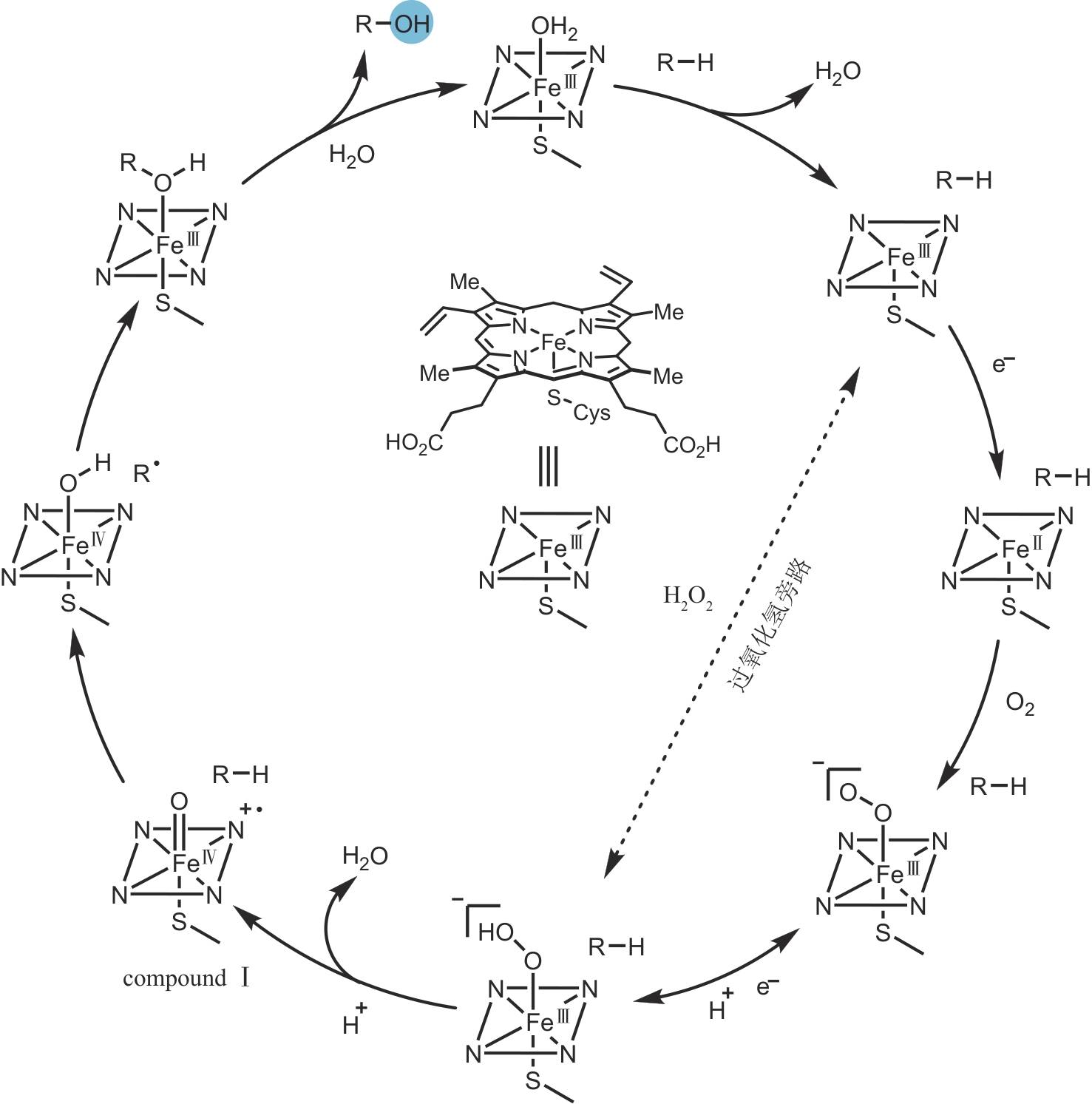
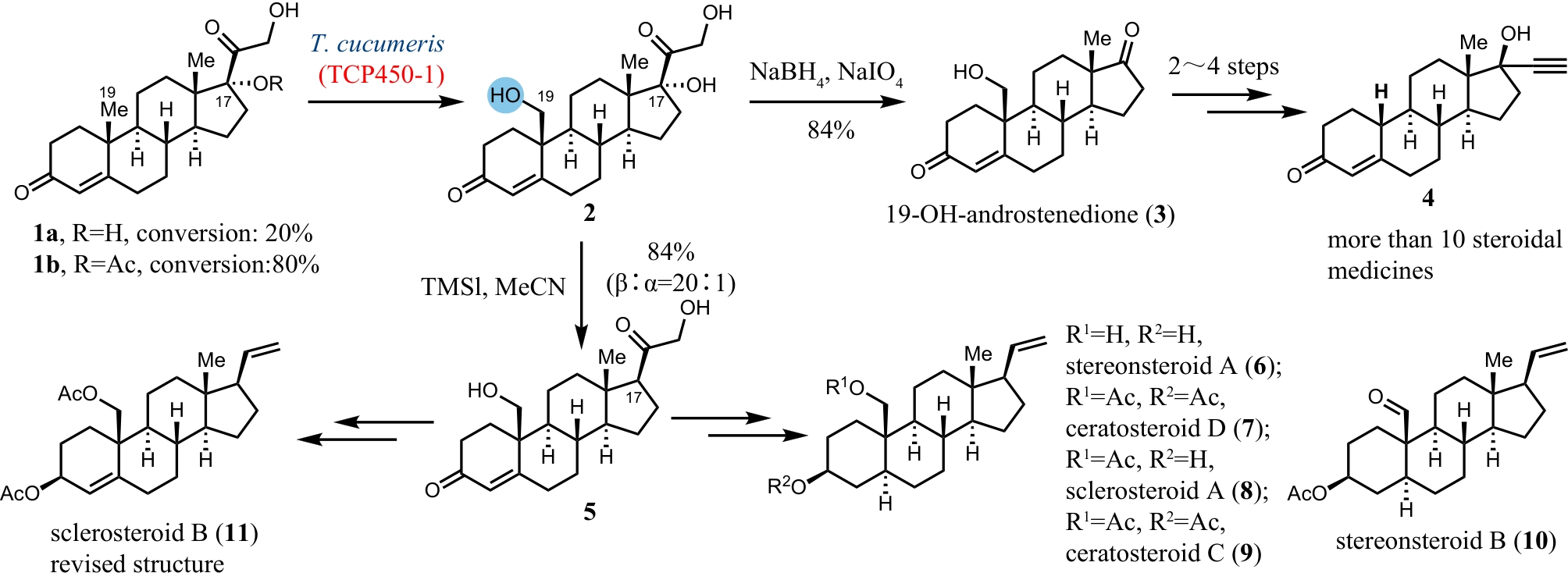
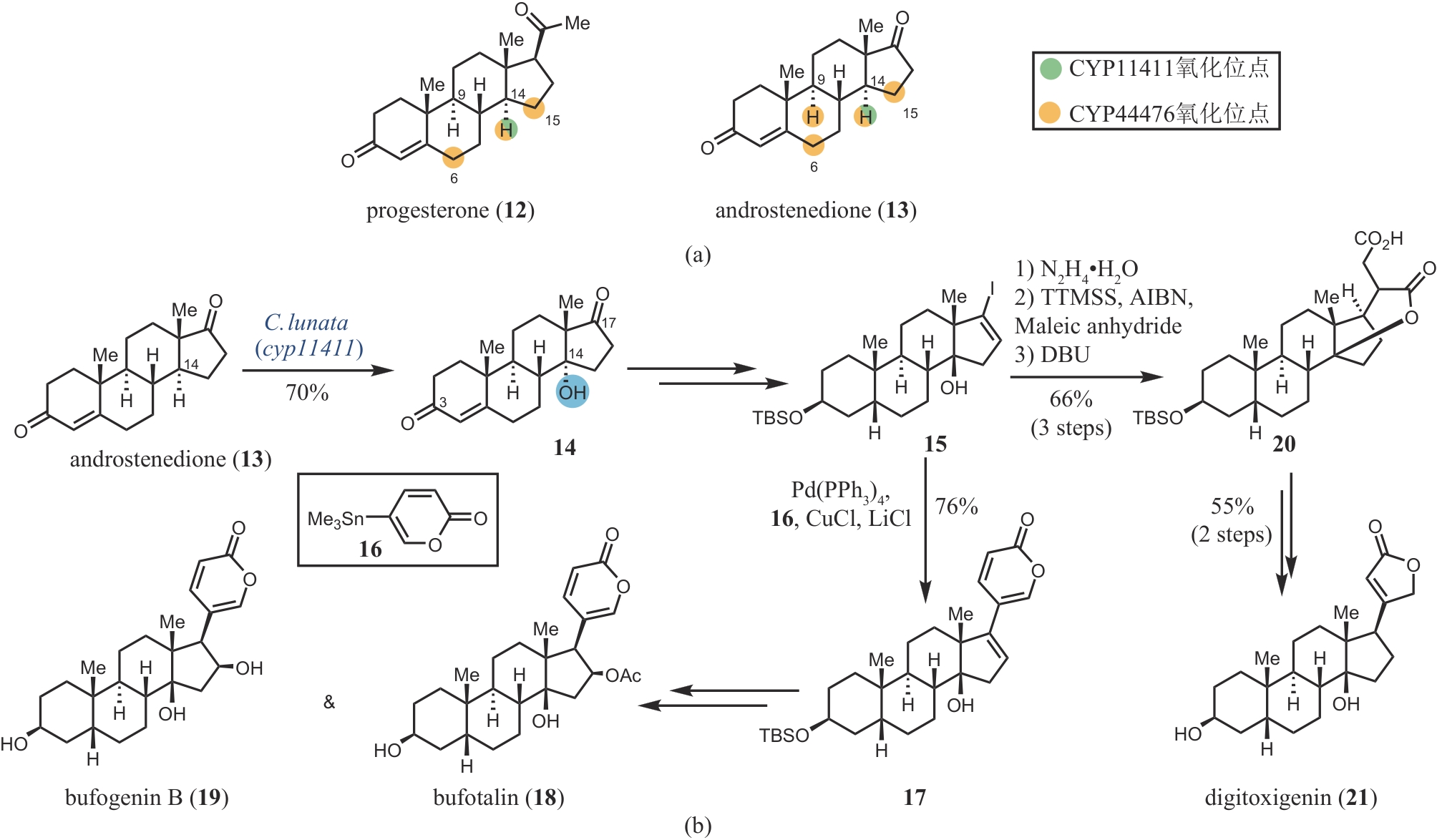
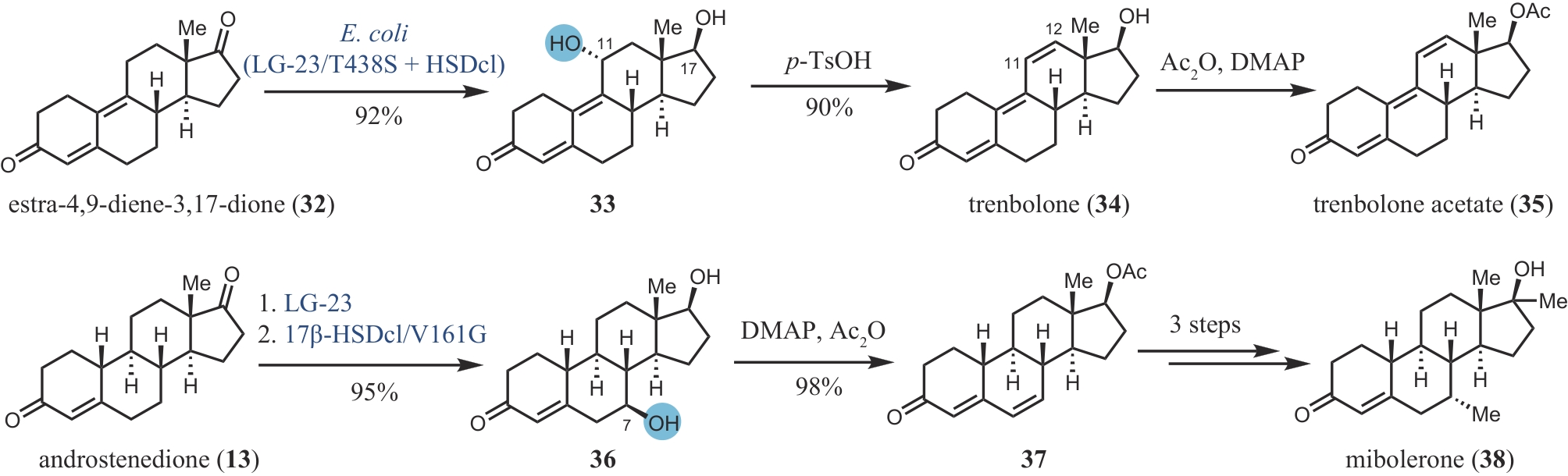
Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 960-980.DOI: 10.12211/2096-8280.2024-017
• Invited Review • Previous Articles Next Articles
Recent advances in chemoenzymatic synthesis of natural products via site- selective P450 oxidation
CHENG Zhongyu, LI Fuzhuo
- Department of Natural Medicine,School of Pharmacy,Fudan University,Shanghai 201203,China
-
Received:2024-02-04Revised:2024-05-21Online:2024-11-20Published:2024-10-31 -
Contact:LI Fuzhuo
基于P450选择性氧化的天然产物化学-酶法合成进展
程中玉, 李付琸
- 复旦大学药学院,天然药物学系,上海 201203
-
通讯作者:李付琸 -
作者简介:程中玉 (1998—),男,研究助理。研究方向为活性天然产物化学-酶法合成。 E-mail:zhongyu_cheng@fudan.edu.cn李付琸 (1990—),男,青年研究员,博士生导师。研究方向为酶催化反应开发,活性天然产物化学-酶法合成等。 E-mail:lifuzhuo@fudan.edu.cn -
基金资助:国家自然科学基金(22301041);上海市自然科学基金(23ZR1412900)
CLC Number:
Cite this article
CHENG Zhongyu, LI Fuzhuo. Recent advances in chemoenzymatic synthesis of natural products via site- selective P450 oxidation[J]. Synthetic Biology Journal, 2024, 5(5): 960-980.
程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-017
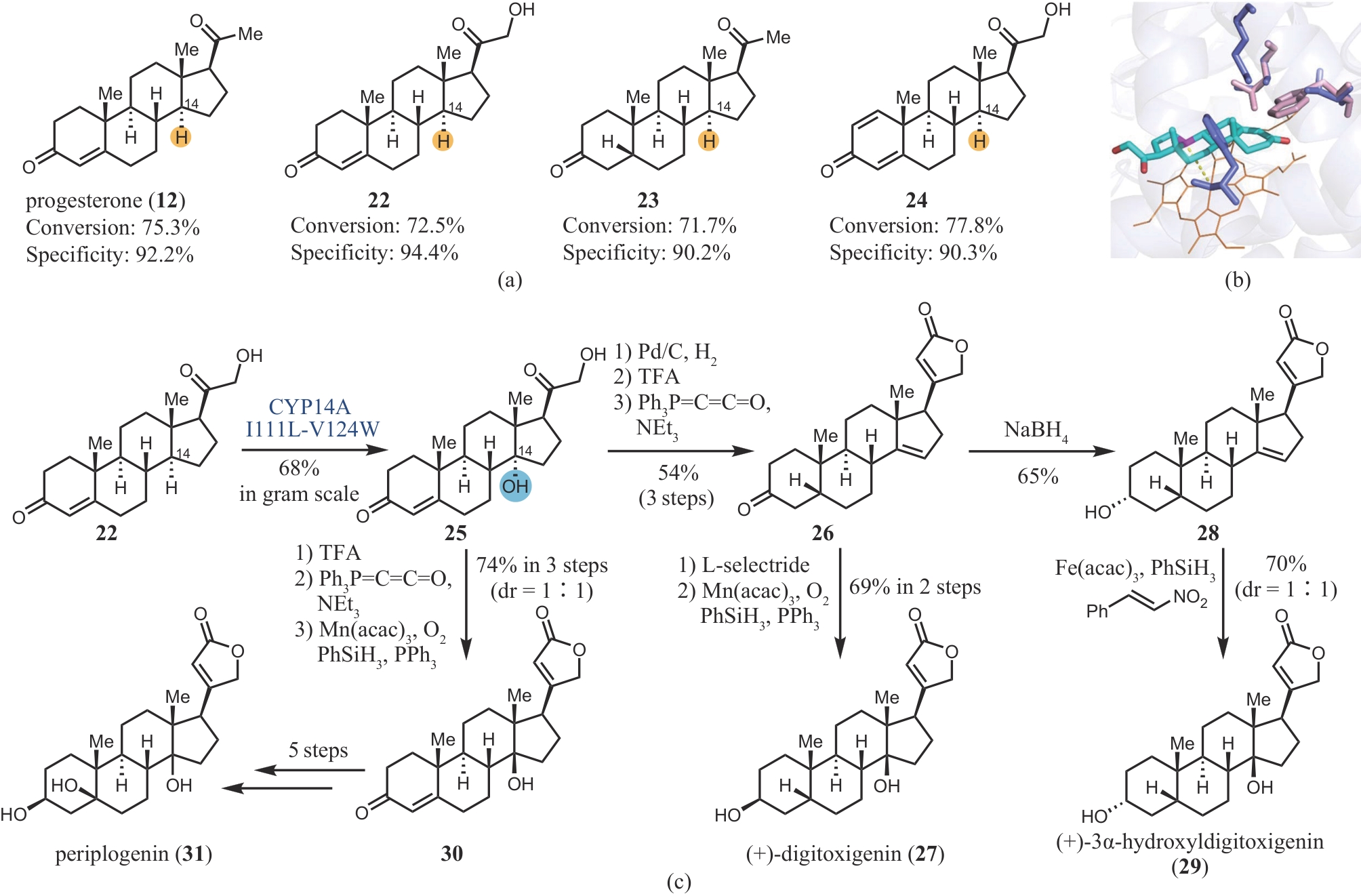
Fig. 4 Regioselectivity of CYP14A I111L-V124W(a), comparison of the wide-type CYP14A and mutant(b), and chemoenzymatic synthesis of digitoxigenin, 3α-hydroxyldigitoxigenin and periplogenin(c)
| 1 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019[J]. Journal of Natural Products, 2020, 83(3): 770-803. |
| 2 | NEWHOUSE T, BARAN P S, HOFFMANN R W. The economies of synthesis[J]. Chemical Society Reviews, 2009, 38(11): 3010-3021. |
| 3 | KUTTRUFF C A, EASTGATE M D, BARAN P S. Natural product synthesis in the age of scalability[J]. Natural Product Reports, 2014, 31(4): 419-432. |
| 4 | ATANASOV A G, ZOTCHEV S B, DIRSCH V M, et al. Natural products in drug discovery: advances and opportunities[J]. Nature Reviews Drug Discovery, 2021, 20(3): 200-216. |
| 5 | LI J, AMATUNI A, RENATA H. Recent advances in the chemoenzymatic synthesis of bioactive natural products[J]. Current Opinion in Chemical Biology, 2020, 55: 111-118. |
| 6 | SIMIĆ S, ZUKIĆ E, SCHMERMUND L, et al. Shortening synthetic routes to small molecule active pharmaceutical ingredients employing biocatalytic methods[J]. Chemical Reviews, 2022, 122(1): 1052-1126. |
| 7 | CHAKRABARTY S, ROMERO E O, PYSER J B, et al. Chemoenzymatic total synthesis of natural products[J]. Accounts of Chemical Research, 2021, 54(6): 1374-1384. |
| 8 | ABRAMS D J, PROVENCHER P A, SORENSEN E J. Recent applications of C—H functionalization in complex natural product synthesis[J]. Chemical Society Reviews, 2018, 47(23): 8925-8967. |
| 9 | SINHA S K, GHOSH P, JAIN S, et al. Transition-metal catalyzed C—H activation as a means of synthesizing complex natural products[J]. Chemical Society Reviews, 2023, 52(21): 7461-7503. |
| 10 | LI F Z, ZHANG X, RENATA H. Enzymatic C—H functionalizations for natural product synthesis[J]. Current Opinion in Chemical Biology, 2019, 49: 25-32. |
