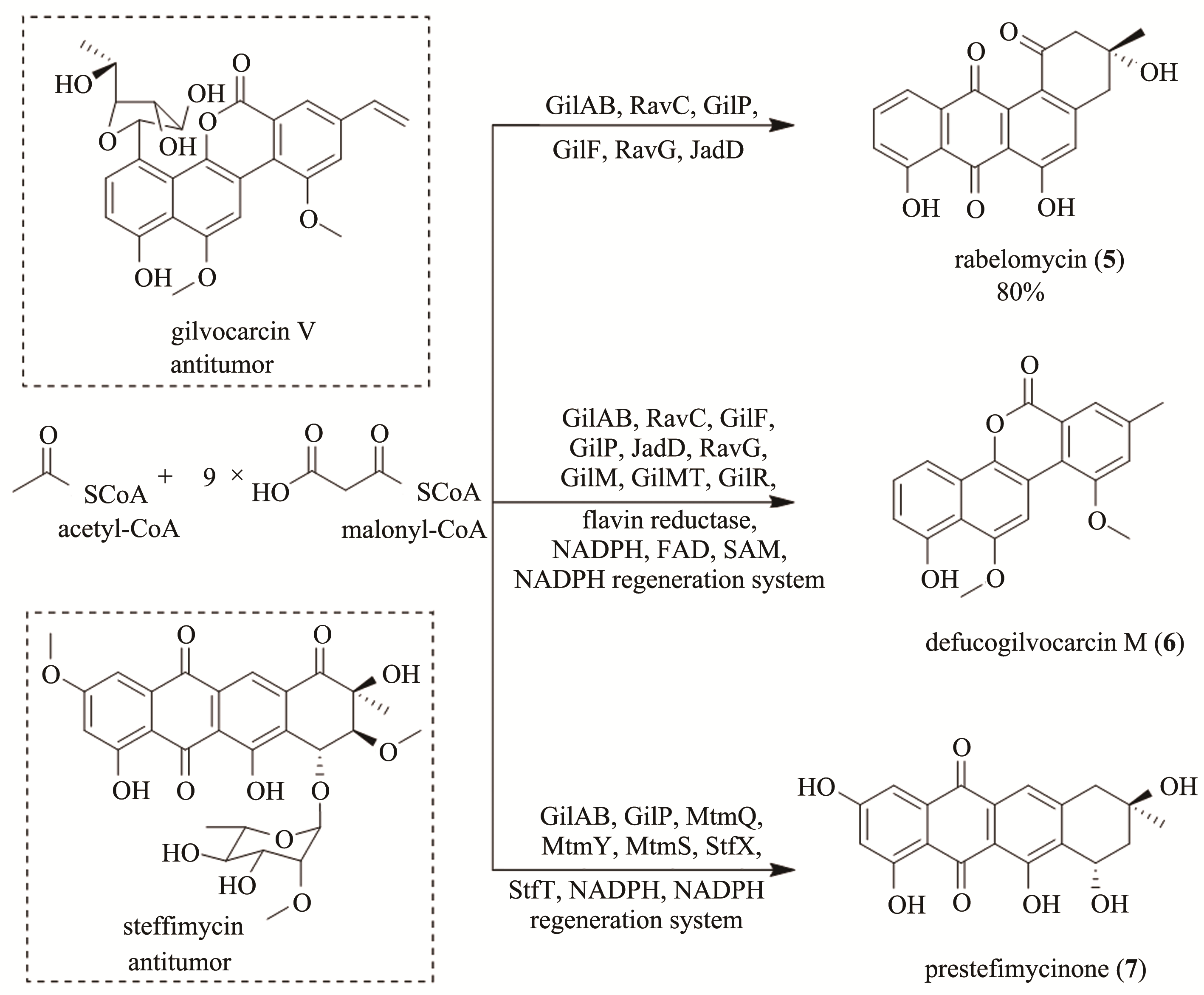
图2 Gilvocarcin V和司替霉素的结构式及多酶催化串联法合成腊伯罗霉素(5)、defucogilvocarcin M(6)和presteffimycinone(7)[flavin reductase,黄素还原酶;FAD,黄素腺嘌呤二核苷酸(flavin adenine dinucleotide)]
Fig. 2 Chemical structures of gilvocarcin V and steffimycin and the multienzyme-catalyzed tandem synthesis of rabelomycin (5), defucogilvocarcin M (6) and presteffimycinone (7)
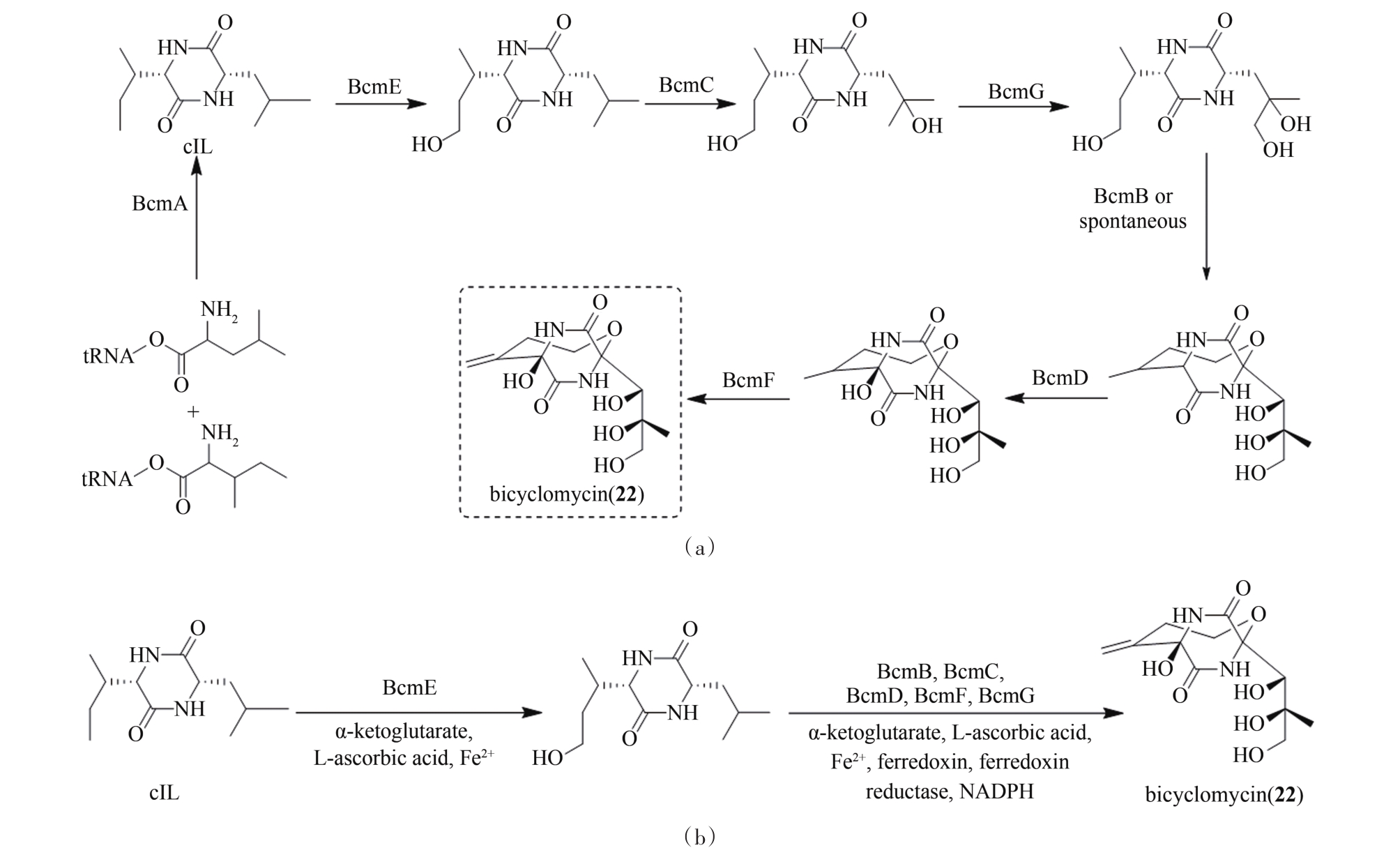
图6 双环霉素(22)的生物合成途径(a)和体外酶催化合成(b)(α-ketoglutarate,α-酮戊二酸;L-ascorbic acid,抗坏血酸)
Fig. 6 Biosynthetic pathway (a) and in vitro multienzyme-catalyzed synthesis (b) of bicyclomycin (22)
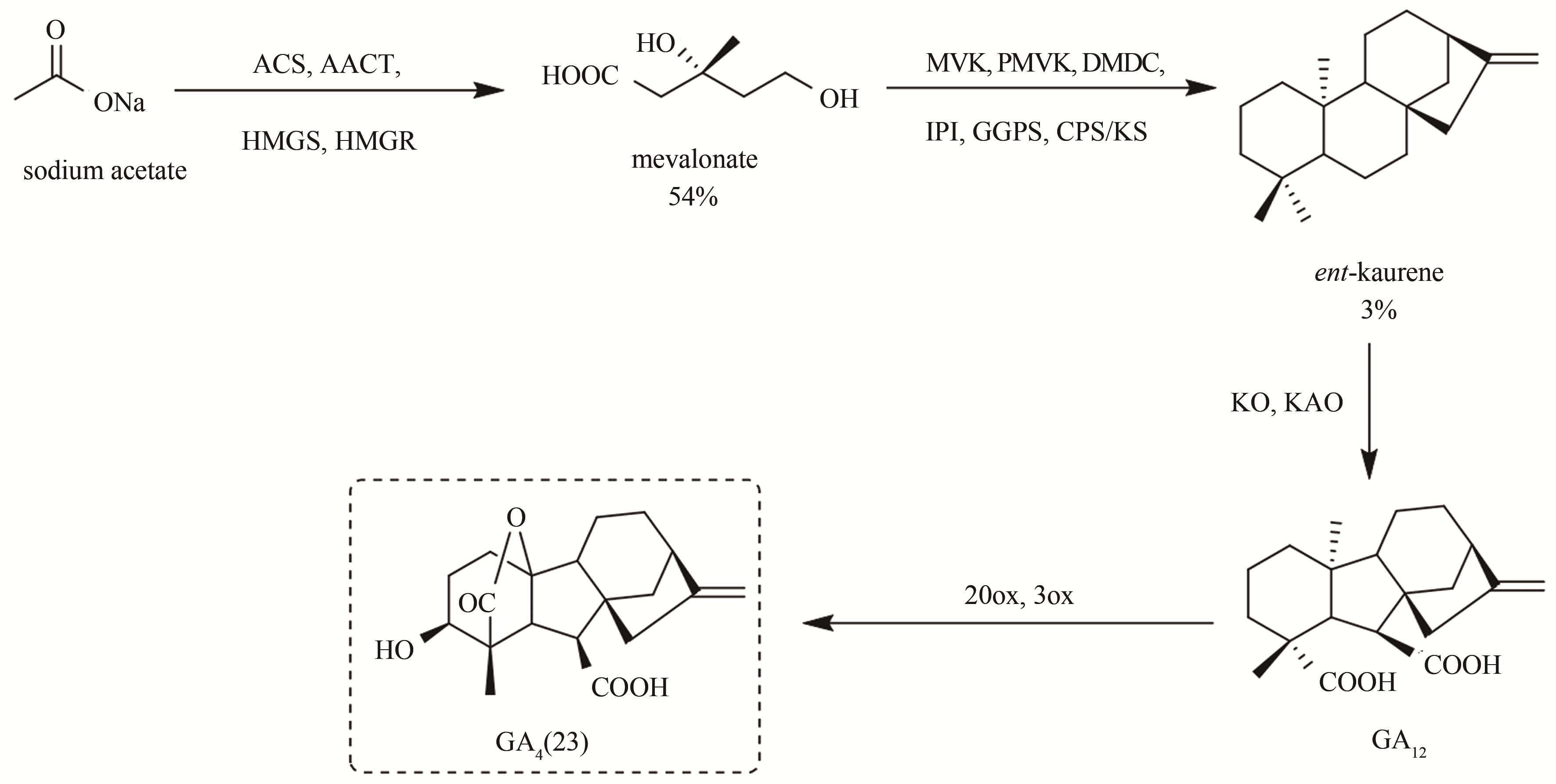
图7 多酶催化串联法合成赤霉素GA4(23)[ACS,乙酰辅酶A合成酶(acetyl-CoA synthase);AACT,乙酰乙酰辅酶A硫解酶(acetoacetyl-CoA thiolase);HMGS,羟甲基戊二酰辅酶A合成酶(hydroxymethylglutaryl-CoA synthase);HMGR,羟甲基戊二酰辅酶A还原酶(hydroxymethylglutaryl-CoA reductase);MVK,甲羟戊酸激酶(mevalonate kinase);PMVK,磷酸甲羟戊酸激酶(phosphomevalonate kinase);DMDC,二磷酸甲羟戊酸脱羧酶(diphosphomevalonate decarboxylase);IPI,异戊烯基焦磷酸异构酶(isopentenyl diphosphate isomerase);GGPS,牻牛儿基牻牛儿基焦磷酸合成酶(geranylgeranyl diphosphate synthase)]
Fig. 7 Multienzyme-catalyzed tandem synthesis of gibberellin A4 (23)

图8 多酶催化串联法合成熊去氧胆酸(24)(lactate,乳酸盐;pyruvate,丙酮酸盐;gluconate,葡萄糖酸盐;glucose,葡萄糖)
Fig. 8 Multienzyme-catalyzed tandem synthesis of ursodeoxycholic acid (24)
| 1 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs from 1981 to 2014[J]. Journal of Natural Products, 2016, 79(3):629-661. |
| 2 | ATANASOV A G, WALTENBERGER B, PFERSCHY-WENZIG E M, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review[J]. Biotechnology Advances, 2015, 33(8): 1582-1614. |
| 3 | PAULI G F, CHEN S N, FRIESEN J B, et al. Analysis and purification of bioactive natural products: the AnaPurNa study[J]. Journal of Natural Products, 2012, 75(6): 1243-1255. |
| 4 | WÖHLER F. Ueber künstliche bildung des harnstoffs[J]. Annalen der Physik, 1828, 87(2):253-256. |
| 5 | NICOLAOU K C, VOURLOUMIS D, WINSSINGER N, et al. The art and science of total synthesis at the dawn of the twenty-first century[J]. Angewandte Chemie International Edition, 2000, 39(1):44-122. |
| 6 | NICOLAOU K C. Inspirations, discoveries, and future perspectives in total synthesis[J]. The Journal of Organic Chemistry, 2009, 74(3):951-972. |
| 7 | HOFFMANN R W. Natural product synthesis: changes over time[J]. Angewandte Chemie International Edition, 2013, 52(1):123-130. |
| 8 | AICHER T D, BUSZEK K R, FANG F G, et al. Total synthesis of halichondrin B and norhalichondrin B[J]. Journal of the American Chemical Society, 1992, 114(8):3162-3164. |
| 9 | KAWANO S, ITO K, YAHATA K, et al. A landmark in drug discovery based on complex natural product synthesis[J]. Scientific Reports, 2019, 9(8656):1-9. |
| 10 | 黄伟, 王健博, 唐功利. 天然产物类药物的合成生物学研究[J]. 生命科学, 2011, 23(9):891-899. |
| HUANG W, WANG J B, TANG G L. Synthetic biology toward medicinal natural products[J]. Chinese Bulletin of Life Sciences, 2011, 23(9):891-899. | |
| 11 | 马大为, 谢卫青. 天然产物全合成——来自大自然的机遇和挑战[J]. 世界科学, 2014,(8):51-52. |
| MA D W, XIE W Q. Total synthesis of natural products-opportunities and challenges from nature[J]. World Science, 2014,(8):51-52. | |
| 12 | 卢志国, 汪建峰, 蒙海林, 等. 合成生物学与天然产物开发[J]. 生命科学, 2011, 23(9):900-911. |
| LU Z G, WANG J F, MENG H L, et al. Synthetic biology and natural products development[J]. Chinese Bulletin of Life Sciences, 2011, 23(9):900-911. | |
| 13 | TIBREWAL N, TANG Y. Biocatalysts for natural product biosynthesis[J]. Annual Review of Chemical and Biomolecular Engineering, 2014, 5(1):347-366. |
| 14 | 潘海学, 袁华, 蹇晓红, 等. 天然产物生物合成与抗肿瘤药物合成生物学研究[J]. 中国科学:生命科学, 2015, 45(10):1027-1039. |
| PAN H X, YUAN H, JIAN X H, et al. Biosynthesis of natural products and synthetic biology of antitumor drugs[J]. Scientia Sinica Vitae, 2015, 45(10):1027-1039 | |
| 15 | SUN H, LIU Z, ZHAO H, et al. Recent advances in combinatorial biosynthesis for drug discovery[J]. Drug Design, Development and Therapy, 2015, 2015(9):823-833. |
| 16 | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2):801-838. |
| 17 | OROZ-GUINEA I, GARCÍA-JUNCEDA E. Enzyme catalysed tandem reactions[J]. Current Opinion in Chemical Biology, 2013, 17(2):236-249. |
| 18 | SPERL J M, SIEBER V. Multienzyme cascade reactions—status and recent advances[J]. ACS Catalysis, 2018, 8(3):2385-2396. |
| 19 | 范宜晓, 王学恭, 刘庆芬. 酶法技术在发酵类制药中的研究与应用[J]. 生物产业技术, 2019 (2):38-48. |
| FAN Y X, WANG X G, LIU Q F. Research and application of enzymatic catalysis technology in fermentative pharmaceuticals[J]. Biotechnology & Business, 2019(2):38-48. | |
| 20 | SHODA S, UYAMA H, KADOKAWA J, et al. Enzymes as green catalysts for precision macromolecular synthesis[J]. Chemical Reviews, 2016, 116(4):2307-2413. |
| 21 | 许凡. 酶催化串联方法合成手性分子研究[D]. 杭州: 浙江大学, 2016. |
| XU F. Study on enzymatic cascade reaction for the synthesis of chiral compounds[D]. Hangzhou: Zhejiang University, 2016. | |
| 22 | LOPEZ-GALLEGO F, SCHMIDT-DANNERT C. Multi-enzymatic synthesis[J]. Current Opinion in Chemical Biology, 2010, 14(2):174-183. |
| 23 | FERNÁNDEZ-LUCAS J. Multienzymatic synthesis of nucleic acid derivatives: a general perspective[J]. Applied Microbiology and Biotechnology, 2015, 99(11):4615-4627. |
| 24 | WALSH C T, MOORE B S. Enzymatic cascade reactions in biosynthesis[J]. Angewandte Chemie International Edition, 2019, 58(21):6846-6879. |
| 25 | SANTACOLOMA P A, SIN G, GERNAEY K V, et al. Multienzyme-catalyzed processes: next-generation biocatalysis[J]. Organic Process Research & Development, 2011, 15(1):203-212. |
| 26 | SÁ A G A, DE MENESES A C, DE ARAÚJO P H H, et al. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries[J]. Trends in Food Science & Technology, 2017, 69(1):95-105. |
| 27 | MIYAIRI N, SAKAI H, KONOMI T, et al. Enterocin, a new antibiotic[J]. The Journal of Antibiotics, 1976, 29(3):227-235. |
| 28 | PIEL J, HOANG K, MOORE B S. Natural metabolic diversity encoded by the enterocin biosynthesis gene cluster[J]. Journal of the American Chemical Society, 2000, 122(22):5415-5416. |