| 11 | TURNER N J. Directed evolution drives the next generation of biocatalysts[J]. Nature Chemical Biology, 2009, 5(8): 567-573. |
| 12 | PACKER M S, LIU D R. Methods for the directed evolution of proteins[J]. Nature Reviews Genetics, 2015, 16(7): 379-394. |
| 13 | DENISOV I G, MAKRIS T M, SLIGAR S G, et al. Structure and chemistry of cytochrome P450[J]. Chemical Reviews, 2005, 105(6): 2253-2278. |
| 14 | GUENGERICH F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity[J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. |
| 15 | 蒋媛媛, 李盛英. 细胞色素P450酶在生物合成及有机合成中的催化功能及其应用[J]. 有机化学, 2018, 38(9): 2307-2323. |
| JIANG Y Y, LI S Y. Catalytic function and application of cytochrome P450 enzymes in biosynthesis and organic synthesis[J]. Chinese Journal of Organic Chemistry, 2018, 38(9): 2307-2323. | |
| 16 | FESSNER N D. P450 monooxygenases enable rapid late-stage diversification of natural products via C—H bond activation[J]. ChemCatChem, 2019, 11(9): 2226-2242. |
| 17 | XIONG L B, LIU H H, ZHAO M, et al. Enhancing the bioconversion of phytosterols to steroidal intermediates by the deficiency of kasB in the cell wall synthesis of Mycobacterium neoaurum [J]. Microbial Cell Factories, 2020, 19(1): 80. |
| 18 | ZHENG M M, LIN Z, LIN S J, et al. Chemoenzymatic synthesis of steroidal products: recent advances[J]. European Journal of Organic Chemistry, 2024: e202301066. |
| 19 | ZHANG X D, PENG Y Q, ZHAO J, et al. Bacterial cytochrome P450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design[J]. Bioresources and Bioprocessing, 2020, 7(1): 2. |
| 20 | RENATA H, ZHOU Q H, BARAN P S. Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide[J]. Science, 2013, 339(6115): 59-63. |
| 21 | RENATA H, ZHOU Q H, DÜNSTL G, et al. Development of a concise synthesis of ouabagenin and hydroxylated corticosteroid analogues[J]. Journal of the American Chemical Society, 2015, 137(3): 1330-1340. |
| 22 | WOLFF M E, MORIOKA T. C-19 functional steroids. Ⅹ.17β-hydroxy-1β,19-cyclo-5α-androstan-2-one and related compounds[J]. Journal of Organic Chemistry, 1965, 30: 2553-2557. |
| 23 | WANG Y, JU W, TIAN H L, et al. Scalable synthesis of cyclocitrinol[J]. Journal of the American Chemical Society, 2018, 140(30): 9413-9416. |
| 24 | CLARK T A, CHONG R, MADDOX I S. An investigation into the 19-hydroxylation of androstenedione, cortexolone and progesterone by selected fungi[J]. Applied Microbiology and Biotechnology, 1985, 21(1): 132-134. |
| 25 | WANG J L, ZHANG Y N, LIU H H, et al. A biocatalytic hydroxylation-enabled unified approach to C19-hydroxylated steroids[J]. Nature Communications, 2019, 10(1): 3378. |
| 26 | PRASSAS I, DIAMANDIS E P. Novel therapeutic applications of cardiac glycosides[J]. Nature Reviews Drug Discovery, 2008, 7(11): 926-935. |
| 27 | DU J, JIANG L J, CHEN F Q, et al. Cardiac glycoside ouabain exerts anticancer activity via downregulation of STAT3[J]. Frontiers in Oncology, 2021, 11: 684316. |
| 28 | ZEITLIN P L, DIENER-WEST M, CALLAHAN K A, et al. Digitoxin for airway inflammation in cystic fibrosis: preliminary assessment of safety, pharmacokinetics, and dose finding[J]. Annals of the American Thoracic Society, 2017, 14(2): 220-229. |
| 29 | RIJSBERGEN M, NIEMEYER-VAN DER KOLK T, HOGENDOORN G, et al. A randomized controlled proof-of-concept trial of digoxin and furosemide in adults with cutaneous warts[J]. British Journal of Dermatology, 2019, 180(5): 1058-1068. |
| 30 | FEJEDELEM Z, CARNEY N, NAGORNY P. Synthesis of cardiotonic steroids oleandrigenin and rhodexin B[J]. The Journal of Organic Chemistry, 2021, 86(15): 10249-10262. |
| 31 | KHATRI H R, BHATTARAI B, KAPLAN W, et al. Modular total synthesis and cell-based anticancer activity evaluation of ouabagenin and other cardiotonic steroids with varying degrees of oxygenation[J]. Journal of the American Chemical Society, 2019, 141(12): 4849-4860. |
| 32 | SHIMIZU S, HAGIWARA K, ITOH H, et al. Unified total synthesis of five bufadienolides[J]. Organic Letters, 2020, 22(21): 8652-8657. |
| 33 | ZHAO Y, ZHANG B, SUN Z, et al. Biocatalytic C14-hydroxylation on androstenedione enabled modular synthesis of cardiotonic steroids[J]. ACS Catalysis, 2022, 12(16): 9839-9845. |
| 34 | SONG F Z, ZHENG M M, WANG J L, et al. Chemoenzymatic synthesis of C14-functionalized steroids[J]. Nature Synthesis, 2023, 2: 729-739. |
| 35 | YARROW J F, MCCOY S C, BORST S E. Tissue selectivity and potential clinical applications of trenbolone (17β-hydroxyestra-4,9,11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity[J]. Steroids, 2010, 75(6): 377-389. |
| 36 | 张沪跃, 陈燕, 施蛟, 等. 高收率合成醋酸群勃龙[J]. 复旦学报(医学版), 2002, 29(3): 211-212. |
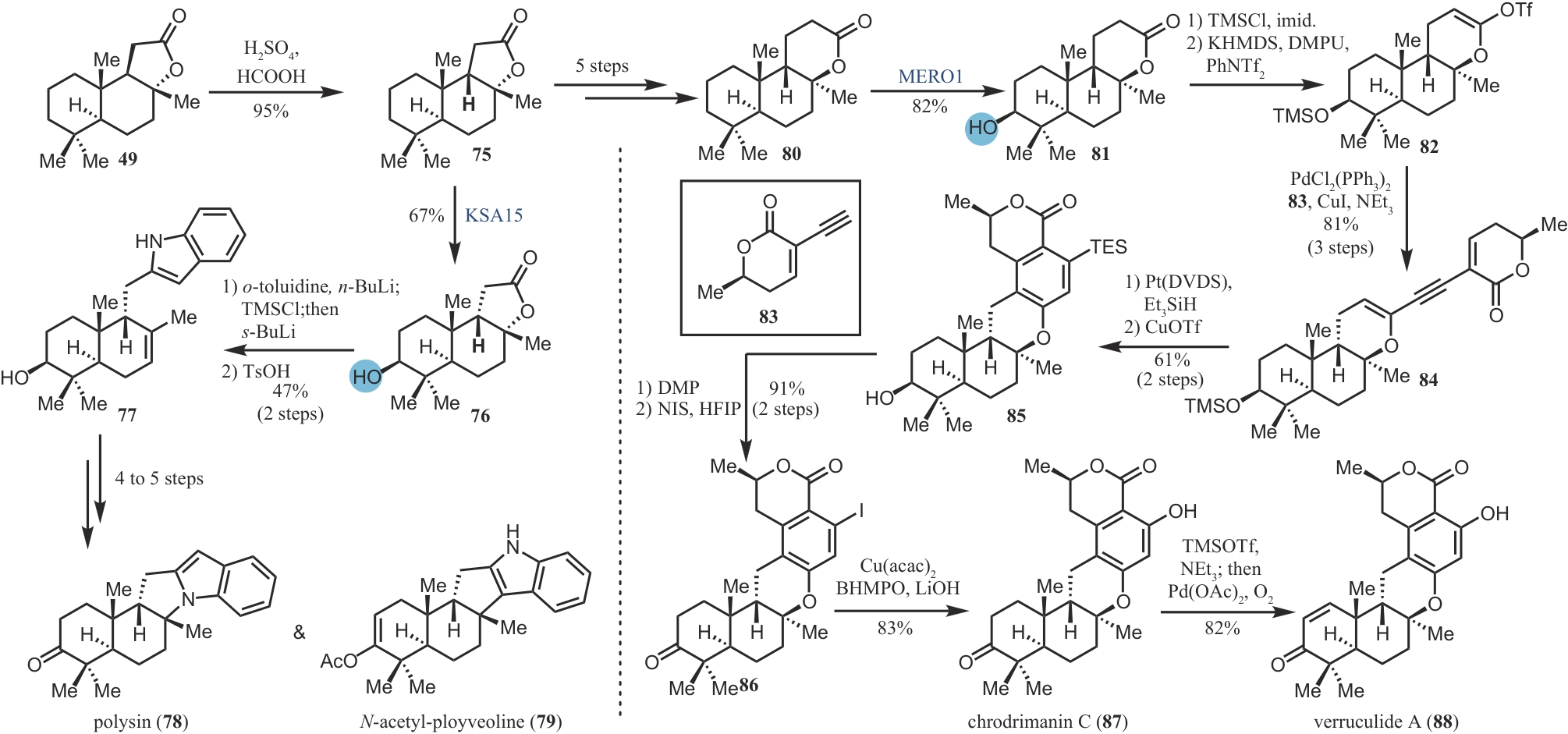
| ZHANG H Y, CHEN Y, SHI J, et al. Synthesis of trenbolone acetate in high yield[J]. Fudan University Journal of Medical Science, 2002, 29(3): 211-212. | |
| 37 | LI A T, ACEVEDO-ROCHA C G, D’AMORE L, et al. Regio- and stereoselective steroid hydroxylation at C7 by cytochrome P450 monooxygenase mutants[J]. Angewandte Chemie International Edition, 2020, 59(30): 12499-12505. |
| 38 | PENG Y Q, GAO C H, ZHANG Z L, et al. A chemoenzymatic strategy for the synthesis of steroid drugs enabled by P450 monooxygenase-mediated steroidal core modification[J]. ACS Catalysis, 2022, 12(5): 2907-2914. |
| 39 | LI C, QIU W W, YANG Z F, et al. Stereoselective synthesis of some methyl-substituted steroid hormones and their in vitro cytotoxic activity against human gastric cancer cell line MGC-803[J]. Steroids, 2010, 75(12): 859-869. |
| 40 | ZHANG Z L, GAO C H, ZHAO J, et al. A designed chemoenzymatic route for efficient synthesis of 6-dehydronandrolone acetate: a key precursor in the synthesis of C7-functionalized steroidal drugs[J]. ACS Catalysis, 2023, 13(19): 13111-13116. |
| 41 | CHEN Q B, XIN X L, YANG Y, et al. Highly conjugated norditerpenoid and pyrroloquinoline alkaloids with potent PTP1B inhibitory activity from Nigella glandulifera [J]. Journal of Natural Products, 2014, 77(4): 807-812. |