| 29 | PIEL J, HERTWECK C, SHIPLEY P R, et al. Cloning, sequencing and analysis of the enterocin biosynthesis gene cluster from the marine isolate 'Streptomyces maritimus': evidence for the derailment of an aromatic polyketide synthase[J]. Chemistry & Biology, 2000, 7(12):943-955. |
| 30 | XIANG L, KALAITZIS J A, MOORE B S. EncM, a versatile enterocin biosynthetic enzyme involved in Favorskii oxidative rearrangement, aldol condensation, and heterocycle-forming reactions[J]. PNAS, 2004, 101(44):15609-15614. |
| 31 | IZUMIKAWA M, MELUZZI D, MOORE B S, et al. Enzymatic total synthesis of enterocin polyketides[J]. Nature Chemical Biology, 2007, 3(9):557-558. |
| 32 | KALAITZIS J A, CHENG Q, THOMAS P M, et al. In vitro biosynthesis of unnatural enterocin and wailupemycin polyketides[J]. Journal of Natural Products, 2009, 72(3):469-472. |
| 33 | 杜凤翔, 杨靖, 李金洋, 等. 非典型角蒽环聚酮氧化开环酶的功能与进化[J]. 微生物学通报, 2019, 46(2):423-433. |
| DU F X, YANG J, LI J Y, et al. Catalysis and evolution of the ring-opening oxygenases in biosynthesis of atypical angucyclines[J]. Microbiology China, 2019, 46(2):423-433. | |
| 34 | MATSUMOTO A, HANAWALT P C. Histone H3 and heat shock protein GRP78 are selectively cross-linked to DNA by photoactivated gilvocarcin V in human fibroblasts[J]. Cancer Research, 2000, 60(14):3921-3926. |
| 35 | FISCHER C, LIPATA F, ROHR J. The complete gene cluster of the antitumor agent gilvocarcin V and its implication for the biosynthesis of the gilvocarcins[J]. Journal of the American Chemical Society, 2003, 125(26):7818-7819. |
| 36 | PAHARI P, KHAREL M K, SHEPHERD M D, et al. Enzymatic total synthesis of defucogilvocarcin M and its implications for gilvocarcin biosynthesis[J]. Angewandte Chemie International Edition, 2012, 51(5):1216-1220. |
| 37 | LIU W, PARKER W L, SLUSARCHYK D S, et al. Isolation, characterization, and structure of rabelomycin, a new antibiotic[J]. The Journal of Antibiotics, 1970, 23(9):437-441. |
| 38 | KHAREL M K, PAHARI P, LIAN H, et al. Enzymatic total synthesis of rabelomycin, an angucycline group antibiotic[J]. Organic Letters, 2010, 12(12):2814-2817. |
| 39 | WANG G, CHEN J, ZHU H, et al. One-pot enzymatic total synthesis of presteffimycinone, an early intermediate of the anthracycline antibiotic steffimycin biosynthesis[J]. Organic Letters, 2017, 19(3):540-543. |
| 40 | GULLÓN S, OLANO C, ABDELFATTAH M S, et al. Isolation, characterization, and heterologous expression of the biosynthesis gene cluster for the antitumor anthracycline steffimycin[J]. Applied and Environmental Microbiology, 2006, 72(6):4172-4183. |
| 41 | REUSSER F. Steffimycin B, a DNA binding agent[J]. Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis, 1975, 383(3):266-273. |
| 42 | SHEN B, HUTCHINSON C R. Enzymatic synthesis of a bacterial polyketide from acetyl and malonyl coenzyme A[J]. Science, 1993, 262(5139):1535-1540. |
| 43 | BAO W, WENDT-PIENKOWSKI E, HUTCHINSON C R. Reconstitution of the iterative type II polyketide synthase for tetracenomycin F2 biosynthesis[J]. Biochemistry, 1998, 37(22):8132-8138. |
| 44 | 邓永坤, 李辉, 袁芳. 赛洛西宾的毒性与应用[J]. 中国药物依赖性杂志, 2007, 2007(6):410-414. |