| 42 | ZHANG S, ZHANG Z Y. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery[J]. Drug Discovery Today, 2007, 12(9-10): 373-381. |
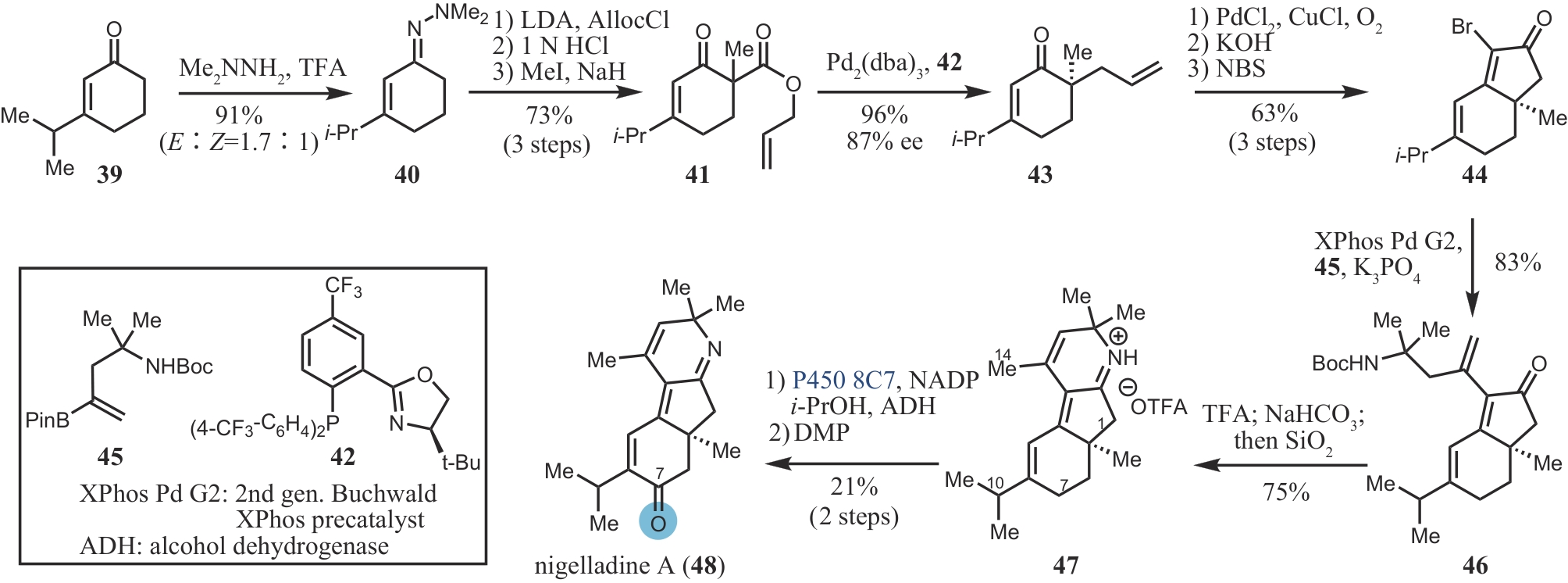
| 43 | LOSKOT S A, ROMNEY D K, ARNOLD F H, et al. Enantioselective total synthesis of nigelladine A via late-stage C—H oxidation enabled by an engineered P450 enzyme[J]. Journal of the American Chemical Society, 2017, 139(30): 10196-10199. |
| 44 | BRUNO N C, TUDGE M T, BUCHWALD S L. Design and preparation of new palladium precatalysts for C—C and C—N cross-coupling reactions[J]. Chemical Science, 2013, 4: 916-920. |
| 45 | LEWIS J C, MANTOVANI S M, FU Y, et al. Combinatorial alanine substitution enables rapid optimization of cytochrome P450BM3 for selective hydroxylation of large substrates[J]. ChemBioChem, 2010, 11(18): 2502-2505. |
| 46 | SUNAZUKA T, ŌMURA S. Total synthesis of α-pyrone meroterpenoids, novel bioactive microbial metabolites[J]. Chemical Reviews, 2005, 105(12): 4559-4580. |
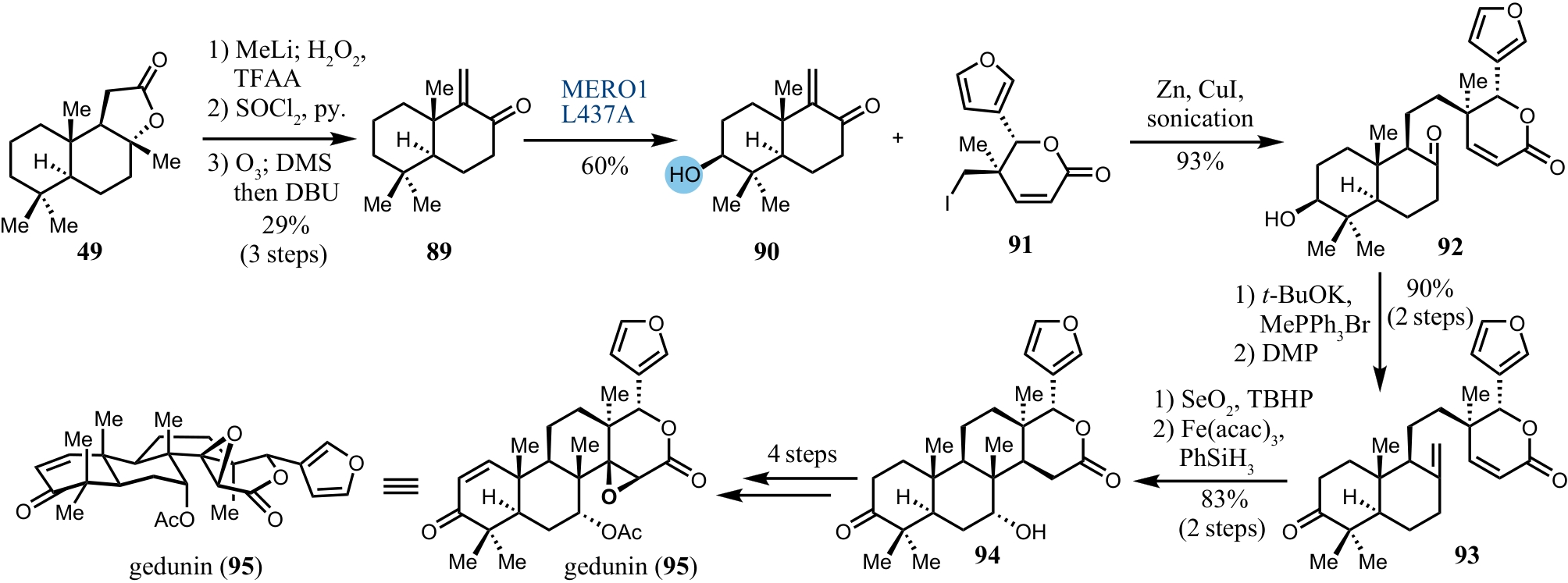
| 47 | COREY E J, NOE M C, LIN S Z. A mechanistically designed bis-cinchona alkaloid ligand allows position- and enantioselective dihydroxylation of farnesol and other oligoprenyl derivatives at the terminal isopropylidene unit[J]. Tetrahedron Letters, 1995, 36(48): 8741-8744. |
| 48 | KUMANIRENG A S, KATO T, KITAHARA Y. Cyclization of polyenes X. Biogenetic type synthesis of dl-taondiol[J]. Chemistry Letters, 1973, 2(10): 1045-1047. |
| 49 | ABAD A, AGULLÓ C, ARNÓ M, et al. An efficient stereoselective synthesis of stypodiol and epistypodiol[J]. The Journal of Organic Chemistry, 1998, 63(15): 5100-5106. |
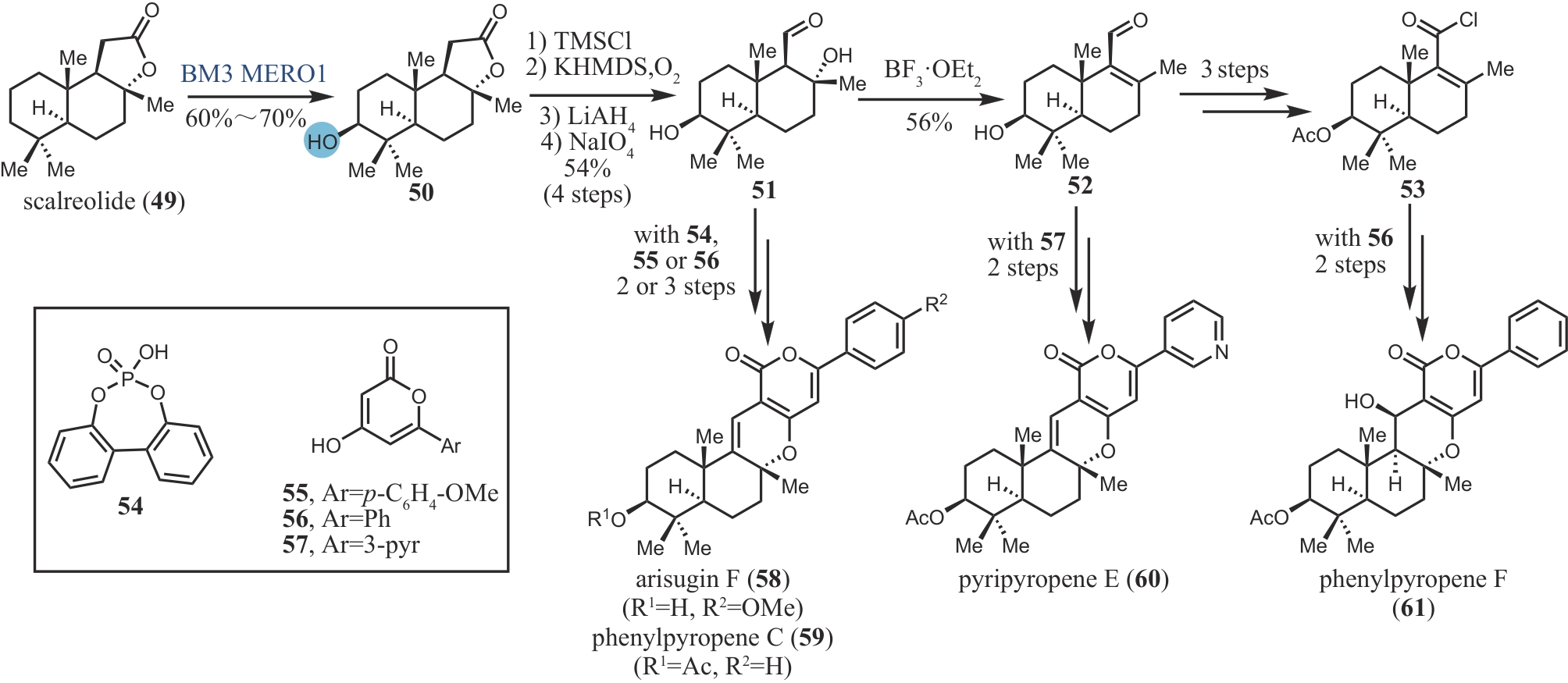
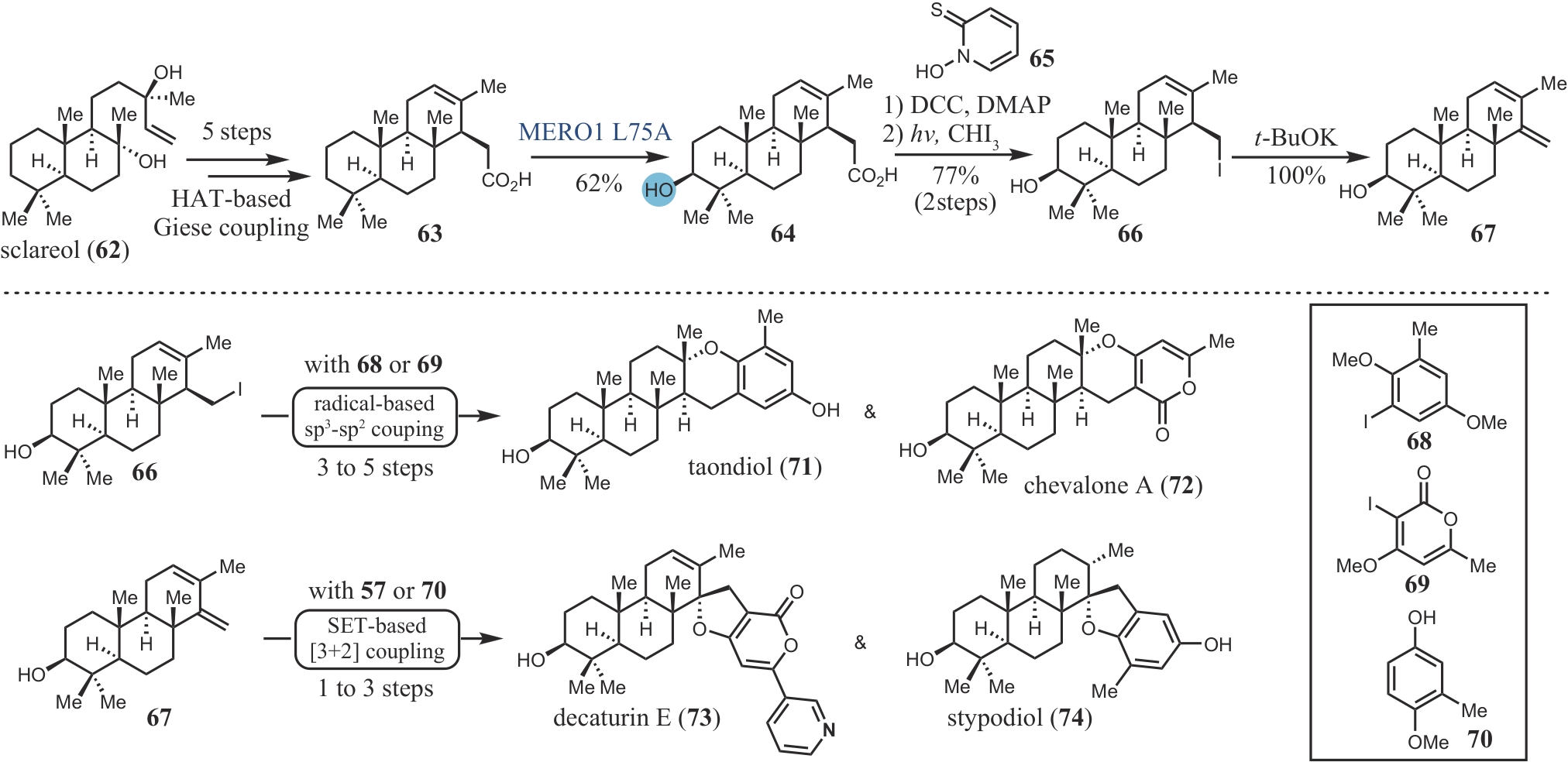
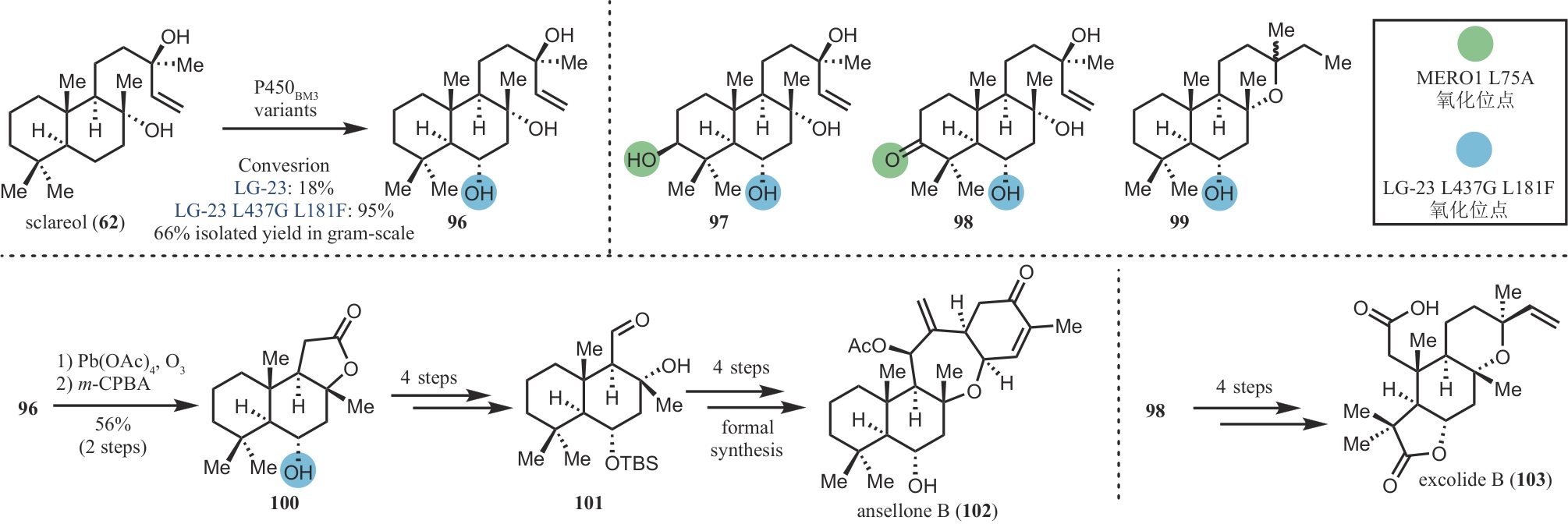
| 50 | TAKIKAWA H, IMAMURA Y, SASAKI M. Synthesis and absolute configuration of brevione B, an allelochemical isolated from Penicillium sp[J]. Tetrahedron, 2006, 62(1): 39-48. |
| 51 | LI J, LI F Z, KING-SMITH E, et al. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids[J]. Nature Chemistry, 2020, 12: 173-179. |
| 52 | EVERSON D A, SHRESTHA R, WEIX D J. Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides[J]. Journal of the American Chemical Society, 2010, 132(3): 920-921. |
| 53 | ANADA M, HANARI T, KAKITA K, et al. Total synthesis of brasilicardins A and C[J]. Organic Letters, 2017, 19(20): 5581-5584. |
| 54 | BOYKO Y D, HUCK C J, NING S, et al. Synthetic studies on selective, proapoptotic isomalabaricane triterpenoids aided by computational techniques[J]. Journal of the American Chemical Society, 2021, 143(4): 2138-2155. |
| 55 | BOYKO Y D, HUCK C J, SARLAH D. Total synthesis of isomalabaricane triterpenoids[J]. Journal of the American Chemical Society, 2019, 141(36): 14131-14135. |
| 56 | POWERS Z, SCHARF A, CHENG A, et al. Biomimetic synthesis of meroterpenoids by dearomatization-driven polycyclization[J]. Angewandte Chemie International Edition, 2019, 58(45): 16141-16146. |
| 57 | YOSHIMURA F, ITOH R, TORIZUKA M, et al. Asymmetric total synthesis of brasilicardins[J]. Angewandte Chemie International Edition, 2018, 57(52): 17161-17167. |
| 58 | MITSUHASHI T, BARRA L, POWERS Z, et al. Exploiting the potential of meroterpenoid cyclases to expand the chemical space of fungal meroterpenoids[J]. Angewandte Chemie International Edition, 2020, 59(52): 23772-23781. |
| 59 | VAN TAMELEN E E, SHARPLESS K B, HANZLIK R P, et al. Enzymic cyclization of trans, trans, trans-18,19-dehydrosqualene 2,3-oxide[J]. Journal of the American Chemical Society, 1967, 89(26): 7150-7151. |
| 60 | LI F Z, RENATA H. A Chiral-pool-based strategy to access trans-syn-fused drimane meroterpenoids: chemoenzymatic total syntheses of polysin, N-acetyl-polyveoline and the chrodrimanins[J]. Journal of the American Chemical Society, 2021, 143(43): 18280-18286. |
| 61 | KILLE S, ZILLY F E, ACEVEDO J P, et al. Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution[J]. Nature Chemistry, 2011, 3(9): 738-743. |
| 62 | SMITH A B, VISNICK M, HASELTINE J N, et al. Organometallic reagents in synthesis: a new protocol for construction of the indole nucleus[J]. Tetrahedron, 1986, 42(11): 2957-2969. |
| 63 | FIER P S, MALONEY K M. Synthesis of complex phenols enabled by a rationally designed hydroxide surrogate[J]. Angewandte Chemie International Edition, 2017, 56(16): 4478-4482. |
| 64 | FU S M, LIU B. Recent progress in the synthesis of limonoids and limonoid-like natural products[J]. Organic Chemistry Frontiers, 2020, 7(14): 1903-1947. |
| 65 | BRANDT G E L, SCHMIDT M D, PRISINZANO T E, et al. Gedunin, a novel Hsp90 inhibitor: semisynthesis of derivatives and preliminary structure-activity relationships[J]. Journal of Medicinal Chemistry, 2008, 51(20): 6495-6502. |
| 66 | ENGLISH A W, LIU K, NICOLINI J M, et al. Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(40): 16217-16222. |
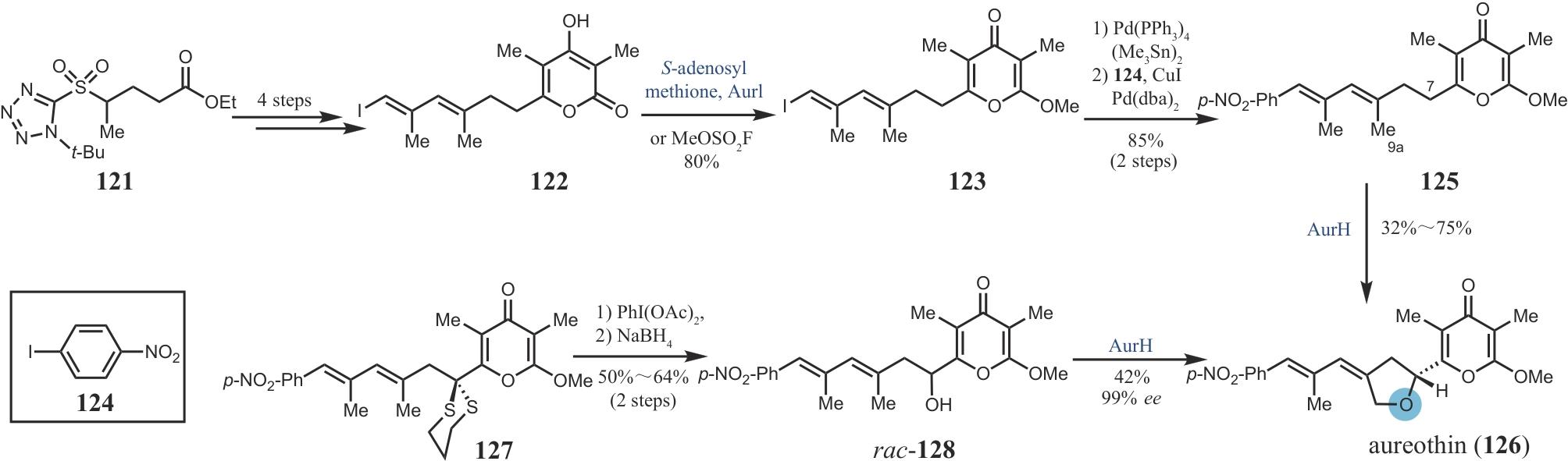
| 67 | GORANTLA N V, DAS R, CHIDAMBARAM H, et al. Basic limonoid modulates chaperone-mediated proteostasis and dissolve Tau fibrils[J]. Scientific Reports, 2020, 10(1): 4023. |
| 68 | BEHENNA D C, COREY E J. Simple enantioselective approach to synthetic limonoids[J]. Journal of the American Chemical Society, 2008, 130(21): 6720-6721. |
| 69 | COREY E J, HAHL R W. Synthesis of a limonoid, azadiradione[J]. Tetrahedron Letters, 1989, 30(23): 3023-3026. |
| 70 | YAMASHITA S, NARUKO A, NAKAZAWA Y, et al. Total synthesis of limonin[J]. Angewandte Chemie International Edition, 2015, 54(29): 8538-8541. |
| 71 | LI J, CHEN F, RENATA H. Concise chemoenzymatic synthesis of gedunin[J]. Journal of the American Chemical Society, 2022, 144(42): 19238-19242. |
| 72 | FENG J J, GARZA V J, KRISCHE M J. Redox-triggered C—C coupling of alcohols and vinyl epoxides: diastereo- and enantioselective formation of all-carbon quaternary centers via tert-(hydroxy)-prenylation[J]. Journal of the American Chemical Society, 2014, 136(25): 8911-8914. |
| 73 | BRILL Z G, CONDAKES M L, TING C P, et al. Navigating the chiral pool in the total synthesis of complex terpene natural products[J]. Chemical Reviews, 2017, 117(18): 11753-11795. |