| DENG Y K, LI H, YUAN F. Toxicity and application of psilocybin[J]. Chinese Journal of Drug Dependence, 2007, 2007(6):410-414. | |
| 45 | HOFMANN A, HEIM R, BRACK A, et al. Psilocybin und psilocin, zwei psychotrope wirkstoffe aus mexikanischen rauschpilzen[J]. Helvetica Chimica Acta, 1959, 42(5):1557-1572. |
| 46 | MAHAPATRA A, GUPTA R. Role of psilocybin in the treatment of depression[J]. Therapeutic Advances in Psychopharmacology, 2017, 7(1):54-56. |
| 47 | CARHART-HARRIS R L, BOLSTRIDGE M, DAY C, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up[J]. Psychopharmacology, 2018, 235(2):399-408. |
| 48 | FRICKE J, BLEI F, HOFFMEISTER D. Enzymatic synthesis of psilocybin[J]. Angewandte Chemie International Edition, 2017, 56(40):12352-12355. |
| 49 | 刘金凤, 黄鹏, 卿志星, 等. 苄基异喹啉类生物碱生物合成与代谢工程研究进展[J]. 基因组学与应用生物学, 2016, 35(8):2194-2200. |
| LIU J F, HUANG P, QING Z X, et al. Advances in the biosynthesis and metabolic engineering research of benzylisoquinoline alkaloids[J]. Genomics and Applied Biology, 2016, 35(8):2194-2200. | |
| 50 | QING Z X, YANG P, TANG Q, et al. Isoquinoline alkaloids and their antiviral, antibacterial, and antifungal activities and structure-activity relationship[J]. Current Organic Chemistry, 2017, 21(18): 1920-1934. |
| 51 | STÖCKIGT J, ANTONCHICK A P, WU F, et al. The Pictet-Spengler reaction in nature and in organic chemistry[J]. Angewandte Chemie International Edition, 2011, 50(37):8538-8564. |
| 52 | FACCHINI P J, DE LUCA V. Opium poppy and madagascar periwinkle: model non‐model systems to investigate alkaloid biosynthesis in plants[J]. The Plant Journal, 2008, 54(4):763-784. |
| 53 | WANG Y, TAPPERTZHOFEN N, MENDEZ-SANCHEZ D, et al. Design and use of de novo cascades for the biosynthesis of new benzylisoquinoline alkaloids[J]. Angewandte Chemie International Edition, 2019, 58(30):10120-10125. |
| 54 | 张宏波. 天然产物Thaxtomin的全合成研究及活性开发[D]. 天津: 南开大学, 2014. |
| ZHANG H B. Total synthesis and biological activities discovery of thaxtomin[D]. Tianjin: Nankai University, 2014. | |
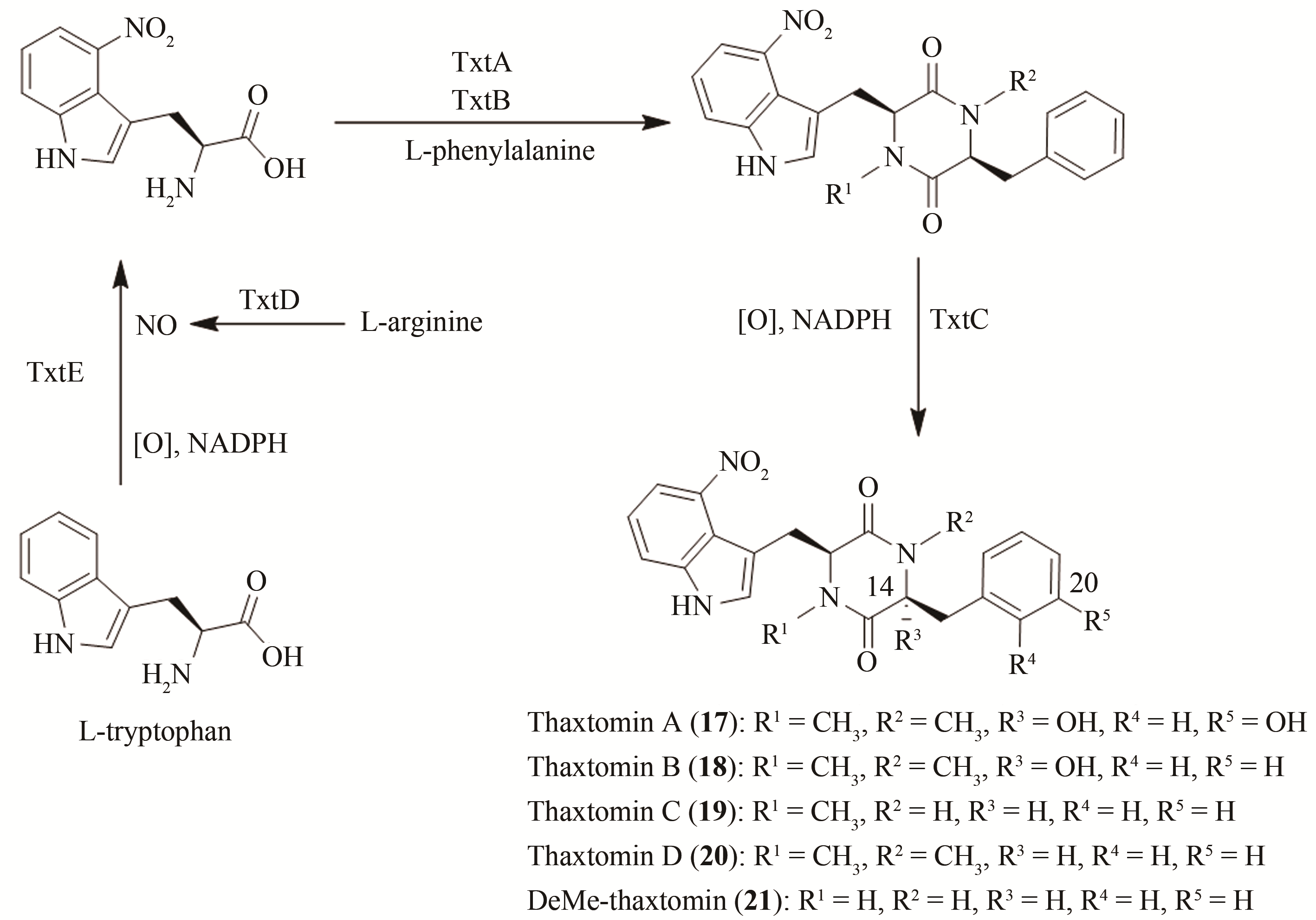
| 55 | FRY B A, LORIA R. Thaxtomin A: evidence for a plant cell wall target[J]. Physiological and Molecular Plant Pathology, 2002, 60(1):1-8. |
| 56 | ZHANG H, NING X, HANG H, et al. Total synthesis of thaxtomin A and its stereoisomers and findings of their biological activities[J]. Organic Letters, 2013, 15(22):5670-5673. |
| 57 | JIANG G, ZUO R, ZHANG Y, et al. One-pot biocombinatorial synthesis of herbicidal thaxtomins[J]. ACS Catalysis, 2018, 8(11):10761-10768. |
| 58 | ZUO R, ZHANG Y, JIANG C, et al. Engineered P450 biocatalysts show improved activity and regio-promiscuity in aromatic nitration[J]. Scientific Reports, 2017, 7(1):1-9. |
| 59 | MIYOSHI T, MIYAIRI N, AOKI H, et al. Bicyclomycin, a new antibiotic[J]. The Journal of Antibiotics, 1972, 25(10):569-575. |
| 60 | KOHN H, WIDGER W. The molecular basis for the mode of action of bicyclomycin[J]. Current Drug Targets-Infectious Disorders, 2005, 5(3):273-295. |