| 74 | LI F Z, DENG H P, RENATA H. Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids[J]. Journal of the American Chemical Society, 2022, 144(17): 7616-7621. |
| 75 | ZHANG W, YAO H L, YU J X, et al. Total syntheses of sesterterpenoid ansellones A and B, and phorbadione[J]. Angewandte Chemie International Edition, 2017, 56(17): 4787-4791. |
| 76 | HE H B, JIANG H, CHEN Y, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity[J]. Nature Communications, 2018, 9(1): 2550. |
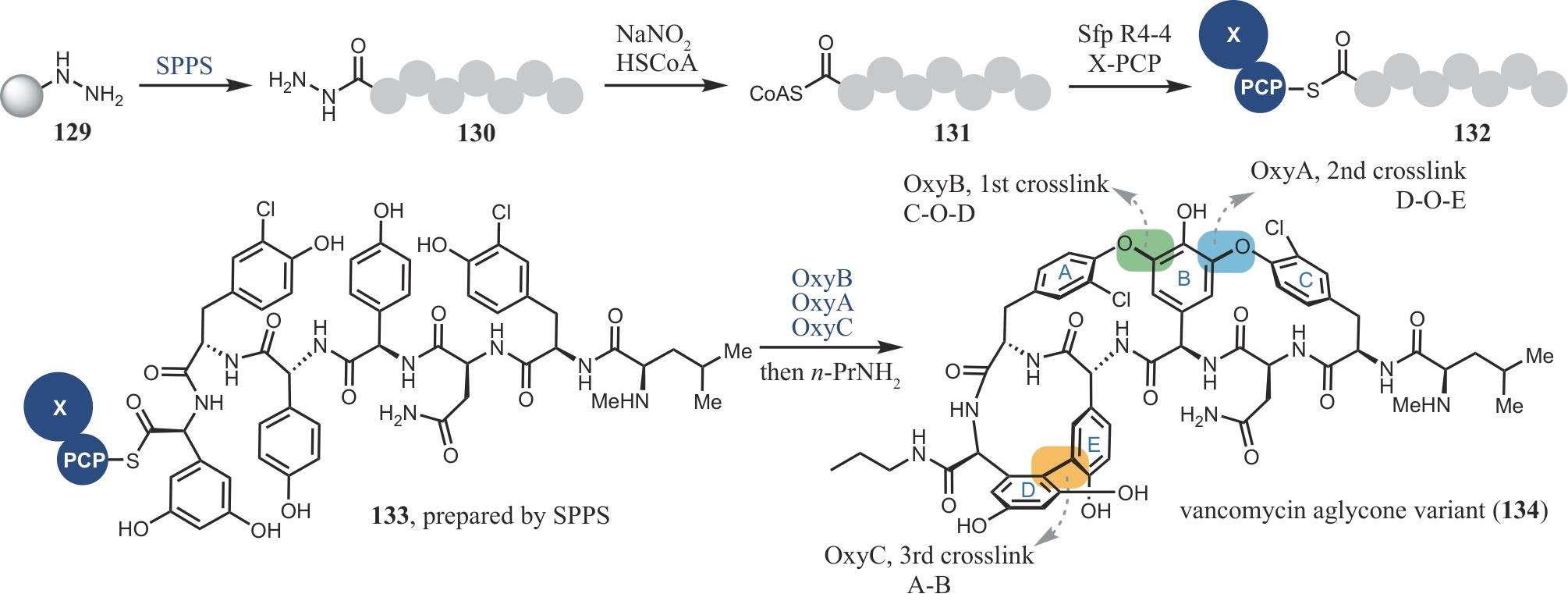
| 77 | LIAO Y J, BAI H Y, LI Z H, et al. Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells[J]. Cell Death & Disease, 2014, 5(3): e1137. |
| 78 | HONG Y J, TANTILLO D J. Formation of beyerene, kaurene, trachylobane, and atiserene diterpenes by rearrangements that avoid secondary carbocations[J]. Journal of the American Chemical Society, 2010, 132(15): 5375-5386. |
| 79 | LAZARSKI K E, MORITZ B J, THOMSON R J. The total synthesis of Isodon diterpenes[J]. Angewandte Chemie International Edition, 2014, 53(40): 10588-10599. |
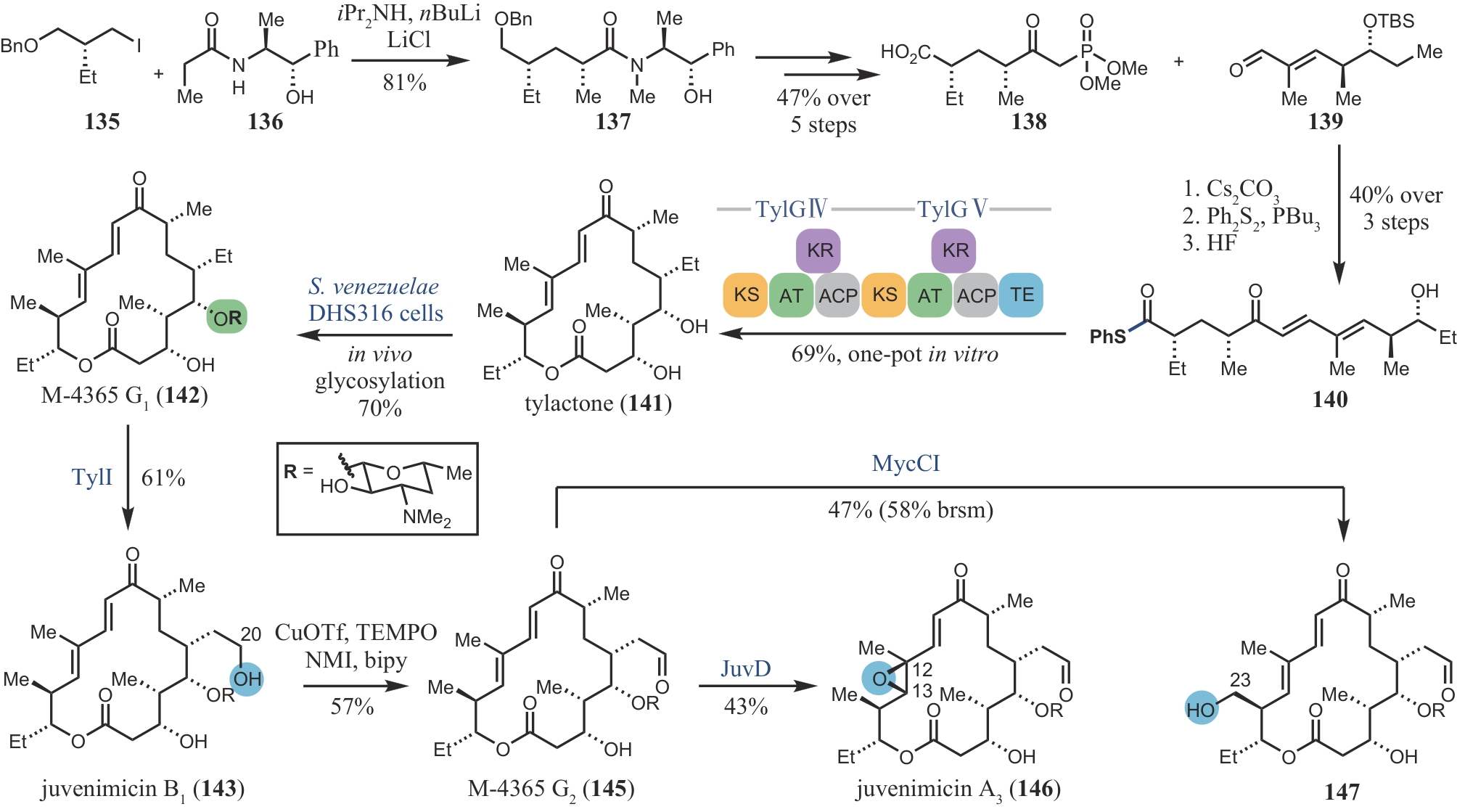
| 80 | RIEHL P S, DEPORRE Y C, ARMALY A M, et al. New avenues for the synthesis of ent-kaurene diterpenoids[J]. Tetrahedron, 2015, 71(38): 6629-6650. |
| 81 | CHERNEY E C, LOPCHUK J M, GREEN J C, et al. A unified approach to ent-atisane diterpenes and related alkaloids: synthesis of (-)-methyl atisenoate, (-)-isoatisine, and the hetidine skeleton[J]. Journal of the American Chemical Society, 2014, 136(36): 12592-12595. |
| 82 | KENNY M J, MANDER L N, SETHI S P. Synthetic studies on rabdosia diterpene lactones Ⅱ: the synthesis of 15-desoxyeffusin[J]. Tetrahedron Letters, 1986, 27(33): 3927-3930. |
| 83 | KOBAYASHI S, SHIBUKAWA K, HAMADA Y, et al. Syntheses of (-)-tripterifordin and (-)-neotripterifordin from stevioside[J]. The Journal of Organic Chemistry, 2018, 83(3): 1606-1613. |
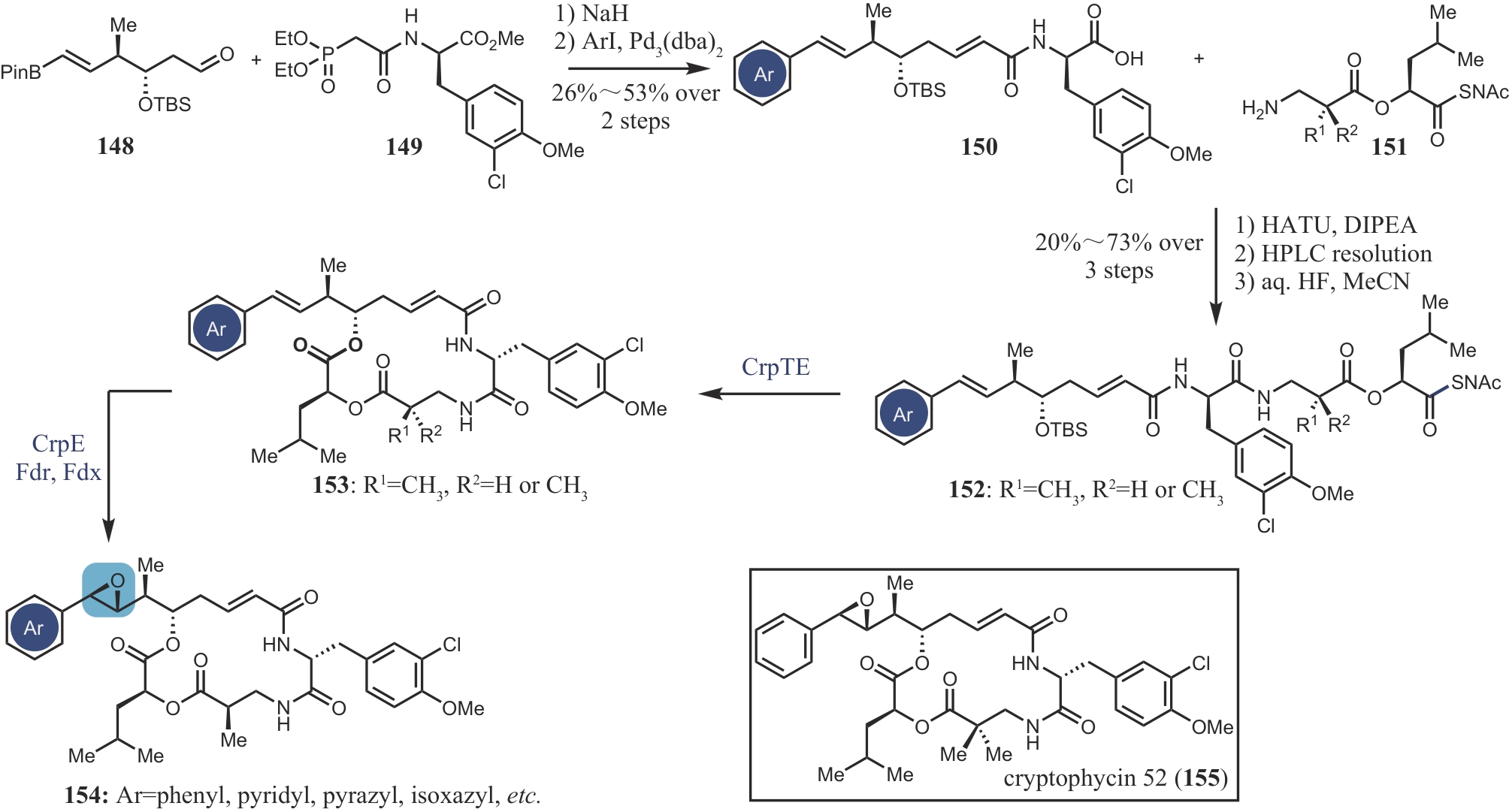
| 84 | DONG L B, ZHANG X, RUDOLF J D, et al. Cryptic and stereospecific hydroxylation, oxidation, and reduction in platensimycin and platencin biosynthesis[J]. Journal of the American Chemical Society, 2019, 141(9): 4043-4050. |
| 85 | RUDOLF J D, DONG L B, ZHANG X, et al. Cytochrome P450-catalyzed hydroxylation initiating ether formation in platensimycin biosynthesis[J]. Journal of the American Chemical Society, 2018, 140(39): 12349-12353. |
| 86 | ZHANG X, KING-SMITH E, DONG L B, et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach[J]. Science, 2020, 369(6505): 799-806. |
| 87 | OISHI H, HOSOKAWA T, OKUTOMI T, et al. Pesticidal activity of aureothin[J]. Agricultural and Biological Chemistry, 1969, 33(12): 1790-1791. |
| 88 | JACOBSEN M F, MOSES J E, ADLINGTON R M, et al. A short total synthesis of aureothin and N-acetylaureothamine[J]. Organic Letters, 2005, 7(4): 641-644. |
| 89 | LIANG G X, SEIPLE I B, TRAUNER D. Stereoselective syntheses of the bioactive polypropionates aureothin, N-acetylaureothamine, and aureonitrile[J]. Organic Letters, 2005, 7(14): 2837-2839. |