| 61 | LAWSON M R, DYER K, BERGER J M. Ligand-induced and small-molecule control of substrate loading in a hexameric helicase[J]. PNAS, 2016, 113(48):13714-13719. |
| 62 | WILLIAMS R M, ARMSTRONG R W, DUNG J S. Stereocontrolled total synthesis of (±)-and (+)-bicyclomycin: new carbon-carbon bond-forming reactions on electrophilic glycine anhydride derivatives[J]. Journal of the American Chemical Society, 1984, 106(19):5748-5750. |
| 63 | WILLIAMS R M, ARMSTRONG R W, DUNG J S. Stereocontrolled total synthesis of (±)-and (+)-bicyclomycin[J]. Journal of the American Chemical Society, 1985, 107(11):3253-3266. |
| 64 | MENG S, HAN W, ZHAO J, et al. A six-oxidase cascade for tandem C-H bond activation revealed by reconstitution of bicyclomycin biosynthesis[J]. Angewandte Chemie International Edition, 2018, 57(3):719-723. |
| 65 | 彭辉, 施天穹, 聂志奎, 等. 微生物发酵产赤霉素的研究进展[J]. 化工进展, 2016, 35(11):3611-3618. |
| PENG H, SHI T Q, NIE Z K, et al. Fermentative production of gibberellins:a review[J]. Chemical Industry and Engineering Progress, 2016, 35(11):3611-3618. | |
| 66 | SUGAI Y, MIYAZAKI S, MUKAI S, et al. Enzymatic total synthesis of gibberellin A4 from acetate[J]. Bioscience, Biotechnology, and Biochemistry, 2011, 75(1):128-135. |
| 67 | POUPON R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action[J]. Clinics and Research in Hepatology and Gastroenterology, 2012, 36(s1):S3-S12. |
| 68 | ZHENG M, WANG R, LI C, et al. Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques [J]. Process Biochemistry, 2015, 50(4):598-604. |
| 69 | ZHENG M M, CHEN F F, LI H, et al. Continuous production of ursodeoxycholic acid by using two cascade reactors with co-immobilized enzymes[J]. Chembiochem, 2018, 19(4):347-353. |
| 70 | ROMERO-FERNÁNDEZ M, PARADISI F. Protein immobilization technology for flow biocatalysis[J]. Current Opinion in Chemical Biology, 2020, 55(1):1-8. |
| 71 | REN S, LI C, JIAO X, et al. Recent progress in multienzymes co-immobilization and multienzyme system applications[J]. Chemical Engineering Journal, 2019, 373(19):1254-1278. |
| 72 | GREUNKE C, GLOCKLE A, ANTOSCH J, et al. Biocatalytic total synthesis of ikarugamycin[J]. Angewandte Chemie International Edition, 2017, 56(15):4351-4355. |
| 73 | JOMON K, KURODA Y, AJISAKA M, et al. A new antibiotic, ikarugamycin[J]. The Journal of Antibiotics, 1972, 25(5):271-280. |
| 74 | ZHANG G, ZHANG W, SAHA S, et al. Recent advances in discovery, biosynthesis and genome mining of medicinally relevant polycyclic tetramate macrolactams[J]. Current Topics in Medicinal Chemistry, 2016, 16(15):1727-1739. |
| 75 | HASUMI K, SHINOHARA C, NAGANUMA S, et al. Inhibition of the uptake of oxidized low‐density lipoprotein in macrophage J774 by the antibiotic ikarugamycin[J]. European Journal of Biochemistry, 1992, 205(2):841-846. |
| 76 | LUO T, FREDERICKSEN B L, HASUMI K, et al. Human immunodeficiency virus type 1 Nef-induced CD4 cell surface downregulation is inhibited by ikarugamycin[J]. Journal of Virology, 2001, 75(5):2488-2492. |
| 77 | ROUSH W R, WADA C K. Application of. eta. 4-diene iron tricarbonyl complexes in acyclic stereocontrol: asymmetric synthesis of the as-indacene unit of ikarugamycin (a formal total synthesis)[J]. Journal of the American Chemical Society, 1994, 116(5):2151-2152. |
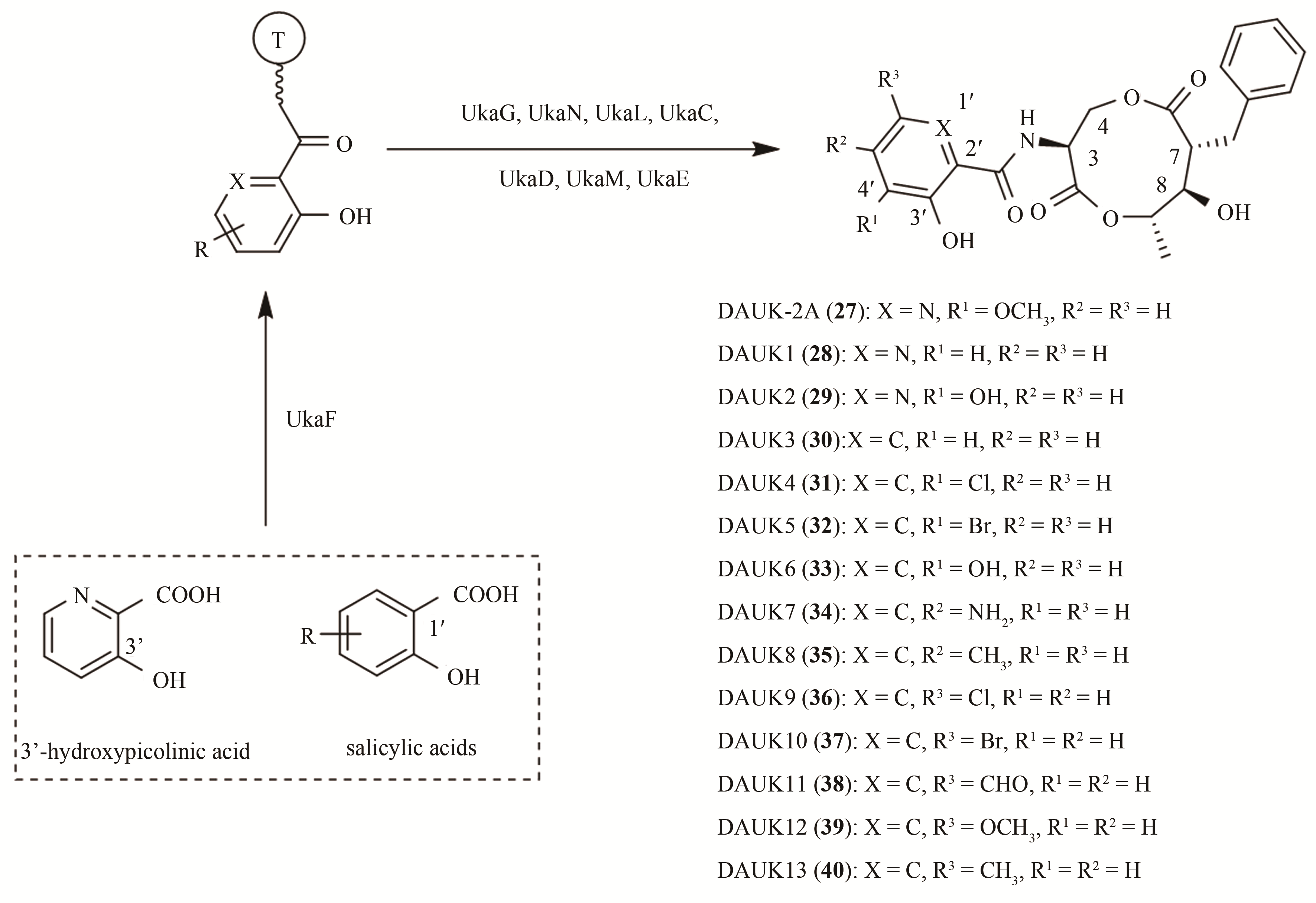
| 78 | OWEN W J, YAO C, MYUNG K, et al. Biological characterization of fenpicoxamid, a new fungicide with utility in cereals and other crops[J]. Pest Management Science, 2017, 73(10):2005-2016. |
| 79 | YOUNG D H, WANG N X, MEYER S T, et al. Characterization of the mechanism of action of the fungicide fenpicoxamid and its metabolite UK-2A[J]. Pest Management Science, 2018, 74(2):489-498. |
| 80 | USUKI Y, MITOMO K, ADACHI N, et al. Semi-synthesis and biological evaluation of analogues of UK-2A, a novel antifungal antibiotic from Streptomyces sp. 517-02[J]. Bioorganic & Medicinal Chemistry Letters, 2005, 15(8):2011-2014. |
| 81 | USUKI Y, ADACHI N, FUJITA K, et al. Structure–activity relationship studies on UK-2A, a novel antifungal antibiotic from Streptomyces sp. 517-02. Part 5: roles of the 9-membered dilactone-ring moiety in respiratory inhibition[J]. Bioorganic & Medicinal Chemistry Letters, 2006, 16(12):3319-3322. |
| 82 | TAN H, YANG X, DAI Q, et al. Unravelling the biosynthetic flexibility of UK-2A enables enzymatic synthesis of its structural variants[J]. ACS Synthetic Biology, 2019, 8(12):2659-2665. |
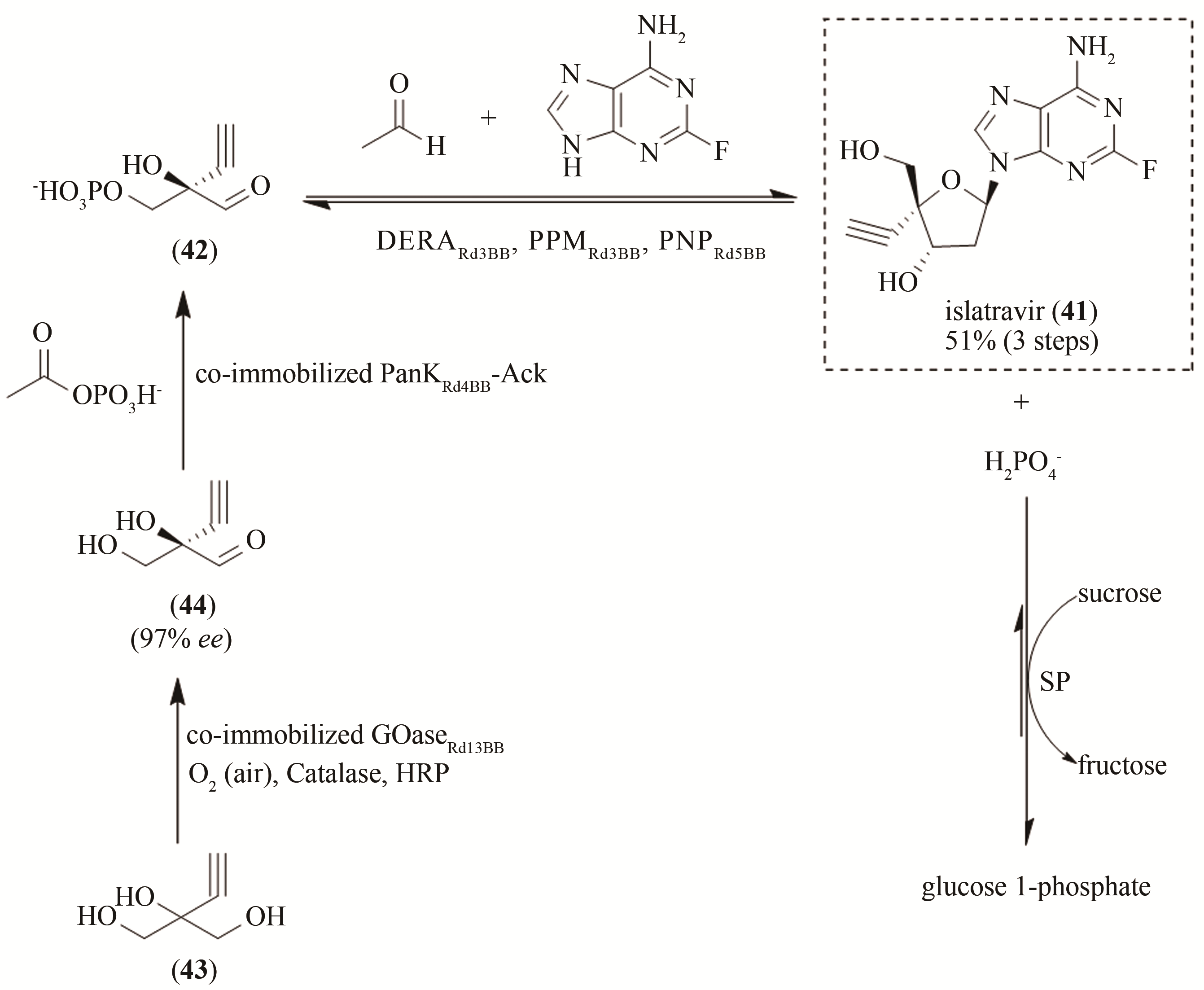
| 83 | MICHAILIDIS E, HUBER A D, RYAN E M, et al. 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms[J]. Journal of Biological Chemistry, 2014, 289(35):24533-24548. |
| 84 | MARKOWITZ M, GROBLER J A. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1[J]. Current Opinion in HIV and AIDS, 2020, 15(1):27-32. |
| 85 | FUKUYAMA K, OHRUI H, KUWAHARA S. Synthesis of EFdA via a diastereoselective aldol reaction of a protected 3-keto furanose[J]. Organic Letters, 2015, 17(4):828-831. |
| 86 | MCLAUGHLIN M, KONG J, BELYK K M, et al. Enantioselective synthesis of 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) via enzymatic desymmetrization[J]. Organic Letters, 2017, 19(4):926-929. |
| 87 | HUFFMAN M A, FRYSZKOWSKA A, ALVIZO O, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir[J]. Science, 2019, 366(6470):1255-1259. |
| 88 | TURNER N J, O'REILLY E. Biocatalytic retrosynthesis[J]. Nature Chemical Biology, 2013, 9(5):285-288. |
| 89 | MIKHAILOPULO I A, MIROSHNIKOV A I. New trends in nucleoside biotechnology[J]. Acta Naturae, 2010, 2(5):36-59. |
| 90 | ROESSNER C A, SPENCER J B, STOLOWICH N J, et al. Genetically engineered synthesis of precorrin-6x and the complete corrinoid, hydrogenobyrinic acid, an advanced precursor of vitamin B12 [J]. Chemistry & Biology, 1994, 1(2):119-124. |
| 91 | CACHO R A, CHOOI Y H, ZHOU H, et al. Complexity generation in fungal polyketide biosynthesis: a spirocycle-forming P450 in the concise pathway to the antifungal drug griseofulvin[J]. ACS Chemical Biology, 2013, 8(10):2322-2830. |