| 90 | ISHIBASHI Y, OHBA S, NISHIYAMA S, et al. Absolute configuration of (+)-aureothin: a toxic metabolite possessing γ-pyrone unit[J]. Bulletin of the Chemical Society of Japan, 1995, 68(12): 3643-3649. |
| 91 | HE J, HERTWECK C. Biosynthetic origin of the rare nitroaryl moiety of the polyketide antibiotic aureothin: involvement of an unprecedented N-oxygenase[J]. Journal of the American Chemical Society, 2004, 126(12): 3694-3695. |
| 92 | HE J, MÜLLER M, HERTWECK C. Formation of the aureothin tetrahydrofuran ring by a bifunctional cytochrome P450 monooxygenase[J]. Journal of the American Chemical Society, 2004, 126(51): 16742-16743. |
| 93 | RICHTER M E, TRAITCHEVA N, KNÜPFER U, et al. Sequential asymmetric polyketide heterocyclization catalyzed by a single cytochrome P450 monooxygenase (AurH)[J]. Angewandte Chemie International Edition, 2008, 47(46): 8872-8875. |
| 94 | WERNEBURG M, HERTWECK C. Chemoenzymatic total synthesis of the antiproliferative polyketide (+)-(R)-aureothin[J]. ChemBioChem, 2008, 9(13): 2064-2066. |
| 95 | HENROT M, RICHTER M E A, MADDALUNO J, et al. Convergent asymmetric synthesis of (+)-aureothin employing an oxygenase-mediated resolution step[J]. Angewandte Chemie International Edition, 2012, 51(38): 9587-9591. |
| 96 | LEVINE D P. Vancomycin: a history[J]. Clinical Infectious Diseases, 2006, 42(): S5-S12. |
| 97 | SHUGRUE C R, MILLER S J. Applications of nonenzymatic catalysts to the alteration of natural products[J]. Chemical Reviews, 2017, 117(18): 11894-11951. |
| 98 | HUBBARD B K, WALSH C T. Vancomycin assembly: nature’s way[J]. Angewandte Chemie International Edition, 2003, 42(7): 730-765. |
| 99 | HUBBARD B K, WALSH C T. Der Aufbau von Vancomycin: so macht es die Natur[J]. Angewandte Chemie, 2003, 115(7): 752-789. |
| 100 | OKANO A, ISLEY N A, BOGER D L. Total syntheses of vancomycin-related glycopeptide antibiotics and key analogues[J]. Chemical Reviews, 2017, 117(18): 11952-11993. |
| 101 | STEGMANN E, PELZER S, BISCHOFF D, et al. Genetic analysis of the balhimycin (vancomycin-type) oxygenase genes[J]. Journal of Biotechnology, 2006, 124(4): 640-653. |
| 102 | FORNERIS C C, SEYEDSAYAMDOST M R. In vitro reconstitution of OxyC activity enables total chemoenzymatic syntheses of vancomycin aglycone variants[J]. Angewandte Chemie International Edition, 2018, 57(27): 8048-8052. |
| 103 | HAUSER N, IRELAND K A, CHIOTI V T, et al. Robust chemoenzymatic synthesis of keratinimicin aglycone analogues facilitated by the structure and selectivity of OxyB[J]. ACS Chemical Biology, 2023, 18(7): 1473-1479. |
| 104 | LOWELL A N, M D Ⅱ DEMARS, SLOCUM S T, et al. Chemoenzymatic total synthesis and structural diversification of tylactone-based macrolide antibiotics through late-stage polyketide assembly, tailoring, and C—H functionalization[J]. Journal of the American Chemical Society, 2017, 139(23): 7913-7920. |