| 92 | TEUFEL R, KAYSSER L, VILLAUME M T, et al. One‐pot enzymatic synthesis of merochlorin A and B[J]. Angewandte Chemie International Edition, 2014, 53(41):11019-11022. |
| 93 | MCKINNIE S, MILES Z D, JORDAN P A, et al. Total enzyme syntheses of napyradiomycins A1 and B1[J]. Journal of the American Chemical Society, 2018, 140(51):17840-17845. |
| 94 | KLEIN A P, ANARAT CAPPILLINO G, SATTELY E S. Minimum set of cytochromes P450 for reconstituting the biosynthesis of camalexin, a major Arabidopsis antibiotic[J]. Angewandte Chemie International Edition, 2013, 52(51):13625-13628. |
| 95 | LIU J, NG T, RUI Z, et al. Unusual acetylation‐dependent reaction cascade in the biosynthesis of the pyrroloindole drug physostigmine[J]. Angewandte Chemie International Edition, 2014, 53(1):136-139. |
| 96 | DU Y, ALKHALAF L M, RYAN K S. In vitro reconstitution of indolmycin biosynthesis reveals the molecular basis of oxazolinone assembly[J]. PNAS, 2015, 112(9):2717-2722. |
| 97 | HEDGES J B, RYAN K S. In vitro reconstitution of the biosynthetic pathway to the nitroimidazole antibiotic azomycin[J]. Angewandte Chemie International Edition, 2019, 58(34):11647-11651. |
| 98 | TAKAHASHI H, LIU Y, LIU H. A two-stage one-pot enzymatic synthesis of TDP-L-mycarose from thymidine and glucose-1-phosphate[J]. Journal of the American Chemical Society, 2006, 128(5):1432-1433. |
| 99 | ZHANG W, NTAI I, BOLLA M L, et al. Nine enzymes are required for assembly of the pacidamycin group of peptidyl nucleoside antibiotics[J]. Journal of the American Chemical Society, 2011, 133(14):5240-5243. |
| 100 | SANKARANARAYANAN K, ANTARIS X X, PALANSKI B A, et al. Tunable enzymatic synthesis of the immunomodulator Lipid IVA to enable structure-activity analysis[J]. Journal of the American Chemical Society, 2019, 141(24):9474-9478. |
| 101 | DENARD C A, HARTWIG J F, ZHAO H. Multistep one-pot reactions combining biocatalysts and chemical catalysts for asymmetric synthesis[J]. ACS Catalysis, 2013, 3(12):2856-2864. |
| 102 | SCHRITTWIESER J H, VELIKOGNE S, HALL M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules[J]. Chemical Reviews, 2018, 118(1):270-348. |
| 103 | 李晓军, 张万斌, 高栓虎. 复杂天然产物全合成:化学合成与生物合成结合的策略[J]. 有机化学, 2018, 38(9):2185-2198. |
| LI X J, ZHANG W B, GAO S H. Total synthesis of complex natural products: combination of chemical synthesis and biosynthesis strategies[J]. Chinese Journal of Organic Chemistry, 2018, 38(9):2185-2198. | |
| 104 | 赵淑玲, 谷耀华, 薛屏. 化学-酶法高选择性合成手性化合物的研究进展[J]. 化学研究与应用, 2014, 26(4):473-482. |
| ZHAO S L, GU Y H, XUE P. Recent progress on chemo-enzymatic synthesis of chiral compounds with high stereoselectivity[J]. Chemical Research and Application, 2014, 26(4):473-482. | |
| 105 | 李龙, 邱贵森, 蒋泰龙, 等. 化学酶法合成盐酸度洛西汀的研究进展[J]. 化工进展, 2014, 33(7):1839-1843. |
| LI L, QIU G S, JIANG T L, et al. Research progresses in chemoenzymatic synthesis of Duloxetine hydrochloride[J]. Chemical Industry and Engineering Progress, 2014, 33(7):1839-1843. | |
| 106 | KING-SMITH E, ZWICK III C R, RENATA H. Applications of oxygenases in the chemoenzymatic total synthesis of complex natural products[J]. Biochemistry, 2018, 57(4):403-412. |