| 105 | CHAGANTY S, GOLAKOTI T, HELTZEL C, et al. Isolation and structure determination of cryptophycins 38, 326, and 327 from the terrestrial cyanobacterium Nostoc sp. GSV 224[J]. Journal of Natural Products, 2004, 67(8): 1403-1406. |
| 106 | SMITH C D, ZHANG X Q, MOOBERRY S L, et al. Cryptophycin: a new antimicrotubule agent active against drug-resistant cells[J]. Cancer Research, 1994, 54(14): 3779-3784. |
| 107 | EDELMAN M J, GANDARA D R, HAUSNER P, et al. Phase 2 study of cryptophycin 52 (LY355703) in patients previously treated with platinum based chemotherapy for advanced non-small cell lung cancer[J]. Lung Cancer, 2003, 39(2): 197-199. |
| 108 | MAGARVEY N A, BECK Z Q, GOLAKOTI T, et al. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from Nostoc cyanobionts[J]. ACS Chemical Biology, 2006, 1(12): 766-779. |
| 109 | SUN H H, ZHANG H F, ANG E L, et al. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates[J]. Bioorganic & Medicinal Chemistry, 2018, 26(7): 1275-1284. |
| 110 | YANG Y, ARNOLD F H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer[J]. Accounts of Chemical Research, 2021, 54(5): 1209-1225. |
| 111 | ZHOU Q, CHIN M, FU Y, et al. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450[J]. Science, 2021, 374(6575): 1612-1616. |
| 112 | MILLER D C, ATHAVALE S V, ARNOLD F H. Combining chemistry and protein engineering for new-to-nature biocatalysis[J]. Nature Synthesis, 2022, 1(1): 18-23. |
| 113 | 杨谦, 程伯涛, 汤志军, 等. 基因组挖掘在天然产物发现中的应用和前景[J]. 合成生物学, 2021, 2(5): 697-715. |
| YANG Q, CHENG B T, TANG Z J, et al. Applications and prospects of genome mining in the discovery of natural products[J]. Synthetic Biology Journal, 2021, 2(5): 697-715. | |
| 114 | WU Z, JENNIFER KAN S B J, LEWIS R D, et al. Machine learning-assisted directed protein evolution with combinatorial libraries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(18): 8852-8858. |
| 115 | JACQUES P, BÉCHET M, BIGAN M, et al. High-throughput strategies for the discovery and engineering of enzymes for biocatalysis[J]. Bioprocess and Biosystems Engineering, 2017, 40(2): 161-180. |
| 116 | KING-SMITH E, FABER F A, REILLY U, et al. Predictive Minisci late stage functionalization with transfer learning[J]. Nature Communications, 2024, 15(1): 426. |
| 117 | FINNIGAN W, LUBBERINK M, HEPWORTH L J, et al. RetroBioCat database: a platform for collaborative curation and automated meta-analysis of biocatalysis data[J]. ACS Catalysis, 2023, 13(17): 11771-11780. |
| [1] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [2] | YANG Haoran, YE Farong, HUANG Ping, WANG Ping. Recent advances in glycoprotein synthesis [J]. Synthetic Biology Journal, 2024, 5(5): 1072-1101. |
| [3] | Faguang ZHANG, Ge QU, Zhoutong SUN, Jun′an MA. From chemical synthesis to biosynthesis: trends toward total synthesis of natural products [J]. Synthetic Biology Journal, 2021, 2(5): 674-696. |
| [4] | Yu LIU, Huiling WEI, Jixiang LIU, Shaojie WANG, Haijia SU. Design and progress of synthetic consortia: a new frontier in synthetic biology [J]. Synthetic Biology Journal, 2021, 2(4): 635-650. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||