| 107 | XU Y, MASUKO S, TAKIEDDIN M, et al. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins[J]. Science, 2011, 334(6055):498-501. |
| 108 | BAKER DOCKREY S A, LUKOWSKI A L, BECKER M R, et al. Biocatalytic site- and enantioselective oxidative dearomatization of phenols[J]. Nature Chemistry, 2018, 10(2):119-125. |
| 109 | MORRILL L A, SUSICK R B, CHARI J V, et al. Total synthesis as a vehicle for collaboration[J]. Journal of the American Chemical Society, 2019, 141(32):12423-12443. |
| 110 | LAZZAROTTO M, HAMMERER L, HETMANN M, et al. Chemoenzymatic total synthesis of deoxy‐, epi‐, and podophyllotoxin and a biocatalytic kinetic resolution of dibenzylbutyrolactones[J]. Angewandte Chemie International Edition, 2019, 58(24):8226-8230. |
| 111 | KIM H J, CHOI S H, JEON B S, et al. Chemoenzymatic synthesis of spinosyn A[J]. Angewandte Chemie International Edition, 2014, 53(49):13553-13557. |
| 112 | KIRST H A. The spinosyn family of insecticides: realizing the potential of natural products research[J]. The Journal of Antibiotics, 2010, 63(3):101-111. |
| 113 | CAI X, BAI Y, DAI M. Total syntheses of spinosyn A[J]. Synlett, 2018, 29(20):2623-2632. |
| 114 | 张敏, 戴均贵. 微生物来源Diels-Alder型加合物生物合成研究进展[J]. 药学进展, 2018, 42(1):21-38. |
| ZHANG M, DAI J G. Progress in the biosynthesis of Diels-Alder adducts of microbial origin[J]. Progress in Pharmaceutical Sciences, 2018, 42(1):21-38. | |
| 115 | WALDRON C, MATSUSHIMA P, ROSTECK JR P R, et al. Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa [J]. Chemistry & Biology, 2001,8(5):487-499. |
| 116 | KIM H J, RUSZCZYCKY M W, CHOI S, et al. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A[J]. Nature, 2011, 473(7345):109-112. |
| 117 | PETITOU M, BOECKEL C A A VAN. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next?[J]. Angewandte Chemie International Edition, 2004, 43(24): 3118-3133. |
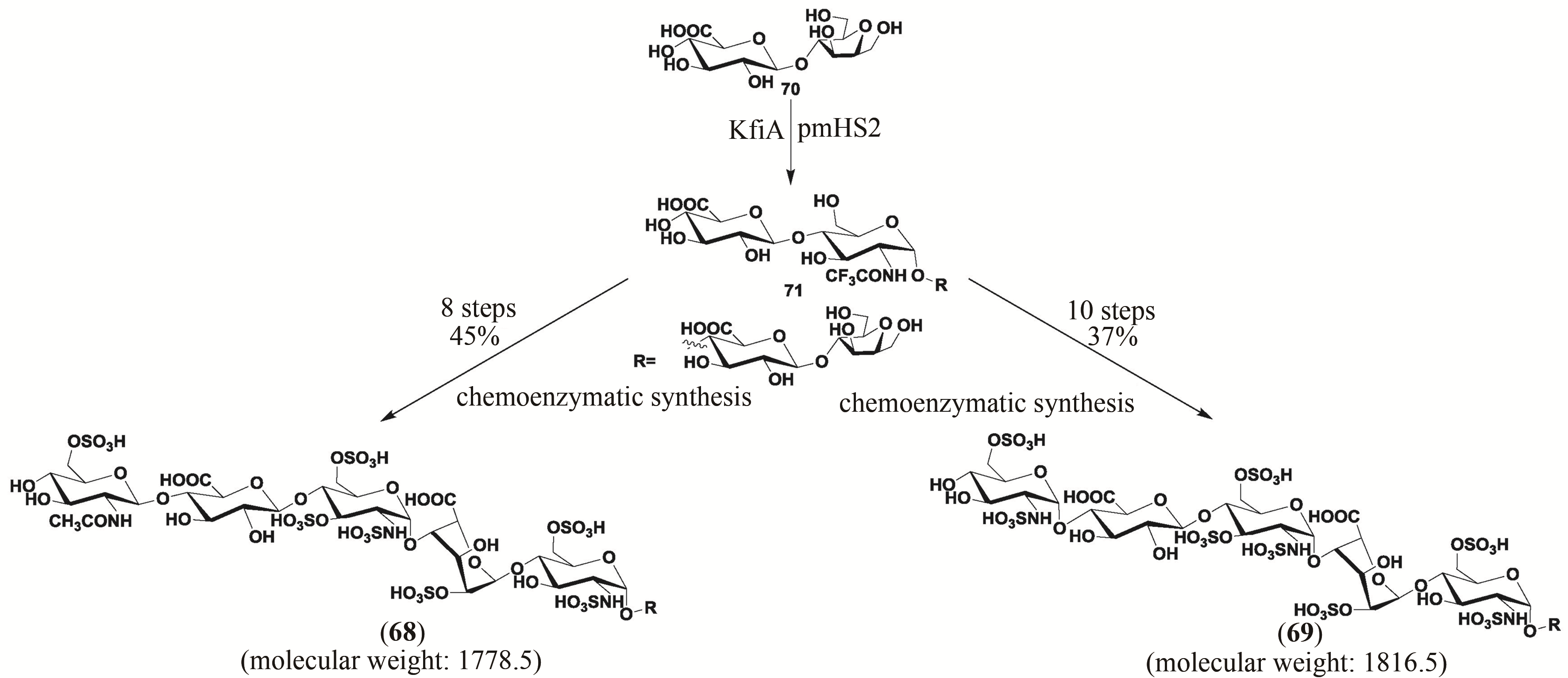
| 118 | LIU J, LINHARDT R J. Chemoenzymatic synthesis of heparan sulfate and heparin[J]. Natural Product Reports, 2014, 31(12): 1676-1685. |
| [1] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [2] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [3] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [4] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [5] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [6] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [7] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [8] | 程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980. |
| [9] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [10] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [11] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [12] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [13] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [14] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| [15] | 虞旭昶, 吴辉, 李雷. 文库构建与基因簇靶向筛选驱动的微生物天然产物高效发现[J]. 合成生物学, 2024, 5(3): 492-506. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||